Introduction
The morbidity and mortality rates associated with
lung cancer have been increasing annually and lung cancer has
become one of the most severe threats to human life and health
(1). Due to the lack of early
diagnostic tools and the absence of disease symptoms in the early
stages of the disease, the majority of patients are diagnosed at an
advanced stage (2,3). Central lung cancer refers to tumors
that originate in the central part of the lung, including the main
bronchus and adjacent structures, and is particularly challenging
to treat. Radiotherapy is a standard of care and a curative
treatment option for patients with lung cancer. To improve
treatment outcomes, dose escalation is routinely used; however, it
is frequently associated with the incidence of radiation
pneumonitis, a dose-volume-dependent side-effect that is associated
with the mean lung dose and the volume of the lung receiving a dose
of at least 20 Gy (V20) (4,5). In
order to address these challenges, it is crucial to carefully
evaluate the risks and benefits of radiotherapy for central lung
cancer. By optimizing treatment planning and using appropriate dose
constraints, clinicians are able to minimize the risk of radiation
pneumonitis and improve disease outcomes for patients with lung
cancer.
Precision radiotherapy has become a routine standard
of care with the purpose of improving patient outcomes, while
mitigating the risk of overdose to target and adjacent organs. The
accurate delineation of the gross target volume (GTV) is a crucial
step in guiding precision radiotherapy. Several non-invasive
imaging procedures have been routinely used for delineating the
target volumes of lung cancer (6,7),
including intensified computed tomography (CT), positron emission
tomography (PET) and diffusion-weighted magnetic resonance imaging
(DW-MRI) (8).
However, the CT and PET procedures have inherent
limitations. The lack of contrast between soft tissues on CT images
can obscure the precision of the radiotherapy by making the
distinction of the target area from the normal tissue challenging
(9,10), particularly for central lung cancer
with pulmonary atelectasis or mediastinal nodes metastasis
(11). Although PET has been
demonstrated to be more suitable than CT in diagnosing the nodal
involvement of lung cancer (7,12,13),
it has been reported to produce an increased rate of false-positive
results (14). The combination of
PET/CT can improve sensitivity and accuracy (7,15,16);
however, the previously mentioned challenges remain, including low
image resolution and the requirement of PET and CT image fusion
(17,18).
DW-MRI is another widely used imaging procedure that
provides information concerning the Brownian movement of the water
molecules in tissues (19-21).
This method can reflect the cellular composition of the tumor and
the integrity of the tumor cell membrane (22,23).
The differential diffusion of water molecules in tumor tissues
enables DW-MRI to detect malignant tumors and differentiate them
from benign tissues (24).
Previous studies have reported that DW-MRI can provide more
accurate delineation for various types of cancer, including
prostate cancer, head and neck squamous cell carcinoma, as well as
cranial tumors (25-28).
The ability of DW-MRI to provide precise and non-invasive imaging
render it an attractive option for delineating target volumes in
patients with lung cancer. Of note, a previous study by the authors
demonstrated that DW-MRI has the potential to reduce exposure doses
to organs at risk (OARs), particularly the lungs, and minimizes the
risk of radiation pneumonitis (29). These findings suggested that DW-MRI
may play a critical role in guiding precision radiotherapy for
patients with lung cancer; further research is required to fully
investigate its potential for improving patient outcomes.
In three-dimensional conformal radiation treatment
(3D-CRT), the accurate delineation of tumor boundaries is
challenging, particularly for central lung cancer with atelectasis,
when relying solely on intensified CT for target volume
delineation. This uncertainty may result in an increased exposure
of the surrounding OARs and incidence rates of complications. A
previous study by the authors demonstrated that DW-MRI surpassed CT
and PET/CT procedures in precise and reproducible delineation of
GTVs for lung tumors (29). In the
present study, a direct comparison of these three imaging methods
is performed in terms of GTV image delineation and the resulting
dosages by the lungs, heart, and spinal cord for patients with lung
cancer with atelectasis.
Materials and methods
Patient selection
The present study (ethics approval reference no.
2015-06-85) was conducted according to a protocol approved by the
Institutional Review Board and the Ethics Committee of Shandong
Cancer Hospital and Institute. Written informed consent was
obtained from all patients prior to their enrollment in the present
study. The critical criterion of patient enrollment is the
histological diagnosis of lung cancer accompanied by a varying
degree of pulmonary atelectasis. All patients were evaluated for
their health condition with a Karnofsky Performance Scale (KPS)
score of ≥70 and were deemed eligible for MRI examination with no
contraindications. To ensure consistency, all of the images for
each patient were collected using the three aforementioned
procedures (CT, PET/CT and MRI) within 1 week.
Patient cohort
The present study recruited 27 patients with central
lung cancer who were scheduled to undergo precision radiotherapy
between October, 2014 and June, 2015, including 23 male and 4
female patients, with the patient age ranging from 37 to 79 years,
with a median of 61 years. The lung cancer types included 12 cases
of squamous cell carcinoma, six cases of adenocarcinoma, six cases
of small cell carcinoma, two cases of atypical carcinoid, and one
case of adenoid cystic carcinoma (rare form of adenocarcinoma).
Among the 27 patients with central lung cancer, 8 patients
presented with tumors in the upper left lung, 4 patients with
tumors in the lower left lung, 5 patients with tumors in the upper
right lung, 4 patients with tumors in the middle right lung, and 6
patients with tumors in the lower right lung (Table I).
 | Table IBaseline characteristics of all
patients. |
Table I
Baseline characteristics of all
patients.
| Patient no. | Sex | Age, years | Cancer type | Tumor location in
lung | Clinical stage |
|---|
| 1 | Male | 62 | Squamous cell
carcinoma | Upper right | IIIB |
| 2 | Male | 49 | Adenoid
carcinoma | Lower right | IIIC |
| 3 | Male | 62 | Squamous cell
carcinoma | Upper left | IIIB |
| 4 | Male | 69 | Squamous cell
carcinoma | Upper right | IIIB |
| 5 | Male | 68 | Small cell
carcinoma | Upper left | IIIB |
| 6 | Male | 37 | Squamous cell
carcinoma | Lower right | IIIC |
| 7 | Female | 41 | Small cell
carcinoma | Upper left | IIIA |
| 8 | Male | 65 | Atypical
carcinoid | Upper left | IIIC |
| 9 | Male | 69 | Squamous cell
carcinoma | Lower left | IIIB |
| 10 | Male | 52 | Adenoid
carcinoma | Middle right | IIIA |
| 11 | Male | 52 | Small cell
carcinoma | Upper right | IIIC |
| 12 | Male | 65 | Small cell
carcinoma | Upper left | IIB |
| 13 | Male | 62 | Squamous cell
carcinoma | Middle right | IIIB |
| 14 | Female | 56 | Adenoid
carcinoma | Lower right | IIIA |
| 15 | Male | 49 | Small cell
carcinoma | Lower right | IIIC |
| 16 | Male | 79 | Squamous cell
carcinoma | Lower right | IIB |
| 17 | Male | 74 | Squamous cell
carcinoma | Lower left | IIIA |
| 18 | Male | 48 | Adenoid
carcinoma | Upper left | IIIB |
| 19 | Male | 61 | Small cell
carcinoma | Upper left | IIIB |
| 20 | Male | 48 | Squamous cell
carcinoma | Lower right | IIIC |
| 21 | Male | 49 | Squamous cell
carcinoma | Upper right | IIIC |
| 22 | Female | 50 | Adenoid cystic
carcinoma | Lower left | IIIA |
| 23 | Female | 67 | Adenoid
carcinoma | Middle right | IIIB |
| 24 | Male | 65 | Atypical
carcinoid | Middle right | IIIC |
| 25 | Male | 53 | Adenoid
carcinoma | Upper left | IIIB |
| 26 | Male | 49 | Squamous cell
carcinoma | Upper right | IIIC |
| 27 | Male | 67 | Squamous cell
carcinoma | Lower left | IIIA |
Image acquisition
The images collected using the CT, PET/CT and DW-MRI
procedures were acquired and fused according to the methods
described in a previous study (29).
GTV delineation on CT, PET/CT and
DW-MRI images
GTV measurements obtained according to CT, PET/CT
and DW-MRI images, were named as GTVCT,
GTVPET and GTVMRI, respectively. All CT,
PET/CT, and DW-MRI images were independently reviewed by 10
radiotherapists, and the contours of the tumors were delineated
according to standard procedures in China (30). To account for the rough edges of
the nodules and clumps in the CT images of lung tumors, the tumor
edges were used as a reference for GTVCT delineation.
The GTV delineation follows the procedure described in a previous
study (29).
Radiotherapy planning
Planning target volume (PTV) was created by
expanding the GTV by 5 mm. For each imaging method, simple 3D-CRT
plans were developed, namely PlanCT, PlanPET
and PlanMRI. When planning the radiotherapy, the
respective center points, the number of shoots, the direction of
the field, the frame angle, and the position of the multi-blade
grating were set. The dose curve encompassing ≤95% of the PTV was
set to receive 95% of the prescribed dose.
All plans were designed for delivery using six MV
X-rays, with conventional radiotherapy-30 fractions of 2 Gy to a
total dose of 60 Gy administered over a period of 6 weeks. The
planning constraints for OARs were set according to the maximum
dose administered to the spinal cord was <45 Gy, the mean dose
to the lungs was <20 Gy, the percentage of the V20
was <30%, the percentage of the total lung volume receiving ≥30
Gy was <20% (V30), and the mean dose to the heart was
<20 Gy. The parameters of the lungs, heart and spinal cord were
measured and recorded in three sets of radiotherapy plans.
Statistical analyses
Statistical analysis was performed using SAS 9.3
software. The differences between the GTVs and the effects on OARs
are summarized as the mean ± mean standard error (SE). The group
means of CT, PET/CT and MRI were compared using one-way ANOVA with
the Bonferroni adjustment. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pairwise comparisons of GTV
delineation using the CT, PET/CT and DW-MRI methods
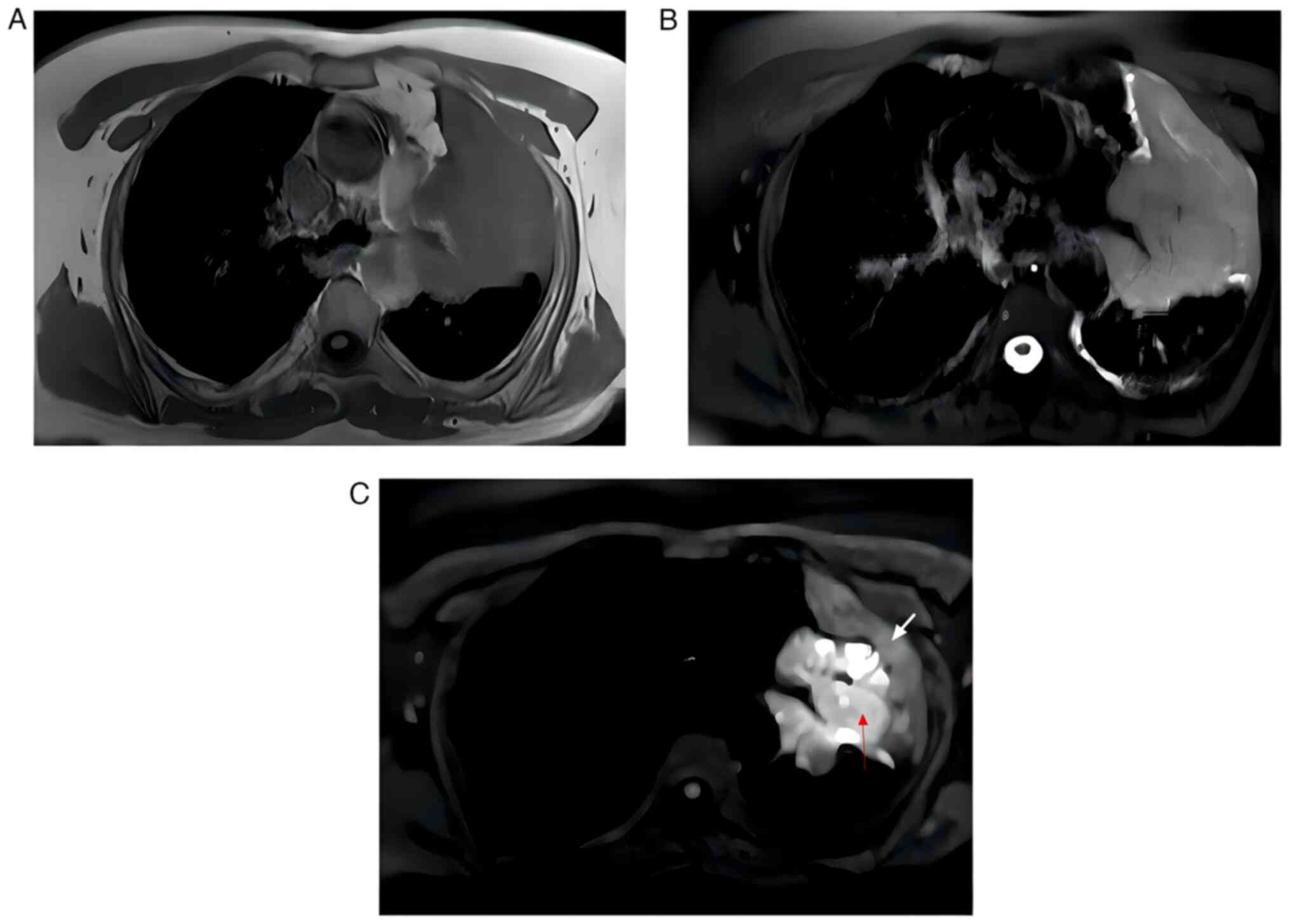
The delineated GTVs for the CT, PET/CT and DW-MRI
images were obtained through image fusions. DW-MRI images were
advantageous in distinguishing central lung cancers from
atelectasis, as compared with the T1 and T2 weighted sequence
(Fig. 1). In a previous study by
the authors (29), examples of
images from two individual patients were presented. Notably, the
GTV delineated from the DW-MRI images was typically smaller in size
with clear edges in comparison to the GTVs obtained from the CT and
PET/CT images.
Effects on OARs
The radiation doses to the lungs, heart, and spinal
cord of cancer patients under various imaging conditions according
to PlanCT, PlanPET, and PlanMRI
were obtained, as described in a previous study (29). As demonstrated in Table II, the proportion of lungs in
PlanMRI was similar to that in PlanPET for
each individual radiation dose, which was significantly reduced as
compared with PlanCT. Similar results were observed in
the dose volume histogram (DVH) of the patients (Fig. 2).
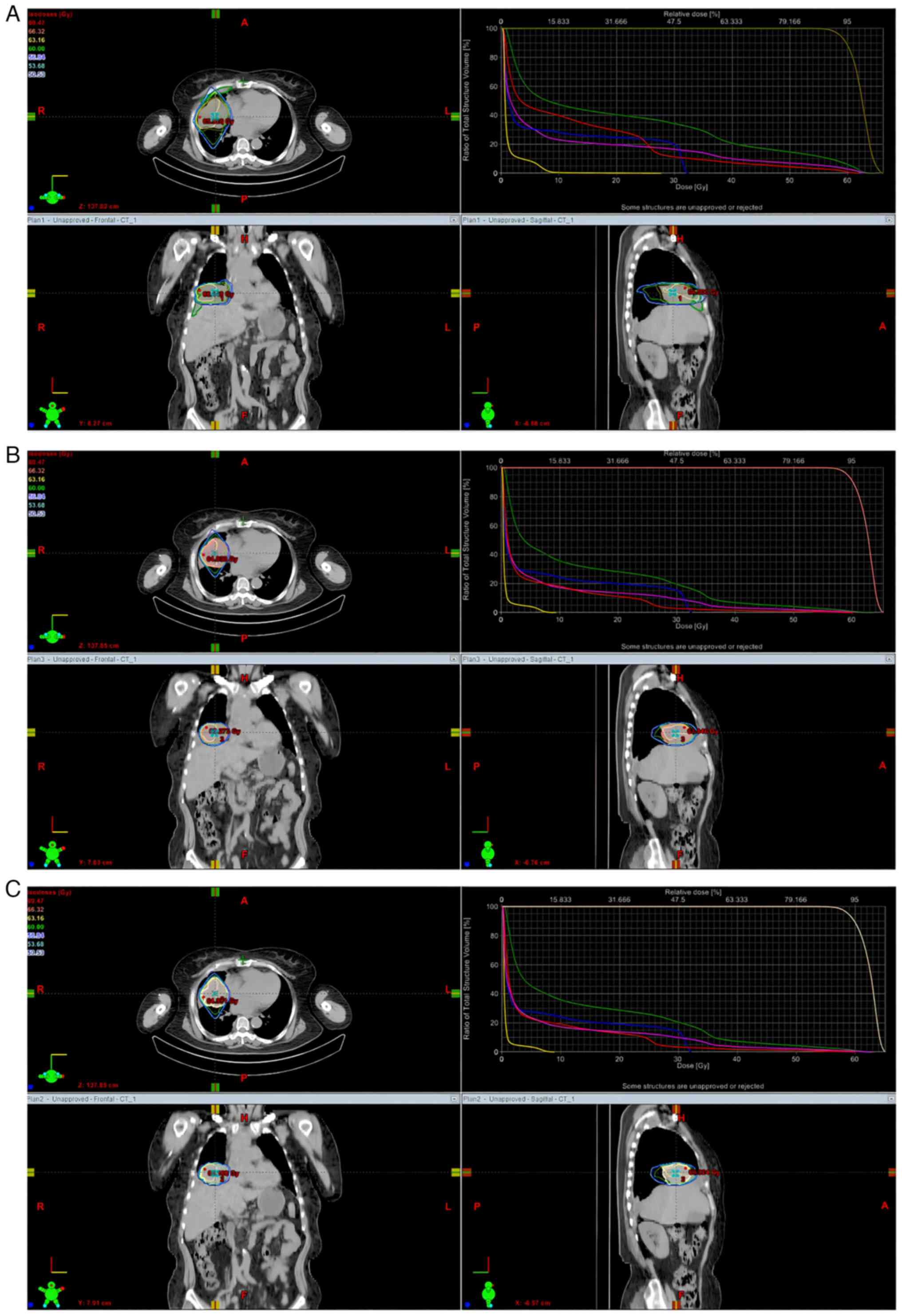 | Figure 2DVH from the (A) PlanCT,
(B) PlanPET and (C) PlanMRI of a 67-year-old
female patient with central lung cancer in right-middle lung.
Differences in volume are depicted at every dose under the three
plans. The yellow line denotes the left lung, while the right lung,
total lung, heart, and spinal cord are respectively represented by
the green line (for the right lung), pink line (for the total
lung), red line (for the heart) and blue line (for the spinal
cord). DVH, dose volume histograms; PlanCT, 3D conformal
plans in CT imaging; PlanPET, 3D conformal plans in
PET/CT imaging; PlanMRI, 3D conformal plans in MRI
imaging; CT, computed tomography; PET, positron emission
tomography; MRI, magnetic resonance imaging. |
 | Table IIPairwise comparisons of CT, PET/CT
and DW-MRI in lung measurements. |
Table II
Pairwise comparisons of CT, PET/CT
and DW-MRI in lung measurements.
| |
P-valuesa |
|---|
| Parameters (mean ±
SE) |
PlanCT |
PlanPET |
PlanMRI | CT vs. PET/CT | CT vs. MRI | PET/CT vs. MRI |
|---|
| V5
(%) | 19.28±2.14 | 15.29±1.98 | 15.28±2.29 | 0.011 | 0.004 | NS |
| V10
(%) | 14.76±1.76 | 11.50±1.62 | 11.57±1.89 | 0.006 | 0.002 | NS |
| V15
(%) | 12.68±1.56 | 9.52±1.37 | 9.72±1.59 | 0.003 | 0.001 | NS |
| V20
(%) | 11.19±1.44 | 8.23±1.21 | 8.49±1.40 | 0.002 | 0.001 | NS |
| V25
(%) | 9.87±1.39 | 7.02±1.10 | 7.32±1.27 | 0.001 | <0.001 | NS |
| V30
(%) | 8.26±1.31 | 5.54±0.93 | 5.94±1.06 | 0.003 | 0.003 | NS |
| V35
(%) | 6.85±1.13 | 4.12±0.68 | 4.57±0.82 | <0.001 | 0.001 | NS |
| V40
(%) | 5.11±0.81 | 2.74±0.45 | 3.20±0.57 | <0.001 | 0.001 | NS |
| Dmean
(cGy) | 6.20±0.73 | 4.50±0.56 | 4.70±0.68 | 0.001 | <0.001 | NS |
Notably, the data for the heart from all three plans
exhibited a similar pattern as that of the lungs, with no
statistically significant differences between the three plans
(Table III). The proportion of
spinal cord volume received in all three plans was similar at the
dose of >40 Gy or higher, and the mean and maximum doses on the
spinal cord are similar in all three plans (Table IV).
 | Table IIIPairwise comparisons of CT, PET/CT,
and MRI in heart measurements. |
Table III
Pairwise comparisons of CT, PET/CT,
and MRI in heart measurements.
| |
P-valuesa |
|---|
| Parameters (mean ±
SE) |
PlanCT |
PlanPET |
PlanMRI | CT vs. PET/CT | CT vs. MRI | PET/CT vs. MRI |
|---|
| V30
(%) | 8.90±3.61 | 6.78±3.71 | 6.59±3.82 | NS | 0.040 | NS |
| V40
(%) | 2.86±1.09 | 1.65±0.88 | 1.87±1.13 | NS | 0.036 | NS |
| V45
(%) | 1.64±0.67 | 0.85±0.47 | 1.09±0.68 | 0.017 | 0.038 | NS |
| V50
(%) | 1.14±0.52 | 0.62±0.36 | 0.83±0.54 | NS | NS | NS |
| V55
(%) | 0.87±0.40 | 0.47±0.28 | 0.65±0.44 | NS | NS | NS |
| Dmean
(cGy) | 8.16±2.29 | 5.39±1.88 | 5.32±1.93 | 0.007 | 0.007 | NS |
| Dmax
(cGy) | 46.60±6.80 | 38.42±7.57 | 38.11±7.72 | NS | NS | NS |
 | Table IVPairwise comparisons of CT, PET/CT,
and MRI in spinal cord measurements. |
Table IV
Pairwise comparisons of CT, PET/CT,
and MRI in spinal cord measurements.
| |
P-valuesa |
|---|
| Parameters (mean ±
SE) |
PlanCT |
PlanPET |
PlanMRI | CT vs. PET/CT | CT vs. MRI | PET/CT vs. MRI |
|---|
| V40
(%) | 1.13±1.13 | 0.90±0.90 | 0.56±0.56 | NS | NS | NS |
| Dmean
(cGy) | 4.91±0.92 | 4.40±0.86 | 4.60±0.88 | NS | NS | NS |
| Dmax
(cGy) | 27.00±3.78 | 27.53±3.90 | 27.93±3.76 | NS | NS | NS |
In summary, pairwise comparisons of observed values
of the lung for all three plans demonstrated that the DW-MRI values
were indistinguishable from those of PET/CT and were differed
significantly from those of CT. These data suggested that DW-MRI is
appropriate for the delineation of GTVs for central lung cancers
with atelectasis.
Discussion
Central lung cancer often simultaneously occurs with
obstructive pulmonary atelectasis (31), which makes it imperative to
distinguish between the central lung cancer and the accompanying
atelectasis when assessing the tumor and delineating the target
volume for radiotherapy (19). The
incorrect delineation of gross tumor volume could lead to lower
survival rates and increased radiation dose on surrounding organs,
especially normal lung tissue (32,33).
Whereas CT remains the only 3D imaging modality used
for dose calculation, there are limitations in accurately
differentiating clinical target volume and gross tumor volume due
to low contrast and lack of functional imaging information
(34). It is challenging to
distinguish lung cancer from pulmonary atelectasis due to
inflammation and effusion (35,36).
The delineated GTV based on the CT scan images was significantly
larger than the pathologic GTV (37). As regards CT, the received dosage
of normal lung tissue and other adjacent organs is significantly
higher than in PET/CT and MRI (38-39).
PET/CT has been proven to significantly enhance the
accuracy of conventional imaging in estimating the full spectrum of
lung cancer, as it can distinguish central lung cancer from
atelectasis. However, PET/CT can also result in higher radiation
exposure and cost (36,38). Deniaud-Alexandre et al
(40) revealed that PET/CT can
provide different GTVs from the traditional CT plan in patients
with non-small cell lung cancer and can also change the estimated
receiving dosage of heart and lungs.
DW-MRI has become an indispensable tool in cancer
research, diagnosis and treatment, and it has been suggested that
DWI combined with MRI can provide important information in
differentiating lung cancer and atelectasis (41).
In the present study, the mean GTV measurements
based on DW-MRI were similar to those based on PET/CT, but smaller
than GTV based on CT significantly (29). The results of the present study are
consistent with those from previous studies (42-46).
DW-MRI outperforms CT in differentiating the central lung cancer
from obstructive pulmonary atelectasis and achieves similar
outcomes with PET/CT, while avoiding the PET/CT scan radiation and
lowering the treatment costs.
In advanced lung cancer precision radiotherapy, the
radiation dose is often limited by the amount of exposure to the
OARs at the target area. By minimizing the receiving dosage of the
OARs, precision radiotherapy can decrease the occurrence of
complications and effectively improve the radiation dose of the
target area under the same toxicity reaction (30).
Bradley et al (47) reported that GTV was positively
associated with the average esophageal dose and lung V20
in PET/CT delineation of the target area. The results of the
present study confirmed that, with the same dose gradient, the
exposure volume of the OARs significantly decreased using the
DW-MRI-based radiotherapy plan, particularly in lungs. In relation
to central lung cancer with atelectasis, with the decrease and
disappearance in air content in the lungs, collapsed lung tissues
and tumors merge in CT images into a solid mass with a similar
density, impeding target delineation in radiotherapy and affecting
the receiving dosage of the OARs (29,48).
Combined with DW-MRI, the lung atelectasis can be differentiated
from the solid lung tumor. The accuracy-boosted delineation of the
tumor tissues and the reduced exposure volume of the OARs
culminates in lower radiation pneumonitis occurrence rate, a more
accomplishable radiotherapy plan, and improved quality of life for
patients with lung cancer with atelectasis. Compared to PET/CT,
DW-MRI achieved the same effect on the protection of normal lung
tissue. The DVH parameters in heart or spinal cord with DW-MRI and
PET/CT exhibited a reduced exposure rate in comparison with CT,
even though the differences were statistically insignificant.
The limitation of the present study was its small
sample size, and further research and larger studies are required
for the confirmation of the findings and the evaluation of the
benefits of DW-MRI. In conclusion, radiotherapy treatment planning
based on DW-MRI plays a crucial role in determining the border of
lung cancer with pulmonary atelectasis, precisely delineating GTV,
reducing potentially toxic reactions, and improving the quality of
life of the patients. Apart from atelectasis, a number of factors
have been reported to affect radiotherapy planning. Patients may
achieve more accurate tumor delineation using DW-MRI without
sacrificing high-dose radiation on the lungs and heart and spinal
cord. DW-MRI is highly recommended since it is radiation-free and
more cost-effective, which is particularly important in developing
countries. With further research and the development of imaging
technologies, DWI-MRI technology may play a more critical role in
lung cancer precision radiotherapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (NSFC; grant nos. 81272699, 81301936
and 81472811), the Shandong Science and Technology Development
Project (grant no. 2014GGC03038) and the International Cooperation
Project of Science and Technology Department (grant no.
2012DFA31560).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, TL, HZ and MZ were involved in the methodology,
data analysis and conceptualization of the study. TL performed the
formal analyses and reviewed the manuscript. XZ and HZ wrote and
drafted the original manuscript. XZ and MZ confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent prior
to enrollment in the study. The study was approved by the
Institutional Review Board and the Ethics Committee of Shandong
Cancer Hospital and Institute (reference no. 2015-06-85).
Patient consent for publication
All patients provided written informed consent
regarding the publication of their data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Edwards BK, Noone AM, Mariotto AB, Simard
EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA,
et al: Annual Report to the Nation on the status of cancer,
1975-2010, featuring prevalence of comorbidity and impact on
survival among persons with lung, colorectal, breast, or prostate
cancer. Cancer. 120:1290–1314. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Shepherd FA, Crowley J, Van Houtte P,
Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw P:
International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions. The
International Association for the Study of Lung Cancer lung cancer
staging project: Proposals regarding the clinical staging of small
cell lung cancer in the forthcoming (seventh) edition of the tumor,
node, metastasis classification for lung cancer. J Thorac Oncol.
2:1067–1077. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Martel MK, Ten Haken RK, Hazuka MB,
Kessler ML, Strawderman M, Turrisi AT, Lawrence TS, Fraass BA and
Lichter AS: Estimation of tumor control probability model
parameters from 3-D dose distributions of non-small cell lung
cancer patients. Lung Cancer. 24:31–37. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kong FM, Ten Haken RK, Schipper MJ,
Sullivan MA, Chen M, Lopez C, Kalemkerian GP and Hayman JA:
High-dose radiation improved local tumor control and overall
survival in patients with inoperable/unresectable non-small-cell
lung cancer: Long-term results of a radiation dose escalation
study. Int J Radiat Oncol Biol Phys. 63:324–333. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jeon TY, Lee KS, Yi CA, Chung MP, Kwon OJ,
Kim BT and Shim YM: Incremental value of PET/CT Over CT for
mediastinal nodal staging of non-small cell lung cancer: Comparison
between patients with and without idiopathic pulmonary fibrosis.
AJR Am J Roentgenol. 195:370–376. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dwamena BA, Sonnad SS, Angobaldo JO and
Wahl RL: Metastases from non-small cell lung cancer: Mediastinal
staging in the 1990s-meta-analytic comparison of PET and CT.
Radiology. 213:530–536. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cobben DC, de Boer HC, Tijssen RH, Rutten
EG, van Vulpen M, Peerlings J, Troost EG, Hoffmann AL and van Lier
AL: Emerging Role of MRI for radiation treatment planning in lung
cancer. Technol Cancer Res Treat. 15:NP47–NP60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van Herk M: Errors and margins in
radiotherapy. Semin Radiat Oncol. 14:52–64. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Patni N, Burela N, Pasricha R, Goyal J,
Soni TP, Kumar TS and Natarajan T: Assessment of three-dimensional
setup errors in image-guided pelvic radiotherapy for uterine and
cervical cancer using kilovoltage cone-beam computed tomography and
its effect on planning target volume margins. J Cancer Res Ther.
13:131–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Devic S: MRI simulation for radiotherapy
treatment planning. Med Phys. 39:6701–6711. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Toloza EM, Harpole L and McCrory DC:
Noninvasive staging of non-small cell lung cancer: A review of the
current evidence. Chest. 123 (1 Suppl):137S–146S. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gould MK, Kuschner WG, Rydzak CE, Maclean
CC, Demas AN, Shigemitsu H, Chan JK and Owens DK: Test performance
of positron emission tomography and computed tomography for
mediastinal staging in patients with non-small-cell lung cancer: A
meta-analysis. Ann Intern Med. 139:879–892. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Roberts PF, Follette DM, von Haag D, Park
JA, Valk PE, Pounds TR and Hopkins DM: Factors associated with
false-positive staging of lung cancer by positron emission
tomography. Ann Thorac Surg. 70:1154–1159; discussion 1159-1160.
2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Silvestri GA, Gould MK, Margolis ML,
Tanoue LT, McCrory D, Toloza E and Detterbeck F: American College
of Chest Physicians. Noninvasive staging of non-small cell lung
cancer: ACCP evidence-based clinical practice guidelines (2nd
edition). Chest. 132 (3 Suppl):178S–201S. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hanna GG, McAleese J, Carson KJ, Stewart
DP, Cosgrove VP, Eakin RL, Zatari A, Lynch T, Jarritt PH, Young VA,
et al: (18)F-FDG PET-CT simulation for non-small-cell lung cancer:
Effect in patients already staged by PET-CT. Int J Radiat Oncol
Biol Phys. 77:24–30. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aristei C, Falcinelli L, Palumbo B and
Tarducci R: PET and PET-CT in radiation treatment planning for lung
cancer. Expert Rev Anticancer Ther. 10:571–584. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Farr KP, West K, Yeghiaian-Alvandi R,
Farlow D, Stensmyr R, Chicco A and Hau E: Functional perfusion
image guided radiation treatment planning for locally advanced lung
cancer. Phys Imaging Radiat Oncol. 11:76–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bourgouin PM, McLoud TC, Fitzgibbon JF,
Mark EJ, Shepard JA, Moore EM, Rummeny E and Brady TJ:
Differentiation of bronchogenic carcinoma from postobstructive
pneumonitis by magnetic resonance imaging: Histopathologic
correlation. J Thorac Imaging. 6:22–27. 1991.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Maheshwari S and Mukherji SK:
Diffusion-weighted imaging for differentiating recurrent
cholesteatoma from granulation tissue after mastoidectomy: Case
report. AJNR Am J Neuroradiol. 23:847–849. 2002.PubMed/NCBI
|
|
21
|
Moon JY, Kim SH, Choi SY, Hwang JA, Lee JE
and Lee J: Differentiating malignant from benign hyperintense
nodules on unenhanced T1-weighted images in patients with chronic
liver disease: Using gadoxetic acid-enhanced and diffusion-weighted
MR imaging. Jpn J Radiol. 36:489–499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koh DM and Collins DJ: Diffusion-weighted
MRI in the body: Applications and challenges in oncology. AJR Am J
Roentgenol. 188:1622–1635. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iima M: Perfusion-driven intravoxel
incoherent motion (IVIM) MRI in Oncology: Applications, challenges,
and future trends. Magn Reson Med Sci. 20:125–138. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Usuda K, Zhao XT, Sagawa M, Matoba M,
Kuginuki Y, Taniguchi M, Ueda Y and Sakuma T: Diffusion-weighted
imaging is superior to positron emission tomography in the
detection and nodal assessment of lung cancers. Ann Thorac Surg.
91:1689–1695. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stieb S, Elgohari B and Fuller CD:
Repetitive MRI of organs at risk in head and neck cancer patients
undergoing radiotherapy. Clin Transl Radiat Oncol. 18:131–139.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Winter RM, Leibfarth S, Schmidt H, Zwirner
K, Mönnich D, Welz S, Schwenzer NF, la Fougère C, Nikolaou K,
Gatidis S, et al: Assessment of image quality of a
radiotherapy-specific hardware solution for PET/MRI in head and
neck cancer patients. Radiother Oncol. 128:485–491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wee CW, Jang BS, Kim JH, Jeong CW, Kwak C,
Kim HH, Ku JH, Kim SH, Cho JY and Kim SY: Prediction of Pathologic
findings with MRI-based clinical staging using the bayesian network
modeling in prostate cancer: A radiation oncologist perspective.
Cancer Res Treat. 54:234–244. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Navarria P, Reggiori G, Pessina F,
Ascolese AM, Tomatis S, Mancosu P, Lobefalo F, Clerici E, Lopci E,
Bizzi A, et al: Investigation on the role of integrated PET/MRI for
target volume definition and radiotherapy planning in patients with
high grade glioma. Radiother Oncol. 112:425–429. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang X, Fu Z, Gong G, Wei H, Duan J, Chen
Z, Chen X, Wang R and Yin Y: Implementation of diffusion-weighted
magnetic resonance imaging in target delineation of central lung
cancer accompanied with atelectasis in precision radiotherapy.
Oncol Lett. 14:2677–2682. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Y, Wang L, Gao L, Jin J, et al:
Radiation Oncology, Version 5.0: 739, 2018.
|
|
31
|
Qi LP, Zhang XP, Tang L, Li J, Sun YS and
Zhu GY: Using diffusion-weighted MR imaging for tumor detection in
the collapsed lung: A preliminary study. Eur Radiol. 19:333–341.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Senan S and De Ruysscher D: Critical
review of PET-CT for radiotherapy planning in lung cancer. Crit Rev
Oncol Hematol. 56:345–351. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yin LJ, Yu XB, Ren YG, Gu GH, Ding TG and
Lu Z: Utilization of PET-CT in target volume delineation for
three-dimensional conformal radiotherapy in patients with non-small
cell lung cancer and atelectasis. Multidiscip Respir Med.
8(21)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pereira GC, Traughber M and Muzic RF Jr:
The role of imaging in radiation therapy planning: Past, present,
and future. Biomed Res Int. 2014(231090)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McAdams HP, Erasums JJ, Patz EF, Goodman
PC and Coleman RE: Evaluation of patients with round atelectasis
using 2-[18F]-fluoro-2-deoxy-D-glucose PET. J Comput Assist Tomogr.
22:601–604. 1998.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schmidt S, Nestle U, Walter K, Licht N,
Ukena D, Schnabel K and Kirsch CM: Optimization of radiotherapy
planning for non-small cell lung cancer (NSCLC) using 18FDG-PET.
Nuklearmedizin. 41:217–220. 2002.PubMed/NCBI(In German).
|
|
37
|
Chan R, He Y, Haque A and Zwischenberger
J: Computed tomographic-pathologic correlation of gross tumor
volume and clinical target volume in non-small cell lung cancer: A
pilot experience. Arch Pathol Lab Med. 125:1469–1472.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shao Y, Wang H, Chen H, Gu H, Duan Y, Feng
A, Li X and Xu Z: Dosimetric comparison and biological evaluation
of PET- and CT-based target delineation for LA-NSCLC using
auto-planning. Phys Med. 67:77–84. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen H, Huang Y, Wang H, Shao Y, Yue NJ,
Gu H, Duan Y, Feng A and Xu Z: Dosimetric comparison and biological
evaluation of fixed-jaw intensity-modulated radiation therapy for
T-shaped esophageal cancer. Radiat Oncol. 16(158)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Deniaud-Alexandre E, Touboul E, Lerouge D,
Grahek D, Foulquier JN, Petegnief Y, Grès B, El Balaa H, Keraudy K,
Kerrou K, et al: Impact of computed tomography and 18F-deoxyglucose
coincidence detection emission tomography image fusion for
optimization of conformal radiotherapy in non-small-cell lung
cancer. Int J Radiat Oncol Biol Phys. 63:1432–1441. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao D, Hu Q, Qi L, Wang J, Wu H, Zhu G
and Yu H: Magnetic resonance (MR) imaging for tumor staging and
definition of tumor volumes on radiation treatment planning in
non-small cell lung cancer: A prospective radiographic cohort study
of single center clinical outcome. Medicine (Baltimore).
96(e5943)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gao Z, Wilkins D, Eapen L, Morash C,
Wassef Y and Gerig L: A study of prostate delineation referenced
against a gold standard created from the visible human data.
Radiother Oncol. 85:239–246. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
McLaughlin PW, Evans C, Feng M and
Narayana V: Radiographic and anatomic basis for prostate contouring
errors and methods to improve prostate contouring accuracy. Int J
Radiat Oncol Biol Phys. 76:369–378. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang RM, Li L, Wei XH, Guo YM, Huang YH,
Lai LS, Chen AM, Liu GS, Xiong WF, Luo LP and Jiang XQ:
Differentiation of central lung cancer from atelectasis: Comparison
of diffusion-weighted MRI with PET/CT. PLoS One.
8(e60279)2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Seppala T, Visapaa H, Collan J, Kapanen M,
Beule A, Kouri M, Tenhunen M and Saarilahti K: Converting from CT-
to MRI-only-based target definition in radiotherapy of localized
prostate cancer: A comparison between two modalities. Strahlenther
Onkol. 191:862–868. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bradley J, Bae K, Choi N, Forster K,
Siegel BA, Brunetti J, Purdy J, Faria S, Vu T, Thorstad W and Choy
H: A phase II comparative study of gross tumor volume definition
with or without PET/CT fusion in dosimetric planning for
non-small-cell lung cancer (NSCLC): Primary analysis of Radiation
Therapy Oncology Group (RTOG) 0515. Int J Radiat Oncol Biol Phys.
82:435–441 e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bradley J, Thorstad WL, Mutic S, Miller
TR, Dehdashti F, Siegel BA, Bosch W and Bertrand RJ: Impact of
FDG-PET on radiation therapy volume delineation in non-small-cell
lung cancer. Int J Radiat Oncol Biol Phys. 59:78–86.
2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Braun LH, Welz S, Viehrig M, Heinzelmann
F, Zips D and Gani C: Resolution of atelectasis during
radiochemotherapy of lung cancer with serious implications for
further treatment. A case report. Clin Transl Radiat Oncol. 9:1–4.
2017.PubMed/NCBI View Article : Google Scholar
|