1. Introduction
Worldwide, Endometrial Cancer (EC) is the second
most common gynecological malignancy after cervical cancer
(1-4).
Its incidence has been steadily increasing in recent years,
especially in developed countries, ranking 7th among the most
lethal malignancies in Western Europe and 3rd in the USA (3,5). Its
rising incidence can be partially attributed to the modern way of
life, with obesity and sedentary lifestyle playing a major role. In
the majority of cases, EC is driven by estrogen dominance, which is
especially prevalent in obese women due to the aromatization in
adipose tissue (2). Alongside its
raising incidence, it seems to be affecting increasingly younger
women. The majority consists of menopausal women, comprising 75% of
all affected women. The other 25% involves premenopausal women,
with 10% pertaining to women <45 years old (3,6) and
4-7% to women aged 20-44 years old (3,7). It
must be noted that, while rare, EC can also affect adolescent
women; two cases of an 11-year-old and a 13-year-old patient have
been reported (7). The standard
surgical treatment for endometrial cancer is total abdominal
hysterectomy, bilateral salpingo-oophorectomy and staging
lymphadenectomy or sentinel node identification, a de facto
fertility cancellation treatment. The steadily increasing worldwide
incidence of EC, combined with a constantly increasing age of
childbearing in the developed countries, means that more and more
young women will be diagnosed with EC in the future (8,9). The
current review aims to highlight issues upon therapeutic strategies
in young women diagnosed with EC, who want to preserve their
fertility potential.
2. Methods
A narrative review was performed focusing on
conservative treatment of endometrial cancer in young women of
reproductive age. A Medline search was performed, using the terms
Conservative Treatment, Endometrial Cancer/EIN, Young Women,
Fertility-Sparing and the boolean operators AND and OR. Articles
published from 2000 onwards were considered and the last search was
performed in February 2023. Only articles published in English were
considered.
3. EC risk factors
The most important risk factors for endometrial
cancer are the increasing age, genetic syndromes, in particular
Lynch Syndrome, also known as HNPCC (hereditary non-polyposis colon
cancer) (1,5) and less so, Turner Syndrome and Cowden
Syndrome (7), familiar history of
EC, individual history of ovarian cancer and, particularly, breast
cancer treated with Tamoxifen, a Selective Estrogen Receptor
Modulator(SERM), which acts as an agonist on endometrial estrogen
receptors, individual history of Polycystic Ovarian Syndrome(PCOS),
unopposed and prolonged estrogenic action, obesity, type II
diabetes mellitus, arterial hypertension, nulliparity, individual
history of endometrial hyperplasia, individual history of
radiotherapy, early menarche and late menopause and geographical
distribution, with European and North American women at greater
risk.
On the contrary, smoking is associated with a lower
risk of endometrial cancer, especially in postmenopausal women,
through a proposed anti-estrogenic mechanism (1,2,5).
4. Selection criteria for conservative
treatment
A summary of the selection criteria, as outlined by
several medical societies, is provided below (10,11):
Presence of complex atypical hyperplasia/endometrioid
intra-epithelial neoplasia (EIN) or well-differentiated (grade 1)
endometrioid EC, FIGO histological stage IA without myometrial
invasion (Table I) (12,13),
no evidence of myometrial invasion as demonstrated by imaging
examinations, preferably Magnetic Resonance Imaging (MRI), no
evidence of lymph node metastases (both pelvic and para-aortic), no
evidence of synchronous ovarian cancer, the patient is thoroughly
informed that the oncological safety is based on retrospective or
non-randomized data and, following treatment, she will have to
undergo a hysterectomy, close collaboration with a fertility expert
in the Gynecologic team is mandatory, as often these patients
undergo immediate in vitro fertilization, following
successful remission of EC and the patient is willing to commit to
the treatment protocol and the appropriate follow-up examinations
required (2,4,14)
 | Table IInternational Federation of
Gynecology and Obstetrics staging for endometrial cancer. |
Table I
International Federation of
Gynecology and Obstetrics staging for endometrial cancer.
| Stage | Features |
|---|
| I | Tumor confined to
the corpus uteri |
| Ιa | No or <50%
myometrial invasion |
| Ib | Invasion ≥50% of
the myometrium |
| II | Tumor invades
cervical stroma but does not extend beyond the uterus |
| III | Local and/or
regional spread of the tumor |
| IIIa | Tumor invades
serosa of the corpus uteri and/or adnexa |
| IIIb | Vaginal and/or
parametrial involvement |
| IIIc1 | Positive pelvic
lymph nodes |
| IIIc2 | Positive
para-aortic lymph nodes with or without pelvic nodes |
| IV | Tumor invades
bladder and/or bowel, and/or distant metastases |
| IVa | Tumor invasion of
bladder and/or bowel mucosa |
| IVb | Distant metastases,
including intra-abdominal and/or inguinal lymph nodes |
5. Histopathology
There are two histological types of EC: type I
(endometrioid carcinoma), affecting younger patients, which is
mostly driven by circulating estrogen excess and has a favorable
prognosis, and type II (mostly serous carcinoma), which affects
more often older women and has a poorer prognosis (8).
The COG-33 study has assessed the risk of nodal
involvement according to the histological grade. Grade 1 tumors
confined to the inner third myometrium presented a 3% risk of lymph
node metastases (LNM), while deep myometrial invasion was
associated with an 11% risk. In contrast, the risk of nodal
involvement increased significantly in the case of grade 3 tumors;
those restricted to the inner third of the myometrium presented a
5% risk, whereas deep myometrial invasion increased the risk up to
34% (15). A more recent study has
shown that the risk of LNM in grade 1 tumors without myometrial
invasion may be as low as 0.5%, rising to 1.6% for higher grade
tumors without any myometrial invasion (16).
Based on the above risk assessment of LNM, women
with an endometrioid grade 1 EC, without any evidence of myometrial
invasion, are considered suitable for conservative treatment.
However, high-grade endometrioid tumors, with any evidence of
myometrial invasion, are considered contraindications for
conservative treatment.
6. Diagnosis
The methods of obtaining histological specimens and
assessing myometrial invasion are key elements for selecting
appropriate candidates for conservative treatment. Pipelle
endometrial biopsy has been reported as presenting a diagnostic
accuracy for EC of 91% in the general population; therefore, it can
be a useful initial tool (17).
However, studies show that it is inferior to dilatation and
curettage (D&C) in defining accurately the histological grade,
possibly due to the small volume of tissue obtained by the pipelle
(18). Hysteroscopic diagnosis is
regarded as the gold standard, having been shown to be superior to
both the pipelle and D&C methods in terms of diagnostic
accuracy (5,7,19,20).
Direct visualization allows real-time evaluation of the endometrial
cavity, with accurate endometrial sampling and more effective
disease removal. Importantly, owing to the high inter-observer
variations in histological grade evaluation, it is mandatory that,
in case of candidates for conservative treatment, tissue samples
should be assessed by two experienced pathologists.
Accurate assessment of myometrial invasion (MI) is
challenging, as it can be done only using a hysterectomy specimen.
However, indirect assessment of myometrial invasion can be
performed using a sensitive imaging technique. Contrast-enhanced
MRI provides a high diagnostic accuracy, although studies show that
transvaginal ultrasound (TVUS) has the same sensitivity at a much
lower cost. MRI with diffuse weight imaging is considered the
method of choice, although a recent meta-analysis concluded that
TVUS is not inferior to MRI for evaluating myometrial invasion,
especially in the case of experienced operators (21). The 3D TVUS has been shown to be of
equal performance in assessing myometrial invasion (22), although this was recently
challenged in a multi-centered study, showing inferior specificity
with TVUS (23). A major advantage
of MRI, compared to ultrasound, is that it provides additional
information regarding cervical stroma involvement, as well as the
lymph node status. Thus, MRI is considered the method of
radiological choice in assessing patients for enrollment in
fertility sparing treatment.
The recent 2023 European guidelines stress the
importance of documenting myometrial invasion with the
Hysteroscopic Resectoscope, which could not only define the depth
of MI but, also, remove the entire lesion endoscopically (24).
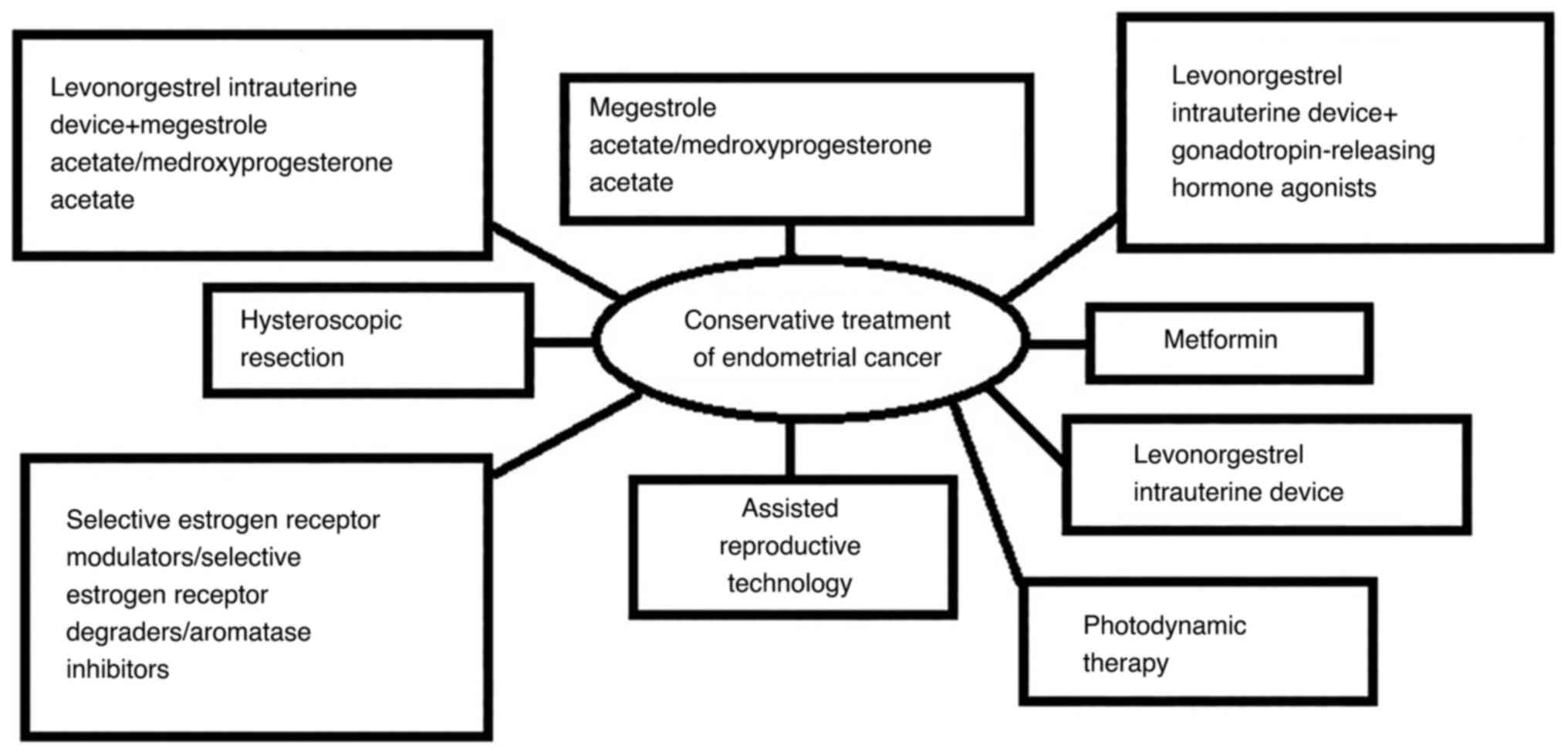
7. Pharmacological fertility-sparing
treatment
i) Oral Progestins, ii) Levonorgestrel-releasing
Intrauterine Device (MIRENA), iii)
Gonadotropin-releasing-hormone-agonists, iv) Selective Estrogen
Receptor Modulators (SERMs), v) Selective Estrogen Receptor
Degraders (SERDs) (5), Aromatase
inhibitors (AIs) (24), vi)
Metformin (Fig. 1) (5,25-28).
8. Progestins
This is the most commonly employed conservative
treatment. Although initially reported in 1961(2), the first study confirming their
effectiveness was published in 1997(29). Their mechanism of action is via
opposition of estrogen-driven endometrial growth, resulting in
thinning of the endometrium and stromal decidualization. This
effect is thought to be exerted through down-regulation of estrogen
receptors, activation of enzymes involved in estrogen mechanism,
regulation of the cell cycle by cyclin-dependent kinases and
enhancement of p27 expression, causing inhibition of cyclin E-Cdk2
function and suppression of the cell cycle (7,30,31).
The two progestins commonly used are megestrol
acetate (MA) and medroxyprogesterone acetate (MPA) (30). The optimal dosage and duration of
the therapy are still under investigation; however, the dose most
often employed is 160 mg/day for MA and 400-600 mg/day for MPA
(7,13,30).
However, treatment with MA has been shown to carry a higher risk of
disease recurrence, highlighting the need for further investigation
(30,32). Also, the optimal duration of
therapy has not been established yet. According to many studies,
the median duration of treatment needed for a complete
histopathological response is 3 months (33). Lack of histologically documented
response to progestins is considered as a failure of conservative
treatment (34). In the case of a
partial response, the dosage can be increased with regular
follow-up at 3-months intervals. In total, a duration of 9-12
months is expected to result in complete response in women who
fulfill the strict criteria, which were outlined earlier in the
text (3,30).
Additionally, many hormonal receptors and other
immunohistochemical markers are being investigated as predictive
markers (35). The most
well-established marker for predicting proportionally the efficacy
of MA/MPA therapy, is the proportion of estrogen and progesterone
receptors in the pathological endometrium. Their implications from
disease pathogenesis to treatment response have been extensively
documented, with the PRB isoform of the progesterone receptor being
the most studied (31,35). Other pathways involving PTEN, MMR,
Dusp6 and GRP78 genes have also been studied, with the hope of
providing targeted therapy options in the future (31,35-37).
Another promising perspective in the conservative
treatment of EC is the addition of metformin to MA/MPA therapy.
Metformin acts synergistically with progestins, inhibiting the
PI3K-AKT-mTor oncogenic pathway and increasing the expression of PR
receptors (13,36,38).
The reported response rate of EC to treatment with
progestins is, approximately, 75%, although in earlier case-studies
no strict criteria selection were used for including patients for
analysis (30). However, high oral
doses of progestin increase the risk of complications, most notably
weight gain, reduction of libido, mood changes, leg cramps,
headaches and thromboembolic events (30). As far as weight gain is concerned,
pre-treatment and post-treatment BMI>25 kg/m2 is
significantly associated with a higher rate of disease recurrence,
due to peripheral aromatization of adipose tissue, underlining the
importance of maintaining a normal BMI during treatment with
progestins (7).
9. Levonorgestrel-releasing intrauterine
device (LNG-IUD) (MIRENA)
In the past few years, a new type of progestin
therapy, free of the above side effects, has been proposed. The
so-called MIRENA is an endometrial device that releases 25 µg/day
of levonogestrel at a continuous rate inside the uterine cavity,
thus avoiding the adverse effects of oral administration (37). Furthermore, higher progestin
concentrations can be achieved locally, increasing their efficacy.
The results are very promising, especially for cases of atypical
endometrial hyperplasia, with regression rates approaching 90%.
Contrary to oral progestin therapy, LNG-IUD should be used with
caution in women with an enlarged uterus, because there is a risk
of misplacement in the endometrial cavity and treatment failure
(39).
10. LNG-IUD and oral progestins
Several studies have suggested that the local usage
of LNG-IUD, combined with a systemic high-dose of progestin, might
be a more effective type of treatment for EC. Oral progestins lead
to high plasma concentrations but low local concentrations in the
endometrium, especially in obese women. Conversely, LNG-IUD
releases highly effective progestins into the endometrium, with a
30-times higher median concentration than in the plasma. According
to the latest ESGO guidelines, the combination of LNG-IUD and oral
progestin therapy is the most effective treatment, providing a low
recurrence rate and a satisfactory pregnancy rate (13). However, the efficacy of this
combination for patients with EC has not been well investigated. It
remains unknown the reason why the combined MA with local LNG-IUD
did not achieve better treatment efficacy than MA alone in some
studies. Oral MA may achieve adequate concentration of progestins
for EC treatment without the additional of local levonogestrel. A
recent Korean study showed that the combined LNG-IUD with oral MPA
(500 mg daily) is more effective than the use of LNG-IUD alone
(9). Also, a gynecologic oncology
group study reported that low-dose MPA (200 mg/day) was more
effective than high-dose treatment (1 g/day) (40). More research is needed to elucidate
what is the ideal monotherapy or combined progestin treatment.
11. LNG-IUD and GnRHa
Another therapeutic option is the combination of
LNG-IUD and GnRHa (gonadotropin-releasing hormone agonists)
(16). A meta-analysis, combining
data from six studies, showed a satisfactory pregnancy and low
recurrence rate (9,14). After one year of LNG-IUD combined
with GnRH analogs, up to 57% of EC and 95% of EIN achieved a
complete response, with a pregnancy rate of 85% and a recurrence
rate of 20% (41).
12. SERMs/SERDs/AIs
There are several anti-estrogenic drugs, which could
be potential therapeutic candidates for the conservative treatment
of EC. These include SERMs (tissue-selective agonist or antagonist
action on ERs) with raloxifene and arzoxifene blocking estrogen
receptors, excluding Tamoxifen, which has both stimulatory and
blocking effects, SERDs (mainly Fulvestrant, which down-regulates
ERs) and aromatase inhibitors, such as letrozole (decrease of
systemic exposure to estrogens by inhibition of the peripheral
conversion of androgens to estrogens).
It has been postulated that any of the above could
be used as a primary treatment for obese EC patients or as a
second-line treatment, after initial failure of progestins alone
(7).
13. Metformin
Metformin has been shown to inhibit proliferation
and migration of endometrial cancer cells in vitro.
Additionally, it has been reported to upregulate progesterone
receptors, making endometrial cancer cells more sensitive to
progesterone interventions (42).
The theory of metformin administration in endometrial cancer
conservative treatment has been developed as a result of the
interaction between glucose metabolism and endometrial cancer
development and progression. Apart from the immediate effect of
metformin on endometrial cancer cells described above, metformin
downregulates circulating insulin-a result of insulin resistance,
which in turn acts as a growth factor for endometrial cancer cells
(28). Furthermore, except for the
direct effect of metformin on endometrial cancer, metformin also
helps with reversing the consequences of metabolic syndrome
Notably, obese women with metabolic syndrome are considered of
poorer prognosis compared to women with normal BMI (43). Furthermore, in the case of
polycystic ovary syndrome (PCOS), reversal of insulin resistance
may lead to normal ovarian function, minimizing the long estrogenic
effect of anovulation to the endometrium. Recently, it was shown
that PCOS acts as a negative prognosticator for conservative
treatment in case of Complex Atypical Endometrial Hyperplasia
(CAEH) or well differentiated endometrial cancer (43). In contrast, metformin has been
shown to accelerate the time of complete remission, especially in
women with increased BMI (27). A
recent meta-analysis reported that metformin, combined with
progestins, was associated with lower relapse rates, without
significant impact on oncological and reproductive outcomes
(26). There are also several
ongoing or forthcoming trials for the use of metformin in EC women.
In 2013, NCT01968317 began comparing metformin plus MA with MA
alone, as an option for fertility-sparing treatment in patients
with EIN and well-differentiated EC. Metformin is cheap and has an
excellent safety profile, so it appears to be an obvious choice for
prospective randomized studies (44).
14. Other fertility-sparing treatments
i) Hysteroscopic Resection, ii) Photodynamic
Therapy, iii) Assisted Reproductive Technology (Fig. 1) (7,14,33).
15. Hysteroscopic resection
Hysteroscopic resection is the gold standard for the
diagnosis and treatment of intracavitary pathology of the uterus.
Hysteroscopic resection can be attempted for localized endometrial
hyperplastic/malignant lesions. This initial approach in
hysteroscopic resection of endometrial cancer has been described as
a 3-step-process: a) resection of the lesion, b) resection of the
adjacent endometrium and c) resection of the underlying myometrium
(45). The resection was
mandatorily followed by treatment with progestins or LNG-IUD
placement (4,16). A more recent approach has added
multiple hysteroscopically-guided endometrial biopsies to increase
further the sensitivity of the method (46). Several small studies have been
published with supportive results. The most robust study in terms
of sample size (140 cases of CAEH and 40 cases of endometrial
cancer) showed that this type of treatment yields better overall
results in a shorter time frame, provided that the initial tumor
size is <2 cm, and the patient's BMI is <25 kg/m2
(47). The produced evidence has
been incorporated in the 2021 ESGO guidelines (13). The excellent regression rates
achieved after hysteroscopic resection are explained by the
hysteroscopic cytoreductive effect on the primary tumor, which may
increase the effectiveness of the progestin therapy.
The latest 2022 ESGO guidelines highlight the utmost
importance of hysteroscopic biopsy for diagnosis and resectoscopic
resection in order to maximize progestin therapy (41).
16. Photodynamic therapy
This is an innovative type of EC treatment that has
also been described as a therapeutic option for malignancies of
other sites, namely vagina, cervix, bladder and esophagus. Besides
the current clinical indications, PDT constitutes a dynamic area of
research with huge potential to be considered as a valid treatment
option for a wide range of diseases. It works by exposing the
affected area to a specific wavelength of light, which is
selectively toxic to the cancer cells. PDT has been tried both as a
primary and as an adjuvant therapy in cases of disease regression
after fertility preservation. The results were encouraging for both
disease regression and fertility preservation. PDT presents
significant advantages; it is a local, highly selective, and
minimally invasive therapy (41).
The single reported side effect was facial angioedema, presenting
at a rate of 25%. Further clinical trials are needed for PDT to be
established as a safe conservative treatment for EC (33).
17. Assisted reproductive technology
(ART)
When hysterectomy with bilateral
salpingo-oophorectomy is considered as the therapeutic option, ART
can be applied before definitive treatment for the patient to
conceive through surrogacy (48).
There are many options, including freezing of oocytes, embryos or
even ovarian tissue. Except for the need for surrogacy, the main
advantages are the quality of the cryopreserved tissues upon using
the innovative method of vitrification and that the procedure can
be very expeditious, by selecting ovarian tissue by laparoscopy, as
one-day hospital procedure, or with random-start protocols for
ovarian stimulation (49). In
these cases, letrozole is the preferred agent, as it does not seem
to stimulate the endometrium. Alternatively, a random-start
protocol consists of the administration of gonadotropins any day of
the menstrual cycle (late follicular, peri-ovulatory or even the
luteal phase). The main advantages of this protocol are shorter
time to complete the fertility preservation treatment and, also, it
can be applied in any patient, even for those who have intrauterine
devices. Leaving the IUD in place has the added benefit of
mitigating the endometrial growth driven by estradiol, which
happens during ovarian stimulation. Another ovarian stimulation
protocol is called ‘duo-stimulation’, consisting of a double egg
retrieval procedure in a 28-day time frame, with the initiation of
a second ovarian stimulation cycle 4 days after the first egg
collection (50). By adopting any
of these methods, delays of definite surgical treatment can be
avoided (7).
18. Fertility outcomes
The strongest motive towards a conservative approach
is the patient's childbearing desire. The rate of women who have
successfully conceived and given birth after treatment cannot be
accurately estimated, fluctuating between 25 and 100%, because not
all the patients who have undergone conservative treatment were
planning to conceive in the first place (51). Despite the fact that women who
choose conservative treatment are determined to achieve a future
pregnancy, there might be several medical or social reasons for the
apparent divide between what is observed in clinical trials and
what happens in real life. Certainly, from this point of view, the
best candidate is a patient in a stable relationship, ready to
conceive after successful regression of the disease. There are
several factors that have been positively associated with a
successful pregnancy, including a normal BMI during conception and
gestation a shorter time interval to complete remission, whereas a
thinner endometrium and disease relapse before conception are
considered to have a negative effect (52). After a histologically documented
disease remission, women can begin their efforts to conceive with
safety. This can be achieved even spontaneously or with the use of
assisted reproductive technology (ART). Most often, young women
with EC already have a documented history of infertility, because
of overlap in the pathogenic mechanisms, mainly PCOS and
anovulation. Despite broad consensus regarding the main oncological
criteria for candidate selection, it has not been extensively
investigated as to how each candidate's reproductive prognosis
should be evaluated and used to support decision-making. Another
important issue is the duration of conservative treatment in women
who are not ready to conceive following complete regression. It is
generally accepted that the patient may carry the LNG-IUD for
longer periods, as long as she undergoes an endometrial biopsy
every six months (13,53). Therefore, the guidance of an
experienced medical professional specializing in infertility is
always advised. Generally, immediate ART is advised to decrease the
risk of recurrence, staying without treatment for a long time.
However, an eventual pregnancy is not a risk factor in itself for
disease recurrence (19,31,36,51,54).
19. Follow-up
After a successful pregnancy, women undergoing
conservative treatment should be advised to proceed to definite
treatment, which is a hysterectomy and bilateral oophorectomy.
However, women who want to achieve a second pregnancy can be
followed up with a strict protocol of 3-6 month examinations,
including hysteroscopic biopsies (25). If a patient wants to delay a second
pregnancy, maintenance therapy using low-dose cyclic progestin, or
an LNG-IUD can be suggested.
20. Progress in basic research
Endometrial biopsy and histopathological examination
can be unreliable in predicting risk of disease recurrence owing to
errors in correct histological sampling and significant variability
of histological interpretation. New biomarkers for EC risk
classification are being investigated. The Cancer Genome Atlas
identified four molecular subtypes of endometrial cancer, which
are: POLE ultramutated (POLEmut), MMR deficient (MMRd), nonspecific
molecular profile and p53 abnormal (p53abn) endometrial cancer, the
POLE variant being the least aggressive molecular type. The
molecular classification is likely to be used in the future and
incorporated even in the management of young patients wishing to
preserve their fertility (32,36,55).
21. Pushing the boundaries
Fertility-sparing treatment in EC has been
investigated without a defined consensus in recent years. The
difficulty of defining its boundaries may be related to many
factors that influence its success. The most important issues are
the assessment of the tumor's clinicopathological profile
(histological type and grade, myometrial invasion, presence of
lymph-vascular space invasion), choosing the optimal type,
duration, and dose of medical treatment, and the appropriate
follow-up (56).
Despite the strong desire of fertility preservation
with certain patients not in the above ideal profile, it has to be
stressed that International Societies Guidelines have to be
implemented. There are certain cases which seem to respond
favorably, and with no extra oncological risk, to treatment. For
example, Endometrioid Tumors of Histological Grade 2 and those with
early myometrial invasion. It is advisable to discuss all cases in
a Multidisciplinary Tumor Board Meeting and to enroll such patients
to appropriate trials, having signed a detailed informed consent
form (57,58).
22. Conclusion
Endometrial cancer can affect a small, albeit
increasing, proportion of nulliparous young women who want to
achieve childbearing. So far, the mounting scientific evidence
shows that, in the case of a very early Endometrioid, Grade 1
Endometrial Cancer, this is possible and oncologically safe.
Conservative treatment for EC is not suitable for
all women, but it can be offered on an individual basis, following
a meticulous medical examination, imaging, hysteroscopic evaluation
and expert histological diagnosis. Such patients, willing to
cooperate with a strict therapeutic protocol, have a high chance of
achieving a successful pregnancy. Preferably, immediate ART
technique is used, to maximize the chances of conceiving and not
leaving the patient without treatment for long periods.
With the available evidence, women who do not want
to conceive immediately after successful pharmaceutical disease
regression, or after a first pregnancy, can wear the LNG-IUD and
undergo endometrial biopsies on a regular basis. In this case, it
is advisable to undergo ovarian tissue preservation, preferably egg
freezing.
All individual cases have to be discussed in a
Multidisciplinary Tumor Board and all patients have to be
thoroughly informed and sign a consent form for the treatment and
follow-up. It has to be clear in their minds that the treatment
does not cure the cancer, but it suppresses it enough to achieve a
pregnancy. It has to be clearly said and written in their consent
form that, after a successful pregnancy, a hysterectomy has to take
place.
Developments of new therapeutic strategies are very
exciting and, especially, metformin appears to be a pivotal agent
to achieve disease regression. Of course, the latter refers to the
majority of these patients, with a typical metabolic profile of
obesity, PCOS and anovulation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TP, MZB, AM and TV conceived the concept and wrote
the manuscript. GM and GV critically revised the manuscript for
intellectual content. TP organized and revised the final version of
the manuscript. Data authentication is not applicable. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ali AT: Risk factors for endometrial
cancer. Ceska Gynekol. 78:448–459. 2013.PubMed/NCBI
|
|
2
|
Corzo C, Barrientos Santillan N, Westin SN
and Ramirez PT: Updates on conservative management of endometrial
cancer. J Minim Invasive Gynecol. 25:308–313. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kalogera E, Dowdy SC and Bakkum-Gamez JN:
Preserving fertility in young patients with endometrial cancer:
Current perspectives. Int J Womens Health. 6:691–701.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Peiretti M, Congiu F, Ricciardi E,
Maniglio P, Mais V and Angioni S: Conservative treatment for
well-differentiated endometrial cancer: When and why it should be
considered in young women. Ecancermedicalscience.
13(892)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oaknin A, Bosse TJ, Creutzberg CL,
Giornelli G, Harter P, Joly F, Lorusso D, Marth C, Makker V, Mirza
MR, et al: Endometrial cancer: ESMO clinical practice guideline for
diagnosis, treatment and follow-up. Ann Oncol. 33:860–877.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Braun MM, Overbeek-Wager EA and Grumbo RJ:
Diagnosis and management of endometrial cancer. Am Fam Physician.
93:468–474. 2016.PubMed/NCBI
|
|
7
|
Obermair A, Baxter E, Brennan DJ, McAlpine
JN, Mueller JJ, Amant F, van Gent MDJM, Coleman RL, Westin SN,
Yates MS, et al: Fertility-sparing treatment in early endometrial
cancer: Current state and future strategies. Obstet Gynecol Sci.
63:417–431. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trojano G, Olivieri C, Tinelli R, Damiani
GR, Pellegrino A and Cicinelli E: Conservative treatment in early
stage endometrial cancer: A review. Acta Biomed. 90:405–410.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moore K and Brewer MA: Endometrial cancer:
Is this a new disease? Am Soc Clin Oncol Educ Book. 37:435–442.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rodolakis A, Biliatis I, Morice P, Reed N,
Mangler M, Kesic V and Denschlag D: European society of
gynecological oncology task force for fertility preservation:
Clinical recommendations for fertility-sparing management in young
endometrial cancer patients. Int J Gynecol Cancer. 25:1258–1265.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sundar S, Balega J, Crosbie E, Drake A,
Edmondson R, Fotopoulou C, Gallos I, Ganesan R, Gupta J, Johnson N,
et al: BGCS uterine cancer guidelines: Recommendations for
practice. Eur J Obstet Gynecol Reprod Biol. 213:71–97.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saleh M, Virarkar M, Bhosale P, El Sherif
S, Javadi S and Faria SC: Endometrial cancer, the current
international federation of gynecology and obstetrics staging
system, and the role of imaging. J Comput Assist Tomogr.
44:714–729. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Concil N, Matias-Guiu X, Vergote I, Cibula
D, Mirza MR, Marnitz S, Ladermann J, Bosse T, Chargari C, Fagotti
A, et al: ESGO/ESTRO/ESP guidelines for the management of patients
with endometrial carcinoma. Int J Gynecol Cancer. 31:12–39.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Terzic M, Norton M, Terzic S, Bapayeva G
and Aimagambetova G: Fertility preservation in endometrial cancer
patients: Options, challenges and perspectives.
Ecancermedicalscience. 14(1030)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Creasman WT, Morrow CP, Bundy BN, Homesley
HD, Graham JE and Heller PB: Surgical pathologic spread patterns of
endometrial cancer. A gynecologic oncology group study. Cancer. 60
(8 Suppl):S2035–S2041. 1987.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gonthier C, Douhnai D and Koskas M: Lymph
node metastasis probability in young patients eligible for
conservative management of endometrial cancer. Gynecol Oncol.
157:131–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dijkhuizen FP, Mol BW, Brölmann HA and
Heintz AP: The accuracy of endometrial sampling in the diagnosis of
patients with endometrial carcinoma and hyperplasia: A
meta-analysis. Cancer. 89:1765–1772. 2000.PubMed/NCBI
|
|
18
|
Leitao MM Jr, Kehoe S, Barakat RR,
Alektiar K, Gattoc LP, Rabbitt C, Chi DS, Soslow RA and Abu-Rustum
NR: Comparison of D&C and office endometrial biopsy accuracy in
patients with FIGO grade 1 endometrial adenocarcinoma. Gynecol
Oncol. 113:105–108. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Casadio P, La Rosa M, Alletto A,
Magnarelli G, Arena A, Fontana E, Fabbri M, Giovannico K, Virgilio
A, Raimondo D, et al: Fertility sparing treatment of endometrial
cancer with and without initial infiltration of myometrium: A
single center experience. Cancers (Basel). 12(3571)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gkorzou F, Dimakopoulos G, Vrekoussis T,
Lavasidis L, Koutlas A, Navrozoglou I, Stefos T and Paschopoulos M:
Hysteroscopy in women with abnormal uterine bleeding: A
meta-analysis on four major endometrial pathologies. Arch Gynecol
Obstet. 291:1347–1354. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Frei KA, Kinkel K, Bonél HM, Lu Y,
Zaloudek C and Hricak H: Prediction of deep myometrial invasion in
patients with endometrial cancer: Clinical utility of
contrast-enhanced MR imaging-a meta-analysis and Bayesian analysis.
Radiology. 216:444–449. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alcázar JL, Gastón B, Navarro B, Salas R,
Aranda J and Guerriero S: Transvaginal ultrasound versus magnetic
resonance imaging for preoperative assessment of myometrial
infiltration in patients with endometrial cancer: A systematic
review and meta-analysis. J Gynecol Oncol. 28(e86)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang T, Tian S, Li Y, Tian X, Wang W, Zhao
J, Pei M, Zhao M, Wang L, Quan S and Yang X: Magnetic resonance
imaging (MRI) and three-dimensional transvaginal ultrasonography
scanning for preoperative assessment of high risk in women with
endometrial cancer. Med Sci Monit. 25:2024–2031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rodolakis A, Scambia G, Planchamp F, Acien
M, Di Spiezio Sardo A, Farrugia M, Grynberg M, Pakiz M, Pavlakis K,
Vermeulen N, et al: ESGO/ESHRE/ESGE guidelines for the
fertility-sparing treatment of patients with endometrial carcinoma.
Int J Gynecol Cancer. 33:208–222. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Alonso S, Castellanos T, Lapuente F and
Chiva L: Hysteroscopic surgery for conservative management in
endometrial cancer: A review of the literature.
Ecancermedicalscience. 9(505)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chae-Kim J, Garg G, Gavrilova-Jordan L,
Blake LE, Kim TT, Wu Q and Hayslip CC: Outcomes of women treated
with progestin and metformin for atypical endometrial hyperplasia
and early endometrial cancer: A systematic review and
meta-analysis. Int J Gynecol Cancer. 31:1499–1505. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mitsuhashi A, Habu Y, Kobayashi T, Kawarai
Y, Ishikawa H, Usui H and Shozu M: Long-term outcomes of progestin
plus metformin as a fertility-sparing treatment for atypical
endometrial hyperplasia and endometrial cancer patients. J Gynecol
Oncol. 30(e90)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sidorkiewicz I, Jóźwik M, Niemira M and
Krętowski A: Insulin resistance and endometrial cancer: Emerging
role for microRNA. Cancers (Basel). 12(2559)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y and Yang JX: Fertility-preserving
treatment in women with early endometrial cancer: The Chinese
experience. Cancer Manag Res. 10:6803–6813. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park JY and Nam JH: Progestins in the
fertility-sparing treatment and retreatment of patients with
primary and recurrent endometrial cancer. Oncologist. 20:270–278.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shao R: Progesterone receptor isoforms A
and B: New insights into the mechanism of progesterone resistance
for the treatment of endometrial carcinoma. Ecancermedicalscience.
7(381)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cavaliere AF, Perelli F, Zaami S,
D'Indinosante M, Turrini I, Giusti M, Gullo G, Vizzielli G, Mattei
A, Scambia G, et al: Fertility sparing treatments in endometrial
cancer patients: The potential role of the new molecular
classification. Int J Mol Sci. 22(12248)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Garzon S, Uccella S, Zorzato PC, Bosco M,
Franchi MP, Student V and Mariani A: Fertility-sparing management
for endometrial cancer: Review of the literature. Minerva Med.
112:55–69. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ruiz MP, Huang Y, Hou JY, Tergas AI, Burke
WM, Ananth CV, Neugut AI, Hershman DL and Wright JD: All-cause
mortality in young women with endometrial cancer receiving
progesterone therapy. Am J Obstet Gynecol. 217:669.e1–669.e13.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Travaglino A, Raffone A, Saccone G,
Insabato L, Mollo A, De Placido G and Zullo F: Immunohistochemical
predictive markers of response to conservative treatment of
endometrial hyperplasia and early endometrial cancer: A systematic
review. Acta Obstet Gynecol Scand. 98:1086–1099. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Knez J, Al Mahdawi L, Takač I and Sobočan
M: The perspectives of fertility preservation in women with
endometrial cancer. Cancers (Basel). 13(602)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pal N, Broaddus RR, Urbauer DL,
Balakrishnan N, Milbourne A, Schmeler KM, Meyer LA, Soliman PT, Lu
KH, Ramirez PT, et al: Treatment of low-risk endometrial cancer and
complex atypical hyperplasia with the levonorgestrel-releasing
intrauterine device. Obstet Gynecol. 131:109–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng
YL, Shan WW, Luo XZ, Zhang HW, Zhu Q, Ma FH, et al: Metformin plus
megestrol acetate compared with megestrol acetate alone as
fertility-sparing treatment in patients with atypical endometrial
hyperplasia and well-differentiated endometrial cancer: A
randomised controlled trial. BJOG. 127:848–857. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen X: The current situation of the
levonorgestrel intrauterine system (LNG-IUS) in conservative
treatment for patients with early-stage endometrial cancer and
atypical hyperplasia. J Gynecol Oncol. 30(e79)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu Z, Yang B, Guan J, Shan W, Liao J, Shao
W and Chen X: Comparison of the effect of oral megestrol acetate
with or without levonorgestrel-intrauterine system on
fertility-preserving treatment in patients with early-stage
endometrial cancer: A prospective, open-label, randomized control
phase II trial (urihttp://ClinicalTrials.govsimpleClinicalTrials.gov
NCT03241914). J Gynecol Oncol. 34(e32)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Uccella S, Zorzato PC, Dababou S, Bosco M,
Torella M, Braga A, Frigerio M, Gardella B, Cianci S, Laganà AS, et
al: Conservative management of atypical endometrial hyperplasia and
early endometrial cancer in childbearing age women. Medicina
(Kaunas). 58(1256)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lee TY, Martinez-Outschoorn UE, Schilder
RJ, Kim CH, Richard SD, Rosenblum NG and Jonshon JM: Metformin as a
therapeutic target in endometrial cancers. Front Oncol.
8(341)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li X, Fan Y and Wang J, Zhou R, Tian L,
Wang Y and Wang J: Insulin resistance and metabolic syndrome
increase the risk of relapse for fertility preserving treatment in
atypical endometrial hyperplasia and early endometrial cancer
patients. Front Oncol. 11(744689)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhao X, Niu J, Shi C and Liu Z:
Levonorgestrel-releasing intrauterine device plus metformin, or
megestrol acetate plus metformin for fertility-sparing treatment of
atypical endometrial hyperplasia and early endometrial carcinoma: A
prospective, randomized, blind-endpoint design trial protocol.
Reprod Health. 19(206)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mazzon I, Corrado G, Masciullo V,
Morricone D, Ferrandina G and Scambia G: Conservative surgical
management of stage IA endometrial carcinoma for fertility
preservation. Fertil Steril. 93:1286–1289. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Giampaolino P, Di Spiezio Sardo A, Mollo
A, Raffone A, Travaglino A, Boccellino A, Zizolfi B, Insabato L,
Zullo F, De Placido G and Bifulco G: Hysteroscopic endometrial
focal resection followed by levonorgestrel intrauterine device
insertion as a fertility-sparing treatment of atypical endometrial
hyperplasia and early endometrial cancer: A retrospective study. J
Minim Invasive Gynecol. 26:648–656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang B, Xu Y, Zhu Q, Xie L, Shan W, Ning
C, Xie B, Shi Y, Luo X, Zhang H and Chen X: Treatment efficiency of
comprehensive hysteroscopic evaluation and lesion resection
combined with progestin therapy in young women with endometrial
atypical hyperplasia and endometrial cancer. Gynecol Oncol.
153:55–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lucchini SM, Esteban A, Nigra MA, Palacios
AT, Alzate-Granados JP and Borla HF: Updates on conservative
management of endometrial cancer in patients younger than 45 years.
Gynecol Oncol. 161:802–809. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Carneiro MM, Lamaita RM, Ferreira MCF and
Silva-Filho AL: Fertility-preservation in endometrial cancer: Is it
safe? Review of the literature. JBRA Assist Reprod. 20:232–239.
2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mutlu L, Manavella DD, Gullo G, McNamara
B, Santin AD and Patrizio P: Endometrial Cancer in Reproductive
Age: Fertility-Sparing Approach and Reproductive Outcomes. Cancers
(Basel). 14(5187)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Harrison RF, He W, Fu S, Zhao H, Sun CC,
Suidan RS, Woodard TL, Rauh-Hain A, Westin SN, Giordano SH and
Meyer LA: National patterns of care and fertility outcomes for
reproductive-aged women with endometrial cancer or atypical
hyperplasia. Am J Obstet Gynecol. 221:474.e1–474.e11.
2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fan Y, Li X and Wang J, Wang Y, Tian L and
Wang J: Analysis of pregnancy-associated factors after
fertility-sparing therapy in young women with early stage
endometrial cancer or atypical endometrial hyperplasia. Reprod Biol
Endocrinol. 19(118)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Herrera Cappelletti E, Humann J, Torrejón
R and Gambaduaro P: Chances of pregnancy and live birth among women
undergoing conservative management of early-stage endometrial
cancer: A systematic review and meta-analysis. Hum Reprod Update.
28:282–295. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chao AS, Chao A, Wang CJ, Lai CH and Wang
HS: Obstetric outcomes of pregnancy after conservative treatment of
endometrial cancer: Case series and literature review. Taiwan J
Obstet Gynecol. 50:62–66. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Britton H, Huang L, Lum A, Leung S, Shum
K, Kale M, Burleigh A, Senz J, Yang W, McConechy M, et al:
Molecular classification defines outcomes and opportunities in
young women with endometrial carcinoma. Gynecol Oncol. 153:487–495.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ronsini C, Mosca L, Iavarone I, Nicoletti
R, Vinci D, Carotenuto RM, Pasanisi F, Solazzo MC, De Franciscis P,
Torella M, et al: Oncological outcomes in fertility-sparing
treatment in stage IA-G2 endometrial cancer. Front Oncol.
12(965029)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Falcone F, Leone Roberti Maggiore U, Di
Donato V, Perrone AM, Frigerio L, Bifulco G, Polterauer S, Casadio
P, Cormio G, Masciullo V, et al: Fertility-sparing treatment for
intramucous, moderately differentiated, endometrioid endometrial
cancer: A gynecologic cancer inter-group (GCIG) study. J Gynecol
Oncol. 31(e74)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Park JY, Kim DY, Kim TJ, Kim JW, Kim JH,
Kim YM, Kim YT, Bae DS and Nam JH: Hormonal therapy for women with
stage IA endometrial cancer of all grades. Obstet Gynecol.
122:7–14. 2013.PubMed/NCBI View Article : Google Scholar
|