1. Introduction
Meningiomas account for the second most frequent
primary central nervous system tumor in adults (1). Despite considerable progress in
current therapies, microsurgical resection is considered the
treatment of choice for a number of patients with meningiomas
(2-6).
Patients undergoing intracranial meningioma removal
have been reported to have an increased risk of venous
thromboembolism (VTE), including pulmonary embolism (PE) and deep
venous thrombosis (DVT), when compared with other intracranial
tumors (7-9).
The published data on patients with postoperative
VTE after meningiomas resection range from 3-72% (7,9,10-12).
The precise mechanism for this result is unknown, but some
hypotheses have included the following: Brain thromboplastin
proliferation during surgical intervention, incited coagulation of
the meningeal surface, steroid therapy and a quantity of
tumor-released hormonal and inflammatory factors (1). Consequently, given the clinical
effects of postoperative VTE in this patient population, reducing
the occurrence of VTE would considerably improve mortality. In
addition, new pre- and postoperative management procedures that
contain chemical prophylaxis, including low-molecular-weight
heparin (LMWH), have poorer effects on VTE compared with patients
who do not receive LMWH (13,14).
However, the benefit of anticoagulants is debatable,
and they are linked to a higher likelihood of intracranial
hemorrhage, making it even more crucial to identify which patients
with meningioma have the greatest risk of postoperative VTE in
order to improve decision-making concerning the risk-benefit ratio
of assertive prophylactic measures (15,16).
The present study performed a meta-analysis for
meningioma operations to ascertain rates of postoperative VTE more
closely and to ascertain the associated parameters with VTE-related
morbidity and mortality in meningioma patients following
resection.
2. Sources and data extraction
Search strategy
The present study searched the comparative articles
involving meningiomas surgery and postoperative VTE (thromboembolic
complications: DVT and PE) through electronic databases, including
the Cochrane Library, Medline (January 1980-January 2021), PubMed
(January 1980-January 2021) and EMBASE (January 1980-January 2021).
Preferred reporting items for systematic reviews and meta-analyses
(PRISMA) were applied for establishing protocol and manuscript
design (17). The present study
used the keywords ‘meningioma’, ‘thrombosis’, and ‘risk of
thrombosis’ in the Medical Subject Headings (MeSH) list.
Selection of studies
Two of the reviewers (GF and VEG) independently
extracted data from the included articles, following the guidelines
of the epidemiology of meta-analysis. The information captured
included the following essentials: The main authors, year of
publication, total case number in the meningiomas surgery (control)
and postoperative VET groups, study type and outcome indicator. The
extracted data was entered into a designed, standardized table
according to the Cochrane Handbook for Systematic Review of
Interventions (v5.1.0) (18).
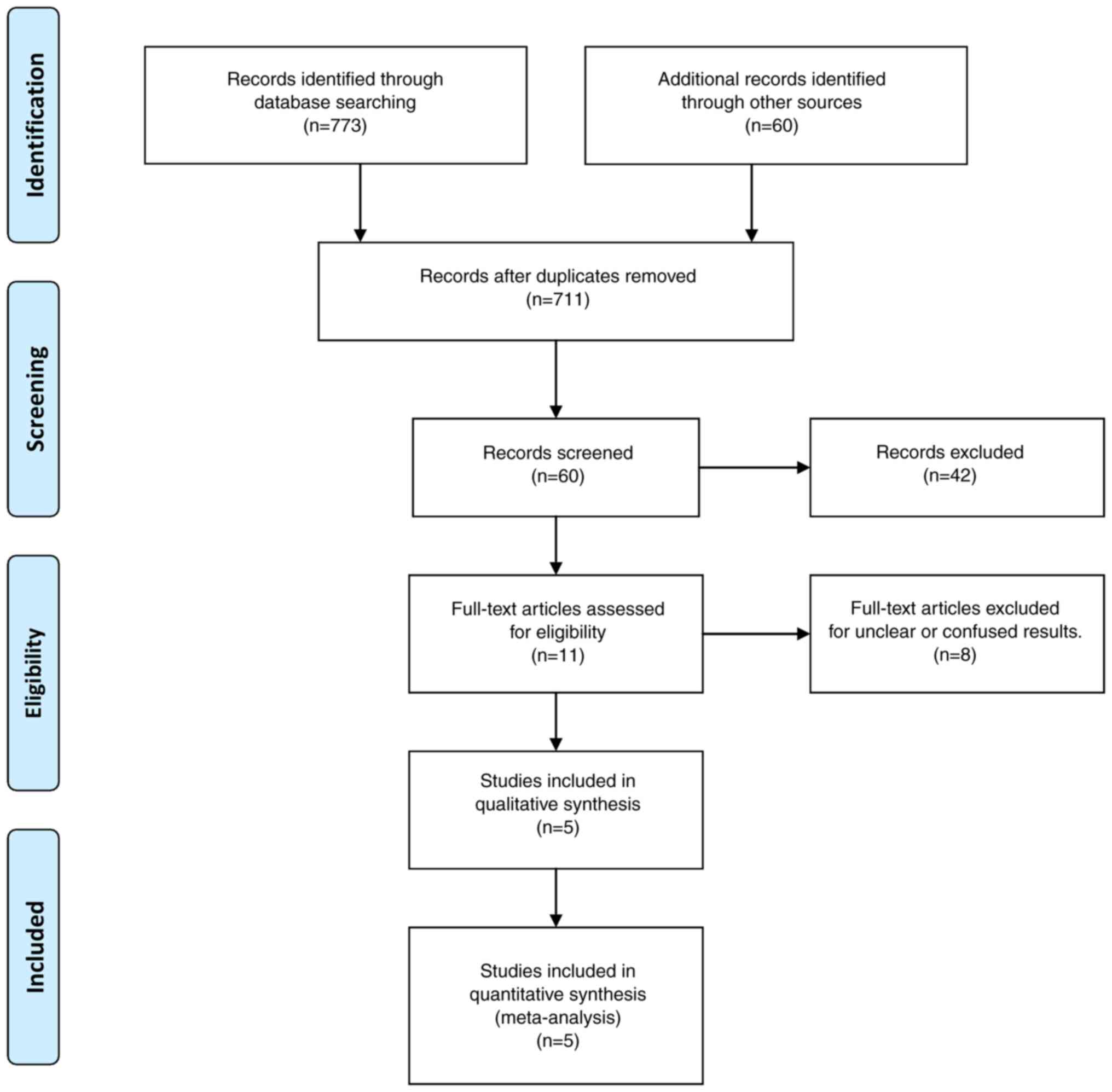
Fig. 1 depicts a flow chart of the
study selection process. If there was disagreement, another of the
authors had the final say.
Inclusion and exclusion criteria
Studies were included in the meta-analysis if the
article met the following criteria, as determined by PICOS: i)
Population: Limited to patients with intracranial meningiomas and
postoperative VTE; ii) intervention: Use of surgical treatment for
intracranial meningiomas; iii) comparison: Compared the outcomes
and iv) outcome measures: One of the primary outcomes, such as
morbidity and mortality, was involved. Tables I and II contain detailed data on these
studies. To avoid publication bias, the final aim was to collect a
homogenous pool of studies, including articles that compare only
two modalities: Intracranial meningioma surgery and postoperative
VTE.
 | Table IDesign of included trials. |
Table I
Design of included trials.
| | Tumor location | Ki-67 | |
|---|
| | Sample size | Mean Age
(year) | Number of
males | Surgical
duration | BMI | Supratentorial | Infratentorial | Skull base | Ki-67 <2% | Ki-67 2-10% | Ki-67 >10% | VTE related
Morbidity | VTE related
Mortality | |
|---|
| Trial, year | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | VTE | Control | (Refs.) |
|---|
| Gerber DE et
al, 2007 | 11 | 224 | 68 | 53 | 7 | 57 | 330 | 300 | 25 | 27 | 9 | 130 | 0 | 9 | 0 | 85 | 10 | 204 | 1 | 18 | 0 | 2 | 6 | 34 | 0 | 0 | (1) |
| Hoefnagel D et
al, 2014 | 89 | 581 | 60.3 | 56.2 | 46 | 180 | 515 | 447 | 28.9 | 26.3 | 58 | 140 | 15 | 115 | 16 | 115 | 71 | 468 | 13 | 88 | 5 | 25 | 69 | 386 | 0 | 0 | (9) |
| Safaee M et
al, 2014 | 12 | 467 | 56 | 58 | 5 | 136 | 454 | 348 | 32 | 28 | NR | NR | NR | NR | NR | NR | 7 | 376 | 5 | 77 | 0 | 31 | NR | NR | NR | NR | (23) |
| Nunno A et
al, 2019 | 170 | 5036 | 54.6 | 58 | 66 | 1682 | - | - | 29 | 28 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 108 | 811 | 10 | 66 | (24) |
| Fluss R et
al, 2021 | 17 | 197 | 62 | 59.63 | 9 | 62 | 417 | 426 | 31 | 302 | 14 | 120 | 0 | 0 | 2 | 59 | 1 | 5 | 12 | 99 | 4 | 35 | 8 | 46 | 2 | 1 | (25) |
 | Table IINewcastle-Ottawa Scale quality
assessment of final article pool. |
Table II
Newcastle-Ottawa Scale quality
assessment of final article pool.
| | Newcastle-Ottawa
Scale | |
|---|
| Trial, year | Study design | Selection | Comparability | Exposure | Total scores | (Refs.) |
|---|
| Gerber et
al, 2007 | Retrospective | 3 | 3 | 3 | 9 | (1) |
| Hoefnagel et
al, 2014 | Retrospective | 3 | 3 | 3 | 9 | (9) |
| Safaee et
al, 2014 | Retrospective | 3 | 3 | 3 | 9 | (23) |
| Nunno et al,
2019 | Retrospective | 3 | 2 | 2 | 7 | (24) |
| Fluss et al,
2021 | Retrospective | 3 | 2 | 2 | 7 | (25) |
The present study included all prospective and
retrospective studies that evaluated at least one of the two
modalities. Excluded were editorials, reviews, case reports,
articles focusing on the pediatric population, unrelated outcomes,
co-morbidities, experimental techniques, or one of the two
modalities from the article pool. In addition, all that had mixed
or unclear results were put into either the meningioma surgery
group (the control group) or the VTE group.
Definition of outcomes
The primary outcomes involved in the present study
included VTE-related mortality and morbidity. In addition, to find
out the association between meningioma surgery and VTE, outcome
measurements such as surgical duration, body mass index (BMI),
location and the proliferation marker for human tumor cells, Ki-67
were collected. The outcomes reported by the included articles were
assessed at least 30 days after the surgical treatment of
meningiomas.
Patient morbidity was scored Karnofsky Performance
Status Scale (KPS) <80(19);
dependent ambulatory (indicating walking with a mobility aid, such
as a cane or walking frame), wheel-chair bound, or bedridden.
VTE-related mortality was defined as mortality within 30 days
following surgery registered with VTE.
The mean surgical duration, defined as the time from
anesthesia induction to skin closure, was >310 min. A
board-certified neuroradiologist's pre-operative magnetic resonance
imaging review described the mean tumor size (mean volume in
cm3) and location (supratentorial, infratentorial and
skull base). Tumor grade and Ki-67 indices were retrieved from
operative pathology reports based on the World Health Organization
(WHO) classification (III) assigned by board-certified
neuropathologists (20).
Additionally, to decrease the risk of bias in poor
articles, a quality assessment tool [the Newcastle Ottawa Scale
(NOS)] was used (Table II)
(21).
Evaluation of the risk of bias
The Cochrane Collaboration's tool to assess the risk
of bias (ROB) was used by two reviewers (GF and VEG) for each study
(22). The evaluation includes
random sequence generation, allocation concealment, blinding of
participants and assessors, blinding of outcome assessment,
incomplete outcome data, selective reporting and other biases. The
assessment results were classified into three levels: Low risk,
high risk and unclear risk. A third reviewer arbitrated any
disagreements.
Data synthesis and assessment of
heterogeneity
All analyses were carried out using Review Manager
Software (RevMan), version 5.4 (https://training.cochrane.org/online-learning/core-software/revman).
Heterogeneity across trials was identified using I2
statistics; I2>50% was considered as high
heterogeneity. A meta-analysis was conducted using a random-effect
model according to the Cochrane Handbook for Systematic Reviews of
Interventions (version 5.1.0) (19). When the model parameters were fixed
or non-random quantities the fixed-effect model was used. The
continuous outcomes were expressed as a weighted mean difference
with 95% confidence intervals (CIs). For discontinuous variables,
odds ratios (OR) with 95% CIs were applied for the assessment.
P<0.05 was considered to indicate a statistically significant
difference.
3. Data on parameters associated with
VTE-related morbidity and mortality in meningioma patients
following resection
Eligibility criteria were met by five articles
(1,9,23-25).
The total number of patients was 6,505 who underwent surgery for
meningiomas and 299 (4.5%) revealed postoperative VTE. The study
sample was based on five studies (Table II). All reports were retrospective
observational studies.
Epidemiological and clinical
features
The mean age of patients was 56.9 (60.1 years for
the VTE sample) and ranged from 18-77 years. The male-to-female
ratio was 1:3.07 (1:1.24 for the VTE group). A total of 1,277
(21.1%) of 6,038/6,505 patients with morbidity data had a KPS of 80
and 191 (63.8%) of 287/299 VTE patients had a poor outcome.
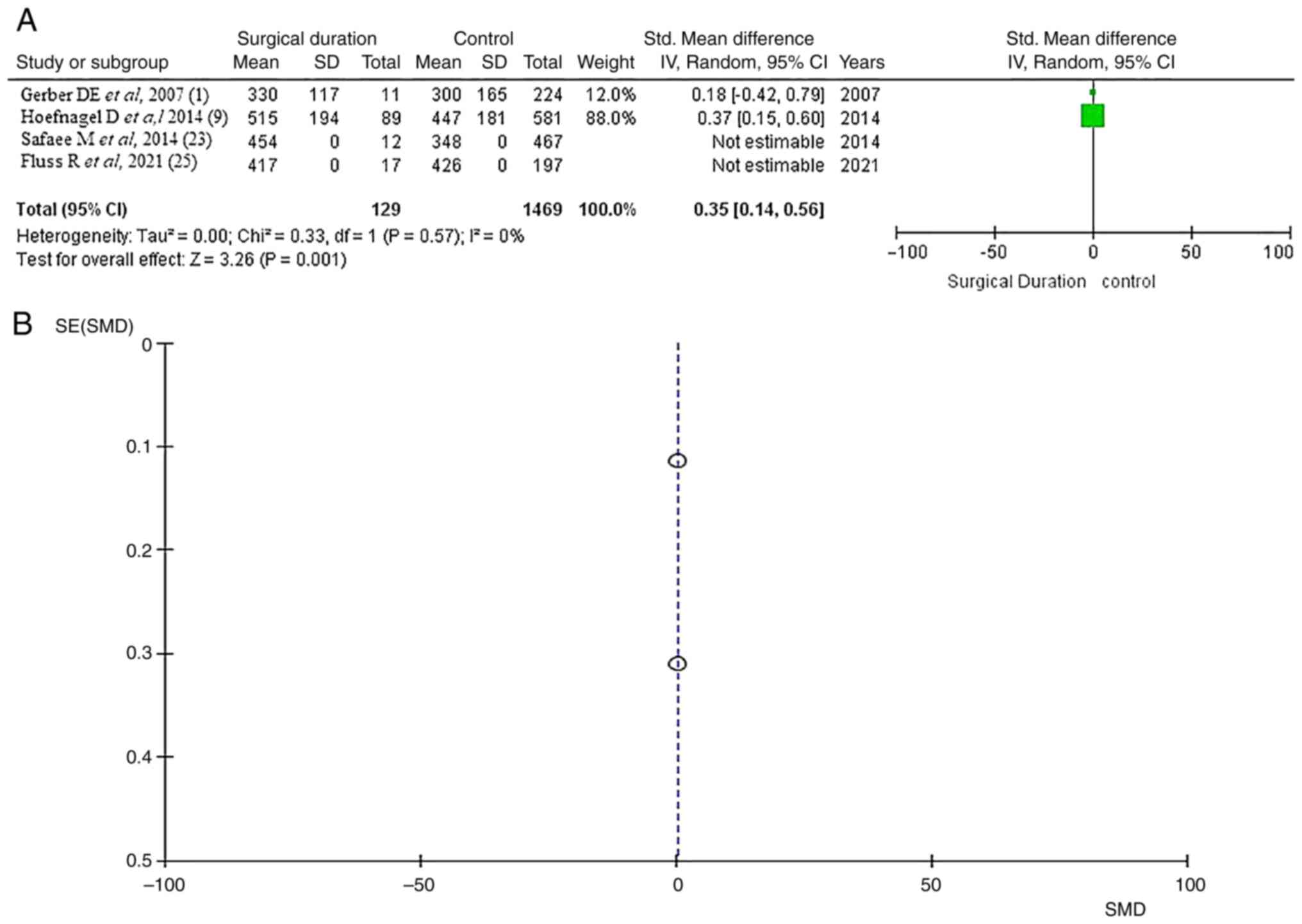
Surgical duration
Information on surgical duration was available in
four of the five articles (1,9,23,25)
with a total of 1,469 patients and 129 with VTE presented with a
mean surgical time of 380/429 min for total patients and VTE
demonstrated a statistically significant result (OR 0.35, CI 95%
0.14-0.56 and P<0.05) with no heterogeneity (P=0.57 and
I2=0%). A very low publication bias was found (Fig. 2).
BMI
Information regarding BMI was available in five
articles (1,9,23-25)
with a total of 6,505 patients and 299 with VTE presented with a
mean BMI of 27.9/29.1 (kg/m2) for total patients and VTE
demonstrated statistically significant results (OR 2.48, CI 95%
1.58-3.38 and P<0.05) with no heterogeneity (P=0.32 and
I2=0%) with no heterogeneity (P=0.32 and
I2=0%). A very low publication bias was found (Fig. 3).
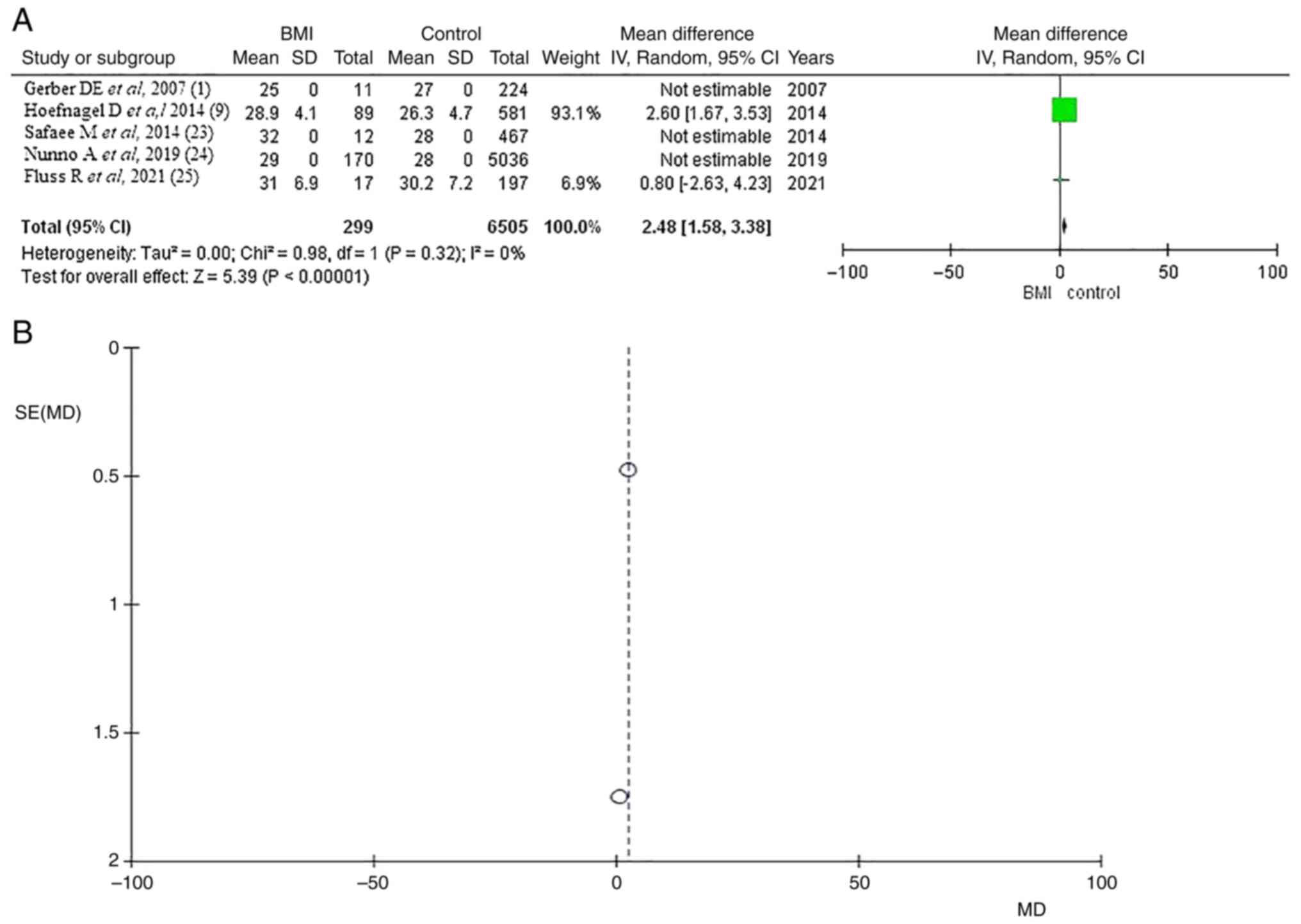 | Figure 3BMI significance. (A) Forest plot
body mass index (BMI): Results demonstrated a statistically
significant result (OR 2.48, CI 95% 1.58-3.38 and P<0.05). (B)
Funnel Plot, testing the sensitivity with funnel plot for BMI,
there was no heterogeneity and thus low publication bias (P=0.32
and I2=0%). BMI, body mass index; OR, odds ratio; CI,
confidence interval; P, P-value; I2, the percentage of
total variation across studies that is due to heterogeneity rather
than chance; SE, standard error; SD, standard deviation; MD, mean
difference. |
Location
Information regarding location was available in
three of five articles (1,9,25).
Supratentorial
Supratentorial location was reported in 390 (38.9%)
of the 1,002 patients in the total sample and in 81 (69.2%) of the
117 patients in the VTE group. The results of the analysis
demonstrated no statistically significant difference (OR 2.13, CI
95% 0.98-4.63 and P=0.06) with heterogeneity (P=0.02 and
I2=75%; (Fig. S1).
Infratentorial
As regards the infratentorial location, the total
number of patients was 124 (12.3%) from 1,002 in the total sample
and 15 (12.8%) from 1,117 in the VTE group. The statistical
analysis demonstrated no potentially significant difference [OR
0.83, CI 95% (0.47-1.48), P=0.54], providing no heterogeneity
(P=0.91 and I2=0%) (Fig.
S2). A very low publication bias was found.
Skull base
In total, 259 (24.8%) of 1,002 patients had skull
base location and 18 (15.3%) of 117 had VTE. The results of the
analysis demonstrated no statistically significant difference (OR
0.58, CI 95% 0.23-1.46 and P=0.25), with low heterogeneity (P=0.23
and I2=33%; Fig.
S3).
Ki-67 is a proliferation marker for
human tumor cells. Ki-67 <2%
Information regarding Ki-67 <2% was available in
four of five articles (1,9,23,25)
and demonstrated no statistical significance (OR 0.98, CI 95%
0.72-1.31 and P=0.87) with no heterogeneity (P=0.76 and I2=0%;
Fig. S4). Ki-67 <2% was found
in 1,053 of 1,469 (71.6%) patients in the total group, compared
with the VTE group, where Ki-67 <2% was diagnosed in 89 of 129
(68.9%) patients.
Ki-67 2-10%
There were 282 (19.1%) of the 1,469 patients with
Ki-67 (2-10%) and 31 (24.0%) of the 129 patients with VTE in the
total group of patients. The results of the analysis demonstrated
no statistically significant difference (OR 1.30, CI 95% 0.84-2.01
and P=0.24), with no heterogeneity (P=0.45 and I2=0%;
Fig. S5).
Ki-67 >10%
As regards the Ki-67 >10%, the total number of
patients was 93 (6.3%) from 1,469 patients and 9 (6.9%) from 129 in
the VTE group. The statistical analysis demonstrated no potentially
significant difference (OR 1.37, CI 95% (0.43-2.80), P=0.38),
providing no heterogeneity (P=0.86 and I2=0%; Fig. S6). A very low publication bias was
found.
Morbidity
Morbidity data were available in four of the five
articles (1,23-25)
with a total of 1,277 (21.1%) patients; poor KPS in 191 (66.5%) of
287 VTE patients, a statistically significant difference (OR 2.58,
CI 95% 1.11-5.99 and P=0.03) and heterogeneity (P=0.05 and
I2=92%; Fig. 4).
Testing the sensitivity, the present study used the ‘leave out one’
model and removed one study at a time (Table III). After removing the article
by Safaee et al (23), a
statistically significant difference result was found (OR 4.19, CI
95% 3.30-5.31 and P<0.05), with no heterogeneity (P=0.37 and
I2=0%; Fig. 4B). It was
found that the study results without the Safaee et al
(23) displayed superior
dispersion, with very low publication bias.
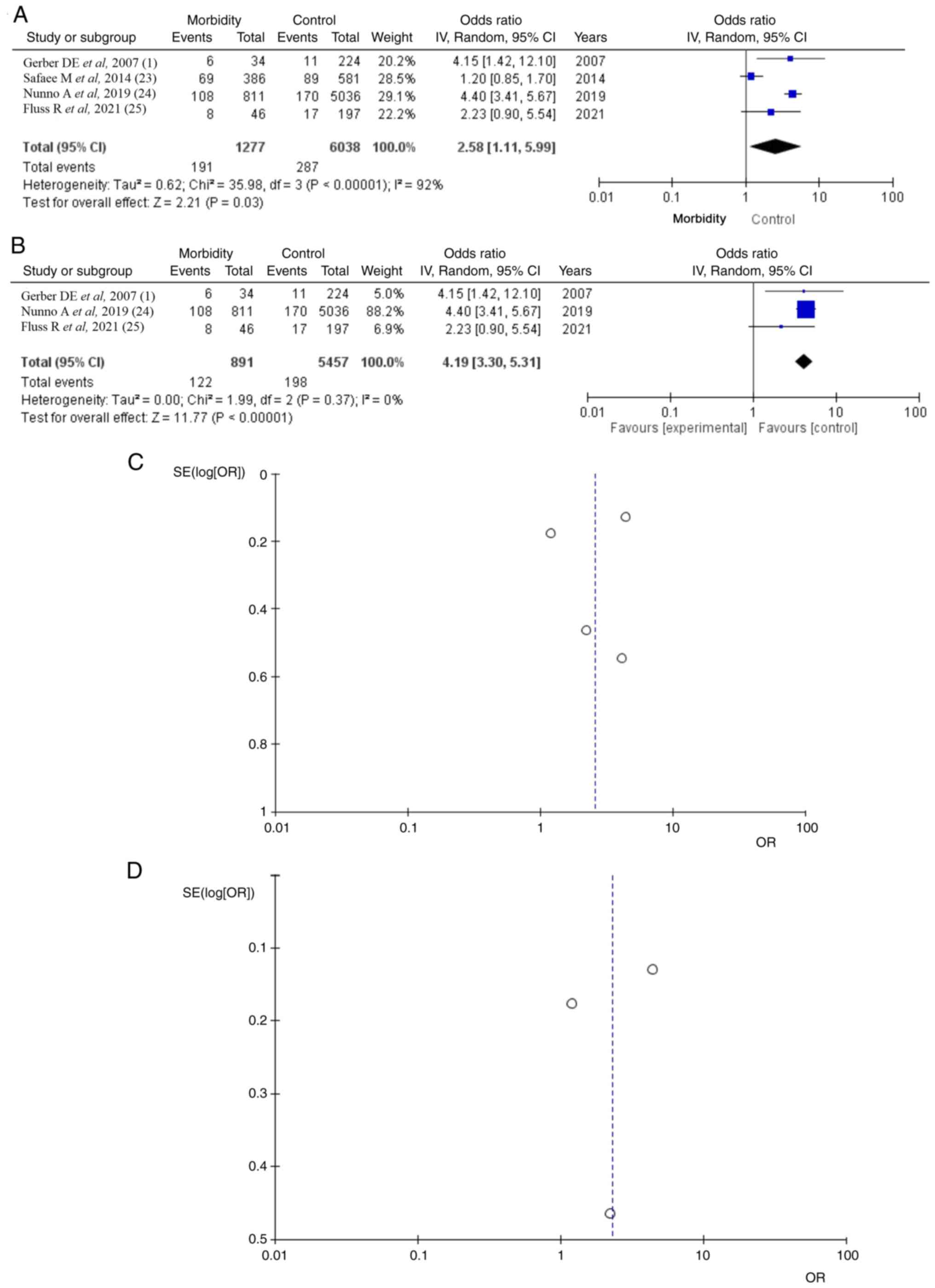 | Figure 4Morbidity. (A) Forest plot morbidity:
Results demonstrated a statistically significant difference between
total surgical meningiomas and VTE groups (OR 0.63, CI
95%-0.06-1.32 and P=0.05). (B) OR forest plot morbidity without
Safaee et al (23): Results
demonstrate again a statistically significant difference result,
(OR 4.19, CI 95% 3.30-5.31 and P<0.05). (C and D) Funnel plots
of the mortality in the total surgical meningiomas and VTE groups,
with (left) or without (right) Safaee et al (23) and with (left) heterogeneity
(P<0.05 and I2=92%) or without (right) heterogeneity
(P=0.37 and I2=0%). VTE, venous thromboembolism; OR,
odds ratio; CI, confidence interval; P, P-value; I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; SE, standard error. |
 | Table IIIMeta-analysis results. |
Table III
Meta-analysis results.
| | Group | Overall effect | Heterogeneity |
|---|
| Outcome | Trial n=5 | VET | Control | Effect
estimate | CI 95% | P-value | I2
(%) | P-value |
|---|
| Surgical duration
(mean) | 4 | 429 | 380 | 0.35 | (0.14-0.56) | <0.05 | 0 | 0.57 |
| BMI (mean) | 5 | 29.1 | 27.9 | 2.48 | (1.58-3.38) | <0.05 | 0 | 0.32 |
| Tumor location | | | | | | | | |
|
Supratentorial | 3 | 81 | 390 | 2.13 | (0.98-4.63 | 0.06 | 75 | 0.02 |
|
Infratentorial | 3 | 15 | 124 | 0.83 | (0.47-1.48) | 0.54 | 0 | 0.91 |
|
Skull
base | 3 | 18 | 259 | 0.58 | (0.23-1.46) | 0.25 | 33 | 0.23 |
| Ki-67 | | | | | | | | |
|
<2% | 4 | 89 | 1,053 | 0.98 | (0.72-1.31) | 0.87 | 0 | 0.76 |
|
2-10% | 4 | 31 | 282 | 1.30 | (0.84-2.01) | 0.24 | 0 | 0.45 |
|
>10% | 4 | 9 | 93 | 1.37 | (0.67-2.80) | 0.38 | 0 | 0.86 |
| Morbidity | 4 | 191 | 1,277 | 2.58 | (1.11-5.99) | 0.03 | 92 | <0.001 |
| | 3 | 185 | 1,243 | 2.29 | (0.85-6.16) | 0.10 | 94 | <0.001 |
| | 3 | 122 | 891 | 4.19 | (3.30-5.31) | <0.05 | 0 | 0.37 |
| | 3 | 83 | 466 | 1.95 | (0.94-4.07) | 0.07 | 65 | 0.06 |
| | 3 | 183 | 1,231 | 2.71 | (0.97-7.52) | 0.06 | 94 | <0.001 |
| Mortality | 4 | 12 | 67 | 29.12 | (7.89-107.38) | <0.05 | 0 | 0.92 |
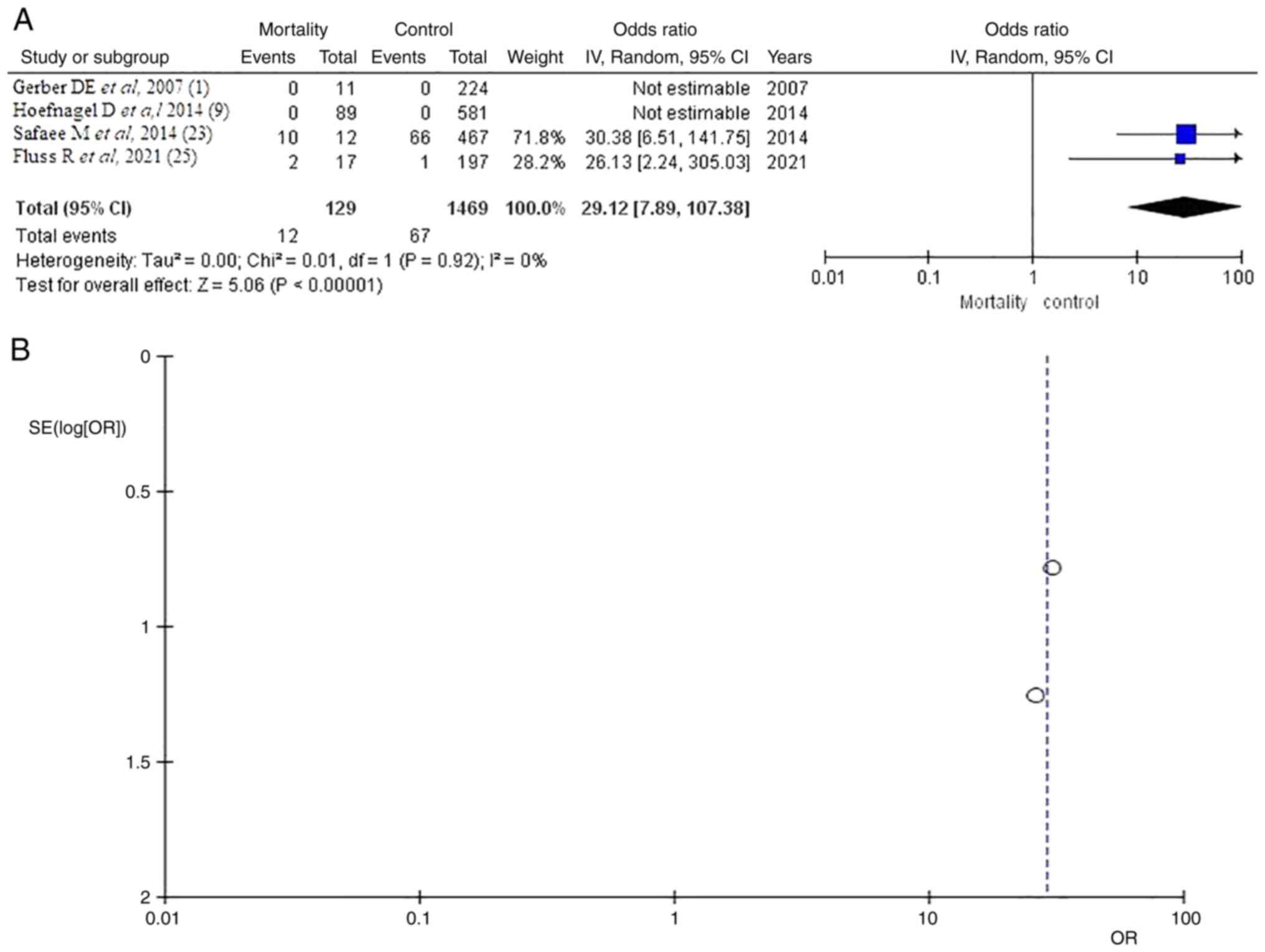
Mortality
As regards the mortality rate, information was
available in four of the five articles (1,9,23,25).
In the total group of patients, there were 129 (8.7%) from 1,469
patients diagnosed and 12 (17.9%) from 67 in the VTE group. The
pooled results demonstrated a statistically significant result (OR
29.12, CI 95% 7.89-107.38 and P<0.05) with no heterogeneity
(P=0.92 and I2=0%; Fig.
5).
4. Discussion
The present study suggested that open surgery for
meningiomas was associated with postoperative VTE in 4.5% of
patients. More precisely, a mean surgical duration time >380 min
and a mean BMI >27.9 kg/m2 were statistically
significant VTE-related parameters in patients who underwent
meningiomas surgery, showing an association with VTE-related
morbidity and mortality. The findings of the present meta-analysis
study suggested that surgical duration and BMI are related to a
high risk of VTE and, thus, an increased risk of postoperative
morbidity (KPS <80) and mortality.
The 4.5% postoperative VTE rate taken out of these
data is certainly lower than rates accounted for in other studies,
which have reported up to 72% of patients with meningiomas
developing VTE (26). However,
this approximation relies on how VTE is defined. Thus, the
literature on symptomatic postoperative VTE mentions a much lower
percentage, with rates between 3.09 and 7.2% (9,11),
which fits the results of the present study.
A number of the risk factors identified in the
present study have been previously reported (24). A mean surgical duration has not
been correlated with patients with meningioma but is a known risk
factor for VTE (27,28). A mean surgical duration of more
than 310 min, defined as the time from anesthesia induction to skin
closure, appears to be associated with poor outcome and a high risk
of mortality and morbidity in patients with postoperative VTE after
meningiomas resection in the present study.
In addition, obesity, defined by BMI, has not been
correlated with patients with meningioma but is known as another
risk factor for VTE (10). The
present study included BMI as one of the main parameters associated
with postoperative meningiomas and VTE-related morbidity and
mortality.
Although a number of risk factors have been found
significant for the development of VTE, such as larger tumor size
and skull base location (8,29),
the present study found no statistically significant results.
Additionally, the Ki-67 proliferation marker for human tumor cells
does not relate to VTE-related mortality and morbidity in patients
who underwent surgical resection for meningiomas.
In the present study, the mortality rate of patients
with postoperative VTE was found to be 17.9%, which is almost
double the 5.88% derived from the literature (9,24).
This inconsistency may be due to measuring the mortality rate in
the different postoperative periods.
Study limitations
There are several limitations to the present study.
First, all eligible reports that were included were retrospective.
These retrospective studies, by definition, rely on imprecision and
can suffer from data loss. Additionally, the methods of the
included studies differed significantly. Among these differences
were the operative technique (e.g., anterior/lateral approach) and
length of follow-up (e.g., 30-90 days). Finally, limitations of the
other studies were the small sample size and that there was not
among the included studies in the current paper a clearly separated
information about receiving LMWH. Thus the identification of VTE
risk in patients receiving LMWH compared with those not receiving
LMWH or other VTE prophylaxis measures could be indicate the aim of
a possible future study.
5. Conclusions
The present study investigated the clinical outcomes
of patients who had postoperative VTE following intracranial
meningioma resection. The findings demonstrated that open surgery
for meningiomas was associated with postoperative VTE. Furthermore,
surgical duration and BMI were statistically significant
VTE-related parameters in patients who underwent meningioma
surgery, showing an association with VTE-related morbidity and
mortality. The findings of the present meta-analysis study
highlighted that surgical duration and BMI are related to a high
risk of VTE and, thus, an increased risk of postoperative morbidity
and mortality.
Supplementary Material
Supratentorial location. (A) OR forest
plot supratentorial location: Results demonstrated no statistically
significant results (OR 2.13, CI 95% 0.98-4.63 and P=0.06). (B)
Funnel plot of the supratentorial location in the group of patients
with surgical management of intracranial meningiomas, demonstrated
very high heterogeneity (P=0.02 and I2=75%). OR, odds
ratio; CI, confidence interval; P, P-value; I2, the
percentage of total variation across studies that is due to
heterogeneity rather than chance; SE, standard error.
Infratentorial location. (A) OR forest
plot infratentorial location: Results demonstrated no statistically
significant results [OR 0.83, CI 95% (0.47-1.48), P=0.54]. (B)
Funnel plot of the infratentorial location in the group of patients
with surgical management of intracranial meningiomas, providing no
heterogeneity (P=0.91 and I2=0%). OR, odds ratio; CI,
confidence interval; P, P-value; I2, the percentage of
total variation across studies that is due to heterogeneity rather
than chance; SE, standard error.
Skull base location. (A) OR Forest
plot skull base location: Results demonstrated no statistically
significant results (OR 0.58, CI 95% 0.23-1.46 and P=0.25). (B)
Funnel plot of the skull base location in the group of patients
with surgical management of intracranial meningiomas, providing low
heterogeneity (P=0.23 and I2=33%). OR, odds ratio; CI,
confidence interval; P, P-value; I2, the percentage of
total variation across studies that is due to heterogeneity rather
than chance; SE, standard error.
Ki-67 <2%. (A) OR Forest plot Ki-67
<2%: Results demonstrated no statistically significant results
(OR 0.98, CI 95% 0.72-1.31 and P=0.87); (B) Funnel plot of the
Ki-67 <2% in the group of patients with surgical management of
intracranial meningiomas, providing no heterogeneity (P=0.76 and
I2=0%). OR, odds ratio; CI, confidence interval; P,
P-value; I2, the percentage of total variation across
studies that is due to heterogeneity rather than chance; SE,
standard error.
Ki-67 2-10%. (A) Forest plot Ki-67
2-10%: Results demonstrated no statistically significant result (OR
1.30, CI 95% 0.84-2.01 and P=0.24). (B) Funnel plot, testing the
sensitivity with funnel plot for Ki-67 2-10% there was no
heterogeneity and thus low publication bias (P=0.45 and
I2=0%). OR, odds ratio; CI, confidence interval; P,
P-value; I2, the percentage of total variation across
studies that is due to heterogeneity rather than chance; SE,
standard error.
Ki-67 >10%. (A) Forest plot Ki-67
>10%: Results demonstrated no statistically significant result
[OR 1.37, CI 95% (0.43-2.80), P=0.38]. (B) Funnel plot, testing the
sensitivity with funnel plot for Ki-67 >10% there was no
heterogeneity and thus low publication bias (P=0.86 and
I2=0%). OR, odds ratio; CI, confidence interval; P,
P-value; I2, the percentage of total variation across
studies that is due to heterogeneity rather than chance; SE,
standard error.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
GF and VEG conceived the study. VEG, AAF, KT, PS,
DAS, GF and NT analyzed the data and wrote and prepared the draft
of the manuscript. VEG and GF provided critical revisions. All
authors contributed to manuscript revision and have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors have no competing interests.
References
|
1
|
Gerber DE, Segal JB, Salhotra A, Olivi A,
Grossman SA and Streiff MB: Venous thromboembolism occurs
infrequently in meningioma patients receiving combined modality
prophylaxis. Cancer. 109:300–305. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fotakopoulos G, Tsianaka E,
Panagiotopoulos V and Fountas K: New Developments in Management of
Meningioma. J Integr Oncol. 4(1000135)2015.
|
|
3
|
Fotakopoulos G, Tsolaki V,
Aravantinou-Fatorou A, Georgakopoulou VE, Spandidos DA, Papalexis
P, Tarantinos K, Trakas N, Sklapani P, Mathioudakis N, et al:
Uncommon and atypical meningiomas and imaging variants: A report of
7 cases. Med Int (Lond). 2(35)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alexiou GA, Vartholomatos E, Goussia A,
Dova L, Karamoutsios A, Fotakopoulos G, Kyritsis AP and Voulgaris
S: DNA content is associated with malignancy of intracranial
neoplasms. Clin Neurol Neurosurg. 115:1784–1787. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Black PM, Morokoff AP and Zauberman J:
Surgery for extra-axial tumors of the cerebral convexity and
midline. Neurosurgery. 62 (6 Suppl 3):S1115–S1123. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ekşi MŞ, Canbolat Ç, Akbaş A, Özmen BB,
Akpınar E, Usseli Mİ, Güngör A, Güdük M, Hacıhanefioğlu M, Erşen
Danyeli A, et al: Elderly patients with intracranial meningioma:
surgical considerations in 228 patients with a comprehensive
analysis of the literature. World Neurosurg. 132:e350–e365.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carrabba G, Riva M, Conte V, Di Cristofori
A, Caroli M, Locatelli M, Castellani M, Bucciarelli P, Artoni A,
Stocchetti N, et al: Risk of post-operative venous thromboembolism
in patients with meningioma. J Neurooncol. 138:401–406.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eisenring CV, Neidert MC, Sabanés Bové D,
Held L, Sarnthein J and Krayenbühl N: Reduction of thromboembolic
events in meningioma surgery: A cohort study of 724 consecutive
patients. PLoS One. 8(e79170)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hoefnagel D, Kwee LE, van Putten EH, Kros
JM, Dirven CM and Dammers R: The incidence of postoperative
thromboembolic complications following surgical resection of
intracranial meningioma. A retrospective study of a large single
center patient cohort. Clin Neurol Neurosurg. 123:150–154.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Karhade AV, Fandino L, Gupta S, Cote DJ,
Iorgulescu JB, Broekman ML, Aglio LS, Dunn IF and Smith TR: Impact
of operative length on post-operative complications in meningioma
surgery: A NSQIP analysis. J Neurooncol. 131:59–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Levi AD, Wallace MC, Bernstein M and
Walters BC: Venous thromboembolism after brain tumor surgery: A
retrospective review. Neurosurgery. 28:859–863. 1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sawaya R and Glas-Greenwalt P:
Postoperative venous thromboembolism and brain tumors: Part II.
Hemostatic profile. J Neurooncol. 14:127–134. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Iorio A and Agnelli G:
Low-molecular-weight and unfractionated heparin for prevention of
venous thromboembolism in neurosurgery: A meta-analysis. Arch
Intern Med. 160:2327–2332. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khan NR, Patel PG, Sharpe JP, Lee SL and
Sorenson J: Chemical venous thromboembolism prophylaxis in
neurosurgical patients: An updated systematic review and
meta-analysis. J Neurosurg. 129:906–915. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hart RG, Boop BS and Anderson DC: Oral
anticoagulants and intracranial hemorrhage. Facts and hypotheses.
Stroke. 26:1471–1477. 1995.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Flaherty ML: Anticoagulant-associated
intracerebral hemorrhage. Semin Neurol. 30:565–572. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Foster RL: Reporting guidelines: Consort,
prisma, and squire. J Spec Pediatr Nurs. 17:1–2. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Higgins JPT and Green S (eds): Cochrane
Handbook for Systematic Reviews of Interventions Version 5.1.0. The
Cochrane Collaboration, 2011. www.cochrane-handbook.org. Updated March 2011.
|
|
19
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. Ottawa Hospital Research Institut, Ottawa, ON, 2014.
http://www.ohri.ca/
programs/clinical_epidemiology/oxford.asp.
|
|
22
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Safaee M, Sun MZ, Oh T, Aghi MK, Berger
MS, McDermott MW, Parsa AT and Bloch O: Use of thrombin-based
hemostatic matrix during meningioma resection: A potential risk
factor for perioperative thromboembolic events. Clin Neurol
Neurosurg. 119:116–120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nunno A, Li Y, Pieters TA, Towner JE,
Schmidt T, Shi M, Walter K and Li YM: Risk factors and associated
complications of symptomatic venous thromboembolism in patients
with craniotomy for meningioma. World Neurosurg. 122:e1505–e1510.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fluss R, Kobets AJ, Inocencio JF, Hamad M,
Feigen C, Altschul DJ and Lasala P: The incidence of venous
thromboembolism following surgical resection of intracranial and
intraspinal meningioma. A systematic review and retrospective
study. Clin Neurol Neurosurg. 201(106460)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sawaya R, Zuccarello M, Elkalliny M and
Nishiyama H: Postoperative venous thromboembolism and brain tumors:
Part I. Clinical profile. J Neurooncol. 14:119–125. 1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim JY, Khavanin N, Rambachan A, McCarthy
RJ, Mlodinow AS, De Oliveria GS Jr, Stock MC, Gust MJ and Mahvi DM:
Surgical duration and risk of venous thromboembolism. JAMA Surg.
150:110–117. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Anderson FA Jr and Spencer FA: Risk
factors for venous thromboembolism. Circulation. 107 (23 Suppl
1):I9–I16. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moussa WMM and Mohamed MAA: Prophylactic
use of anticoagulation and hemodilution for the prevention of
venous thromboembolic events following meningioma surgery. Clin
Neurol Neurosurg. 144:1–6. 2016.PubMed/NCBI View Article : Google Scholar
|