Introduction
Gastric fundus glands, also known as oxyntic glands,
are normally distributed in the fundus and body of the stomach and
include the chief, parietal, cervical mucus, endocrine, and stem
cells. In 2003 and 2005, Müller-Höcker and Rellecke (1) and Matsukawa et al (2) reported on fundic dysplastic polyps of
the chief cell-predominant type. Tsukamoto et al (3) first described gastric adenocarcinoma
with chief cell differentiation in 2007. In 2019, the WHO
classification of digestive system tumors (fifth edition) defined
intramucosal gastric fundus gland tumors as oxyntic gland adenomas,
while those with submucosal infiltration were classified as gastric
adenocarcinoma of the fundic gland type (GAFG) (4). GAFG is a rare type of gastric
neoplasm with an incidence of <0.1% among the patients
undergoing gastroscopy (5), with
the majority of case reports originating from Japan. According to
previous studies, patients with GAFG are primarily middle-aged or
elderly (aged 36-87 years), with a slight male majority (3,6,7).
GAFG patients generally have mild symptoms, such as abdominal
discomfort and acid reflux, or no symptoms. Additionally, clinical
tests have identified only a single case with significant
abnormalities, in which slightly increased C-reactive protein and
carcinoembryonic antigen levels were observed (1). In brief, no specific clinical
manifestations or laboratory test results are known to be
associated with the GAFG, to the best of our knowledge.
Endoscopic examinations have revealed that the
majority of GAFG cases involve solitary lesions in the upper and
middle third of the stomach; multiple lesions were observed in only
a small number of individual patients (8). The mean tumor size was ~10 mm, and
the maximum reported diameter was 85 mm (9). The lesions may appear as raised,
flat, or concave (10-12).
Changes in the color of the mucosa, such as from pink to white,
yellow, or black, can contribute to an early diagnosis (13). Dilated branching vessels have been
observed in approximately half of the reported cases (14). Pathological examination of tumor
specimens is necessary for a correct diagnosis. Morphologically,
GAFG is divided into three subcategories: Chief cell-predominant
(~99% of the reported cases), parietal cell-predominant, and mixed
phenotypes (15). Most tumors
exhibit mild to moderate dysplasia, even when submucosal
infiltration occurs (7).
Helicobacter pylori infection, intestinal metaplasia, and
mucosal atrophy in GAFG are infrequent compared with traditional
gastric adenocarcinoma (5,10,16).
Immunohistochemistry analyses have revealed the presence of
pepsinogen I and mucin-6 (MUC6). Severe cellular dysplasia,
lymphovascular invasion, lymph node metastasis, and atypical
cellular differentiation may be markers of invasion and have been
suggested to indicate poor prognosis (7,9,17-19).
High-risk patients can be treated with total or segmental
gastrectomy plus lymph node dissection (7,18).
GAFG has unique pathological features compared to
traditional gastric adenocarcinoma; however, few studies have
investigated the GNAS, KRAS, and Wnt signaling
pathways at the molecular level in GAFG. Here, molecular analysis
of 10 Chinese GAFG specimens was performed and the relevant
literature was reviewed to improve our understanding of the
molecular characteristics of GAFG. The molecular results were
combined with clinicopathological information, first covering EBV
infection and HER2 status, considering that EBV-positive
gastric cancer tends to occur in the fundus or body of the stomach
(20), and HER2 has
predictive value as it can be used to evaluate the efficacy of
trastuzumab and lapatinib in the treatment of HER2-positive
gastric cancer patients (21). The
results of this study have implications for future explorations
into the factors underlying tumor occurrence, development, and
identification of clinical prognostic biomarkers and potential
therapeutic targets. To the best of our knowledge, this is the
first study on GAFG in a Chinese cohort. Novel findings surrounding
the genetic factors underlying the disease are presented.
Materials and methods
Case selection and clinicopathological
characteristics of patients
Tumor samples from 10 Chinese patients with GAFG
were collected from Peking University International Hospital and
Liaocheng People's Hospital between January 2015 and March 2022.
The patients included 4 males and 6 females, aged 46-75 years, with
a mean age of 62.5 years. Samples from 9 patients were obtained
during complete endoscopic resection, and samples for the remaining
one case were obtained by biopsy. The size of the tumors observed
during endoscopy ranged from ~0.3-1.2 cm. All postoperative
specimens were examined and diagnosed by two senior pathologists.
All cases were classified as chief cell-predominant, intruding into
the submucosa by 100-300 µm, with mild to moderate dysplasia, with
no lymphovascular or perineural involvement. Immunohistochemical
investigations found that the tumor cells were diffusely positive
for pepsinogen I and MUC6, focally reactive for MUC5ac and
H+/K+ ATPase, and negative for MUC2 and CD10.
Moreover, β-catenin protein expression was observed only in the
cell membranes. All samples were identified as negative or
partially weakly positive for p53 protein. All tumors were 1-15%
diffusely distributed, as measured by the Ki-67 index. After a
follow-up period of 16-48 months, no recurrence or metastasis was
observed in any of the patients.
Epstein Barr virus-encoded RNA
(EBV-EBER) testing
Specimens were fixed with 4% neutral buffered
formalin solution at room temperature for 12 h before being
embedded in paraffin and cut into 4 µm sections. EBER was detected
using an in situ hybridization kit (cat. no. ISH-7001,
OriGene Technologies, Inc.), and non-keratinizing nasopharyngeal
carcinoma was used as a positive control. Finally, the sections
were stained with DAB at room temperature for 5 min, counterstained
with hematoxylin at room temperature for 5 min, and then observed
under a Nikon light microscope (maximum magnification, x400, Nikon
Corporation). Positive nuclei were stained brown with DAB and the
negative nuclei were stained blue with hematoxylin.
Gene analysis
All tissue samples included in this study were
pathologically confirmed to contain at least 20% tumor cells. Tumor
tissues embedded in paraffin were cut into wax rolls which were
analyzed with Next-Generation Sequencing (NGS, Gene+ Smart
Laboratory). This high-throughput DNA panel sequencing technology
allowed mutation information for numerous genes to be obtained.
Here, GNAS, KRAS, NRAS, BRAF,
PIK3CA, TP53, APC, CTNNB1, HER2,
MLH1, MSH2, MSH6, and PMS2, alongside
other genes (see Table SI for a
detailed list of all 73 genes) were examined for point mutations,
insertions, deletions, fusions, and amplifications; adjacent
non-tumor tissues were used as the control.
DNA was extracted from paraffin-embedded tumor
tissues using a QIAamp DNA Mini Kit (Qiagen GmbH). DNA was then
fragmented into ~300 bp fragments and a library was constructed
using the KAPA Library Preparation Kit (Kapa Biosystems, Inc.). The
SeqCapEZ Library (Roche Diagnostics) was used to enrich the
fragments for the target regions of 73 common genes involved in
tumor development. After processing the enriched library using the
TruSeq PE Cluster Generation Kit v3 and TruSeq SBS Kit v3 reagent
kits (Illumina, Inc.), sequencing was performed using an Illumina
HiSeq 3000 sequencing platform (Illumina, Inc.). After removing the
terminal connector sequence and filtering out low-quality
sequences, the reads were mapped to the human genome. The Genome
Analysis Tool Kit (https://www.broadinstitute.org/gatk/; GATK) and MuTect
tools were used to detect insertions/deletions and single
nucleotide mutations. Contra (22)
was used to identify copy number variation detection and
BreakDancer (23) was used to
detect tumor-related structural variations. The results were
manually verified.
Statistical analysis
Statistical analysis was performed using SPSS
version 22.0. P<0.05 was considered to indicate a statistically
significant difference. A student's t-test was used to identify any
association between the presence of GNAS missense mutations
and both the tumor size under endoscopy and the depth of submucosal
infiltration.
Results
EBER status
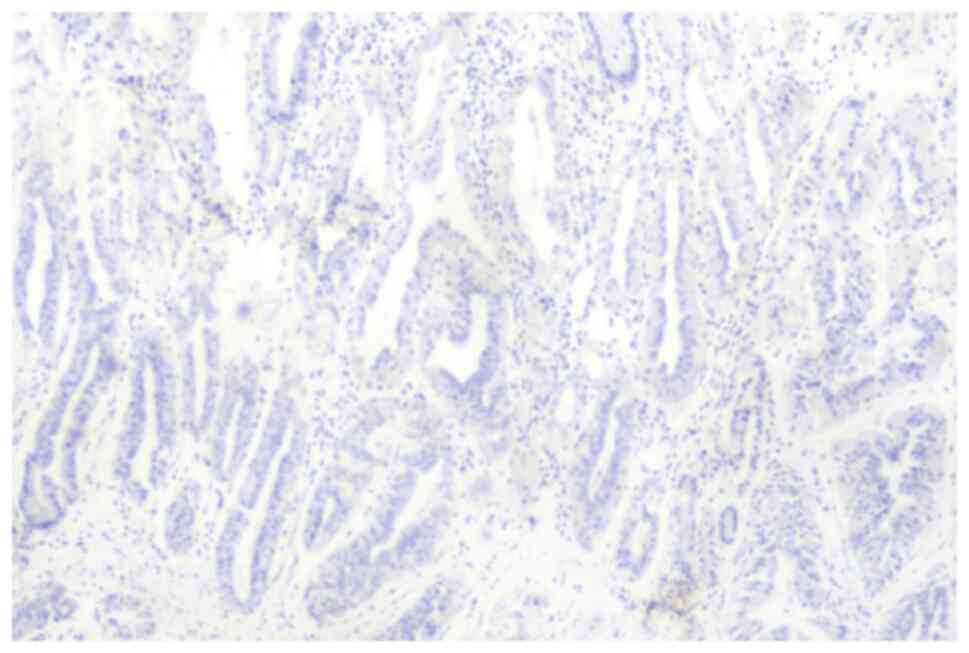
All nuclei stained blue with hematoxylin during EBER
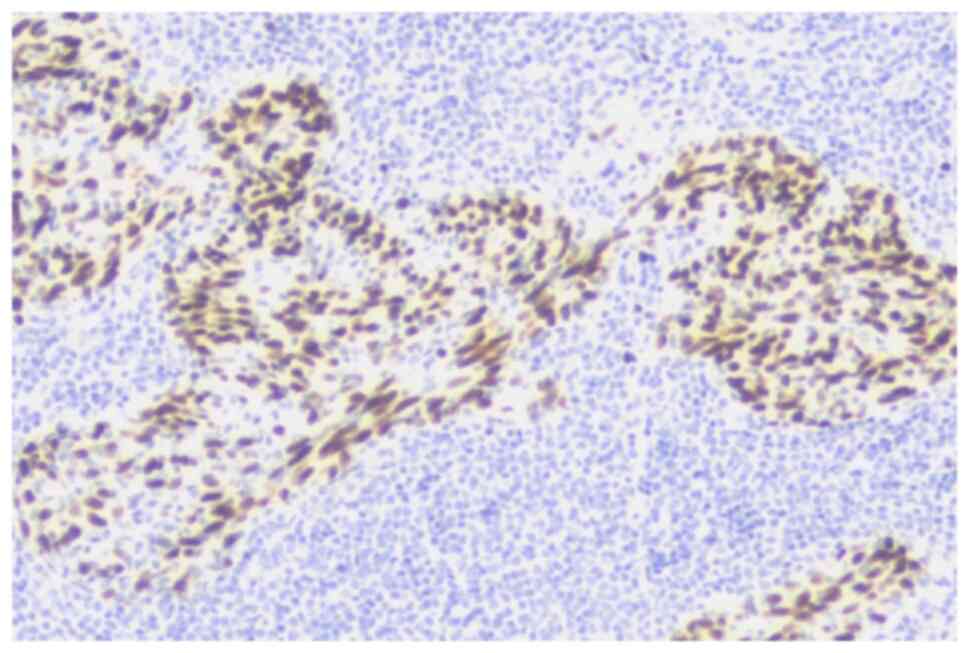
testing, indicating all 10 cases were negative for EBER (Fig. 1). The positive control,
non-keratinizing nasopharyngeal carcinoma, stained brown due to
DAB, as expected (Fig. 2).
NGS analysis
The results of NGS for the detection of mutations
are summarized in Table I. A total
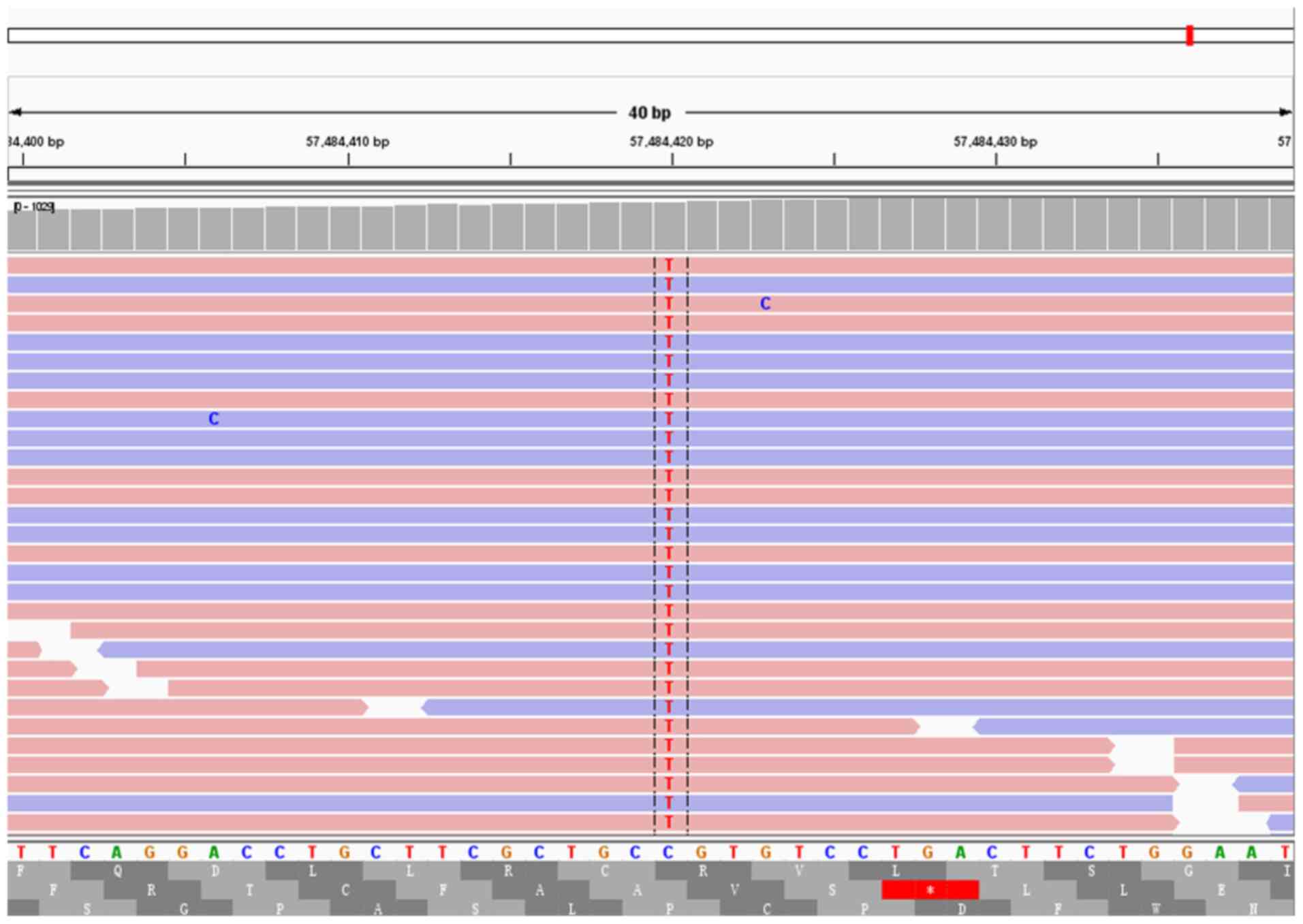
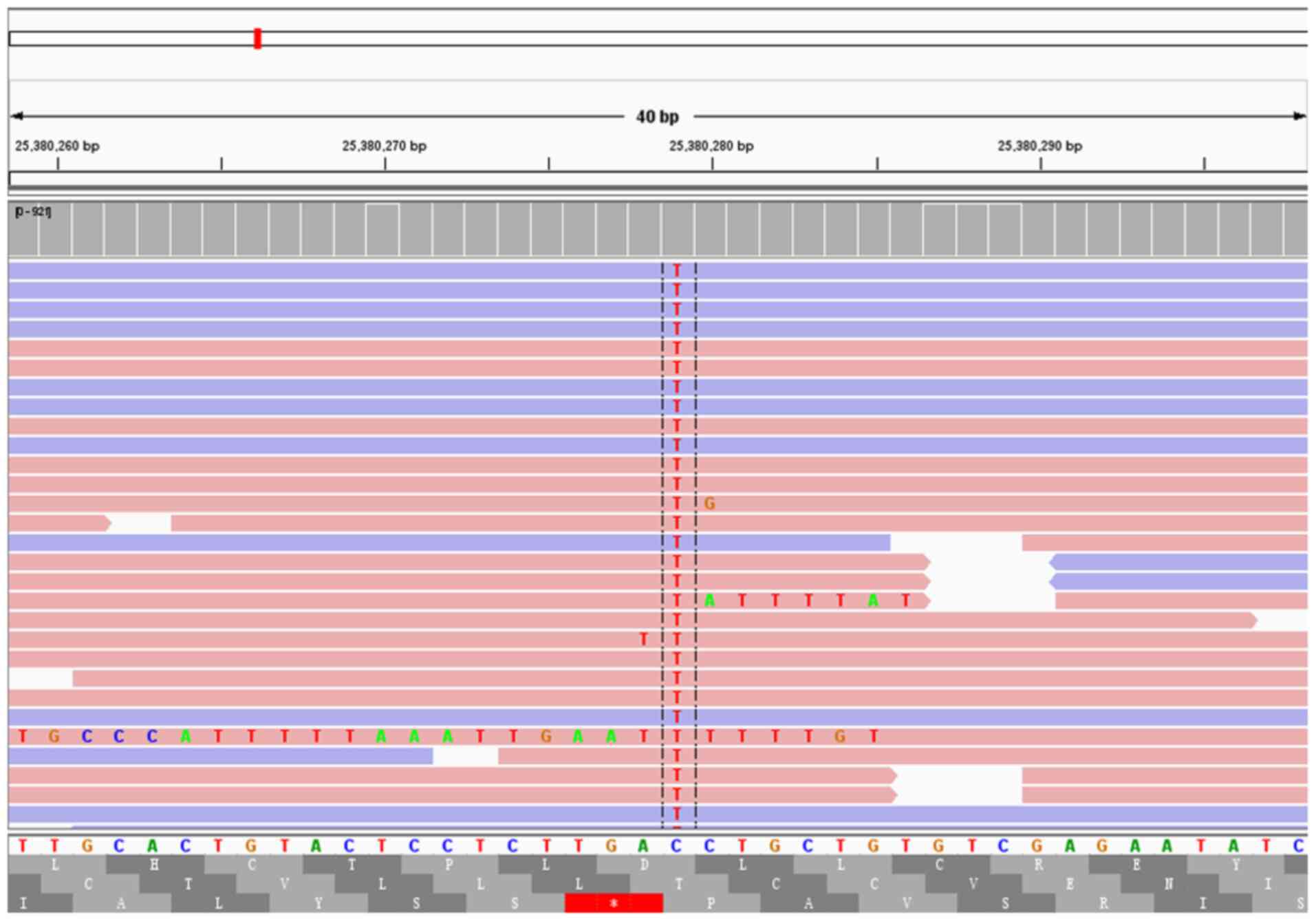
of seven cases were found to carry GNAS missense mutations,
and two cases were found to carry KRAS missense mutations.
Of these cases, two were found to carry both GNAS and
KRAS missense mutations (Figs.
3 and 4). Other instances of
missense mutations included two cases carrying an FGFR
mutation, and one case carrying a TP53 mutation. Another two
cases had no missense mutation. Synonymous mutations were observed
in APC, KRAS, NRAS, and FGFR (case #3),
as well as in MSH6 and BRCA1 (case #1). In case #1,
CDK4 amplification with a copy number of 1.4 was detected
but not considered relevant. No genetic fusion or frameshift
mutations were detected in any of the samples and no mutations were
detected in CTNNB1, BRAF, PIK3CA, HER2,
MLH1, MSH2, and PMS2.
 | Table IGene mutations detected in the 10
cases of gastric adenocarcinoma of the fundic-gland type by
Next-Generation Sequencing. |
Table I
Gene mutations detected in the 10
cases of gastric adenocarcinoma of the fundic-gland type by
Next-Generation Sequencing.
| Case number | Genes containing
missense mutations | Genes containing
coding-synonymous mutations | Genes containing
gain of function mutations | Genes containing
coding segment deletion mutations | Size of the tumor
under an endoscope, cm | Depth of submucosal
infiltration, µm |
|---|
| 1. | TP53 | MSH6 | CDK4 | AR | 1.2 | 300 |
| | FGFR2 | BRCA1 | | | | |
| | IDH1 | | | | | |
| 2. | GNAS | | | | 0.8 | 100 |
| | FGFR1 | | | | | |
| 3. | GNAS | NRAS | | | 0.6 | 300 |
| | ESR1 | KRAS | | | | |
| | | APC | | | | |
| | | CDK6 | | | | |
| | | PTCH1 | | | | |
| | | PTEN | | | | |
| | | FGFR2 | | | | |
| | | NF1 | | | | |
| 4. | | | | | 0.4 | 100 |
| 5. | | | | | 0.4 | 180 |
| 6. | GNAS | | | | 0.4 | 280 |
| 7. | GNAS | | | | 0.6 | 220 |
| | MAP2K1 | | | | | |
| 8. | GNAS | | | | 0.3 | 100 |
| | FBXW7 | | | | | |
| | CCND1 | | | | | |
| 9. | GNAS | | | | 0.4 | 300 |
| | KRAS | | | | | |
| 10. | GNAS | | | | 0.5 | 150 |
| | KRAS | | | | | |
Association between GNAS mutations and
tumor properties
No significant association was identified between
the presence of GNAS missense mutations and either the tumor
size under endoscopy or the depth of submucosal infiltration
(P>0.05).
Discussion
Despite numerous histopathological reports relating
to GAFG, to date, few studies have presented data on the molecular
features of the disease. More than 300 cases of GAFG have been
reported, of which ~one-third have been analyzed by genetic
sequencing (7,9,24-30).
Considerable research on GAFG has focused on Wnt/β-catenin-related
signaling pathways (9,24-27),
and mutations in GNAS and KRAS (7,9,28-30).
β-Catenin is a key protein involved in the Wnt
signaling pathway. Typically, cytoplasmic expression of β-catenin
is maintained at low levels through degradation. However, when
genes related to the Wnt signaling pathway, such as CTNNB1,
AXIN, APC, and PPP2R1A, are activated by
mutations or methylation, β-catenin accumulates leading to nuclear
translocation, which in turn activates downstream genes implicated
in the occurrence and development of tumors. This pathway has
received widespread attention in gastric cancer, and the
development of treatments targeting different molecular components
in this pathway has been explored (24,31,32).
While genetic mutations may not necessarily lead to β-catenin
overexpression (25), the mutation
rate in GAFG is high and variable. Previous studies have shown that
~85% of GAFG tumors are positive for nuclear β-catenin expression
and the mutation rate of Wnt signaling pathway-related genes is
~45% (9,26). The labeling index of nuclear
β-catenin immunoexpression, the number of cases in which it is
overexpressed, and the mutation rate of related genes are higher in
GAFG than in traditional gastric adenocarcinoma (26). However, nuclear β-catenin
immunolabeling and related gene mutations were not observed in the
present study nor in previously published reports on Chinese
patients (33,34). This may be due to the small number
of cases, regional factors, or other mechanisms requiring further
investigation.
Lee et al (25) found that in oxyntic gland adenoma
(OGA), the nuclear β-catenin immunolabeling index and the rate of
related gene mutations were lower than those in GAFG, with
approximate rates of 27% for nuclear expression and 36% for
mutations in APC, AXIN, or PPP2R1A. In
addition, these measures exhibited no significant correlation with
the clinicopathological variables in OGA. However, other studies
have noted that nuclear β-catenin staining preferentially appears
in deeper sections of tumors (invading surface) (7,9) and
that the process of submucosal infiltration may require β-catenin
nuclear transition to activate the Wnt signaling pathway (25,26).
Due to the lack of overexpression in the samples examined in the
current study, similar conclusions could not be drawn.
Murakami et al (27) analyzed the methylation of
Wnt/β-catenin signal-associated genes (including sfrp,
APC, and AXIN2) and found that high methylation
levels were more common in GAFG than in OGA, which may be related
to the occurrence and progression of GAFG. However, the findings of
this study were limited in this regard, as gene methylation testing
was not performed.
The missense mutation rates of GNAS and
KRAS from previous sequencing reports on GAFG (7,9,28-30)
were analyzed here, resulting in mutation rates of 20.2% (19/94)
and 6.2% (5/81), respectively. In particular, all studies reported
GNAS mutations at base locus 601 or 602 and amino acid locus
201, except for one case that had an additional GNAS
mutation at base locus 680 and amino acid locus 227(30). In line with previous results, in
the present study, the missense mutation rate of GNAS (70%) was
significantly higher than that of KRAS (20%). In traditional
gastric adenocarcinoma, KRAS mutations are more common and
correlate with the clinical stage, differentiation degree, lymph
node metastasis, distant metastasis, and depth of invasion, whereas
GNAS mutations are absent or rare. Mutations of GNAS
and KRAS could present simultaneously in both GAFG and
traditional gastric adenocarcinoma (9,35).
In the present study, there were two cases of GAFG with missense
mutations in both GNAS and KRAS.
Furthermore, Kushima et al (28) and Nomura et al (9) evaluated the relationship between the
clinicopathological characteristics of GAFG and GNAS
mutations. They found that tumors with GNAS mutations were
more likely to invade the submucosa and were larger than those
without mutations, although the differences were not statistically
significant. These results suggested that GNAS mutations
play a role in promoting tumor progression and invasion. Although
the missense mutation rate of GNAS in the present study was
70% (7/10 cases), there was no significant correlation between
GNAS mutation status and tumor invasion depth or size.
Therefore, the prognostic significance of GNAS mutation
status in GAFG requires further study and evaluation.
Nomura et al (9) reported two cases of KRAS
mutations in a group of patients with GAFG, where one had the
largest tumor size and lymphatic infiltration and the other had the
highest submucosal infiltration depth compared to the rest of the
cohort. In the present study, the two instances of KRAS
mutations also occurred in tumors with the highest submucosal
infiltration depth (~300 µm). However, OGA occasionally presents
with missense mutations in GNAS (2/11 cases) (7,30)
but never in KRAS (0/5 cases) (28,30).
These results support the novel idea that the KRAS mutations
may serve as a more valuable marker of tumor aggressiveness than
GNAS. Although it is currently impossible to draw
conclusions owing to the limited number of samples; this topic
warrants further study.
As part of The Cancer Genome Atlas project,
researchers have conducted molecular identification in 295 primary
gastric cancer samples (20) and
classified them into four molecular subtypes: EBV-positive,
microsatellite instability, genomically stable, and chromosomal
instability. This classification expands our understanding of the
pathogenesis of gastric cancer and provides a screening basis for
patient stratification, targeted treatment, and clinical trials in
patients with gastric cancer. However, the features of each subtype
do not accurately reflect the genetic characteristics of GAFG. EBER
is the most abundant EBV transcript in long-term latent infections
and promotes cell growth, apoptosis inhibition, and
immunoregulatory activities through a variety of signal pathways
(36). Although GAFG may also
develop in the fundus or body of the stomach (5-15),
the results from in situ hybridization analysis of the 10
samples studied here found all samples to be EBV-EBER negative. In
addition, there have been no previous reports of EBV in this type
of tumor. Microsatellite instability is more common in the gastric
antrum or pylorus, and abnormal DNA repair mechanisms result in a
high mutation rate in genes such as PIK3CA and HER2
(37). To date, only one study has
identified such mutations in GAFG cases, finding only two cases of
PIK3CA missense mutations among 34 cases (30). However, these mutations were not
accompanied by microsatellite instability as was the case in the
gastric antrum and pylorus tumors.
Notably, Yang (38)
reported the first case of ulcerative GAFG with microsatellite
instability. The lesion invaded the subserosa and exhibited lymph
node infiltration and distant metastasis. However, GNAS and
CTNNB1 mutations were not detected. The AXIN2
mutation rate and expression of nuclear β-catenin, as detected
through immunohistochemistry, were significantly higher in tumor
cells than in normal cells. Moreover, PD-1, PD-L1, and CD8
positivity have been observed in lesions with high microsatellite
instability (38). These findings
provide crucial information to aid in the discovery of novel
targeted therapies. No microsatellite instability was observed in
the 10 patients studied in the present report.
A meta-analysis showed that overexpression of
HER2 in patients with gastric cancer was associated with
cell proliferation, apoptosis, migration, and a poor prognosis
(39). In a retrospective
analysis, only three cases of parietal cell-type adenocarcinoma of
the fundus were found to be negative for HER2 by
immunohistochemistry or in situ hybridization (40,41).
In the present study, no HER2 mutations were detected in the
10 patients with chief cell-predominant GAFG. Whether a negative
HER2 mutation status is associated with early GAFG or a
favorable prognosis requires further clarification.
TP53, which encodes p53, plays key roles in
cell cycle regulation and apoptosis. Mutations in TP53 and
p53 overexpression are important biomarkers for predicting the
prognosis of patients with gastric cancer. However, this has rarely
been observed in published GAFG case reports. Of the 10 patients
studied here, a missense mutation in TP53 was found only in
one case (case #1). This case showed the deepest infiltration and
was negative for p53 protein expression. Compared to traditional
gastric adenocarcinoma, the incidence of p53 overexpression in GAFG
is extremely low (30). However,
the prognostic significance of p53 mutations remain unclear.
In addition, Ke et al (42) revealed that the Sonic Hedgehog
(Shh) signaling pathway may be independent of the Wnt/β-catenin
signaling pathway, which is also involved in the progression and
prognosis of GAFG. Ueyama et al (30) reported a case of GAFG with a
CDNK2A missense mutation. The present study showed, for the
first time, that FGFR and other mutations occur in GAFG. The
results of these individual cases highlight the genetic variety of
GAFG.
In summary, samples obtained vis endoscopy from 10
Chinese patients with GAFG, a unique pathological tumor type, were
retrospectively analyzed. GAFG differs from traditional gastric
cancers in that it exhibits diversity at the molecular level, much
of which requires further investigation. In the present study,
non-synonymous mutations in the Wnt/β-catenin pathway were not
detected. The missense mutation rate of GNAS was found to be
much higher than that of KRAS, whereas mutations in
TP53 and microsatellite instability were rare. To date, no
study has demonstrated a positive EBV or HER2 status for
GAFG. Although this is the first study of Chinese patients on the
molecular factors underlying GAFG, it was limited by the small
number of cases. In addition to screening the cases for mutations
in a large number of genes, the relationship between the
pathogenesis, genetic alterations, and clinicopathological
characteristics of GAFG were assessed. Determining the associations
between specific genetic alterations and patient prognoses is the
aim of future research as no statistically relevant factors were
identified in the present study.
Supplementary Material
Detailed list of all 73 genes assessed
by Next-Generation Sequencing.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Peking University
International Hospital Research Grant (grant no. YN2020QN14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The raw sequence data reported in this paper have been
deposited in the Genome Sequence Archive (Genomics, Proteomics
& Bioinformatics 2021) in National Genomics Data Center
(Nucleic Acids Res 2022), China National Center for
Bioinformation/Beijing Institute of Genomics, Chinese Academy of
Sciences (GSA-Human: HRA005206) and are publicly accessible at
https://ngdc.cncb.ac.cn/gsa-human.
Authors' contributions
LL was responsible for the conception and design of
the study. XZ, XF, and XZ contributed to the acquisition and
interpretation of the data. LL drafted the manuscript. LL and XZ
confirm the authenticity of all the raw data. XZ revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by Biomedical Ethics
Committee of Peking University International Hospital (approval on.
2020-KY-0011-02); the need for informed consent from patients was
waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Müller-Höcker J and Rellecke P: Chief cell
proliferation of the gastric mucosa mimicking early gastric cancer:
An unusual variant of fundic gland polyp. Virchows Arch.
442:496–500. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Matsukawa A, Kurano R, Takemoto T,
Kagayama M and Ito T: Chief cell hyperplasia with structural and
nuclear atypia: A variant of fundic gland polyp. Pathol Res Pract.
200:817–821. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tsukamoto T, Yokoi T, Maruta S, Kitamura
M, Yamamoto T, Ban H and Tatematsu M: Gastric adenocarcinoma with
chief cell differentiation. Pathol Int. 57:517–522. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
WHO Classification of Tumours Editorial
Board. WHO classification of tumours. Digestive system tumours 5th
edition. Lyon, IARC Press, 83, 2019.
|
|
5
|
Tohda G, Osawa T, Asada Y, Dochin M and
Terahata S: Gastric adenocarcinoma of fundic gland type: Endoscopic
and clinicopathological features. World J Gastrointest Endosc.
89:244–251. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chan K, Brown IS, Kyle T, Lauwers GY and
Kumarasinghe MP: Chief cell-predominant gastric polyps: A series of
12 cases with literature review. Histopathology. 68:825–833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ushiku T, Kunita A, Kuroda R,
Shinozaki-Ushiku A, Yamazawa S, Tsuji Y, Fujishiro M and Fukayama
M: Oxyntic gland neoplasm of the stomach: Expanding the spectrum
and proposal of terminology. Mod Pathol. 33:206–216.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen O, Shao ZY, Qiu X and Zhang GP:
Multiple gastric adenocarcinoma of fundic gland type: A case
report. World J Clin Cases. 7:2871–2878. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nomura R, Saito T, Mitomi H, Hidaka Y, Lee
SY, Watanabe S and Yao T: GNAS mutation as an alternative mechanism
of activation of the Wnt/β-catenin signaling pathway in gastric
adenocarcinoma of the fundic gland type. Hum Pathol. 45:2488–2496.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chiba T, Kato K, Masuda T, Ohara S, Iwama
N, Shimada T and Shibuya D: Clinicopathological features of gastric
adenocarcinoma of the fundic gland (chief cell predominant type) by
retrospective and prospective analyses of endoscopic findings. Dig
Endosc. 28:722–730. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fujii M, Uedo N, Ishihara R, Aoi K,
Matsuura N, Ito T, Yamashina T, Hanaoka N, Takeuchi Y, Higashino K,
et al: Endoscopic features of early stage gastric adenocarcinoma of
fundic gland type (chief cell predominant type): A case report.
Case Rep Clin Pathol. 2:17–22. 2015.
|
|
12
|
Fukatsu H, Miyoshi H, Ishiki K, Tamura M
and Yao T: Gastric adenocarcinoma of fundic gland type (chief cell
predominant type) treated with endoscopic aspiration mucosectomy.
Dig Endosc. 23:244–246. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Imagawa A and Sano N: Gastric
adenocarcinoma of the fundic gland (chief cell predominant type)
with brownish pigmentation. Gastrointest Endosc. 87:1358–1359.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ueyama H, Matsumoto K, Nagahara A, Hayashi
T, Yao T and Watanabe S: Gastric adenocarcinoma of the fundic gland
type (chief cell predominant type). Endoscopy. 46:153–157.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
WHO Classification of Tumours Editorial
Board. WHO classification of tumours. Digestive system tumours 5th
edition. Lyon, IARC Press, 92, 2019.
|
|
16
|
Lee TI, Jang JY, Kim S, Kim JW, Chang YW
and Kim YW: Oxyntic gland adenoma endoscopically mimicking a
gastric neuroendocrine tumor: A case report. World J Gastroenterol.
21:5099–5104. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Miyazawa M, Matsuda M, Yano M, Hara Y,
Arihara F, Horita Y, Matsuda K, Sakai A and Noda Y: Gastric
adenocarcinoma of fundic gland type: Five cases treated with
endoscopic resection. World J Gastroenterol. 21:8208–8214.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ueo T, Yonemasu H and Ishida T: Gastric
adenocarcinoma of fundic gland type with unusual behavior. Dig
Endosc. 26:293–294. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Okumura Y, Takamatsu M, Ohashi M, Yamamoto
Y, Yamamoto N, Kawachi H, Ida S, Kumagai K, Nunobe S, Hiki N and
Sano T: Gastric adenocarcinoma of fundic gland type with aggressive
transformation and lymph node metastasis: A case report. J Gastric
Cancer. 18:409–416. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cancer genome atlas research network.
Comprehensive molecular characterization of gastric adenocarcinoma.
Natrue. 513:202–209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kong F, Yao Y, Deng R, Li X and Jia Y:
Hopes and failures in front-line advanced HER2-positive gastric
cancer therapy. Anticancer Drugs. 32:675–680. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li J, Lupat R, Amarasinghe KC, Thompson
ER, Doyle MA, Ryland GL, Tothill RW, Halgamuge SK, Campbell IG and
Gorringe KL: CONTRA: Copy number analysis for targeted
resequencing. Bioinformatics. 28:1307–1313. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen K, Wallis JW, McLellan MD, Larson DE,
Kalicki JM, Pohl CS, McGrath SD, Wendl MC, Zhang Q, Locke DP, et
al: BreakDancer: An algorithm for high-resolution mapping of
genomic structural variation. Nat Methods. 6:677–681.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu Z, Jiang X, Qin L, Deng H, Wang J, Ren
W, Li H, Zhao L, Liu H, Yan H, et al: A novel UBE2T inhibitor
suppresses Wnt/β-catenin signaling hyperactivation and gastric
cancer progression by blocking RACK1 ubiquitination. Oncogene.
40:1027–1042. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee SY, Saito T, Mitomi H, Hidaka Y,
Murakami T, Nomura R, Watanabe S and Yao T: Mutation spectrum in
the Wnt/β-catenin signaling pathway in gastric fundic
gland-associatedneoplasms/polyps. Virchows Arch. 467:27–38.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hidaka Y, Mitomi H, Saito T, Takahashi M,
Lee SY, Matsumoto K, Yao T and Watanabe S: Alteration in the
Wnt/beta-catenin signaling pathway in gastric neoplasias of fundic
gland (chief cell predominant) type. Hum Pathol. 44:2438–2448.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Murakami T, Mitomi H, Yao T, Saito T,
Shibuya T and Watanabe S: Epigenetic regulation of Wnt/β-catenin
signal-associated genes in gastric neoplasia of the fundic gland
(chief cell-predominant) type. Pathol Int. 67:147–155.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kushima R, Sekine S, Matsubara A,
Taniguchi H, Ikegami M and Tsuda H: Gastric adenocarcinoma of the
fundic gland type shares common genetic and phenotypic features
with pyloric gland adenoma. Pathol Int. 63:318–325. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tajima Y, Murakami T, Saito T, Hiromoto T,
Akazawa Y, Sasahara N, Mitomi H, Yao T and Watanabe S: Distinct
involvement of the sonic hedgehog signaling pathway in gastric
adenocarcinoma of fundic gland type and conventional gastric
adenocarcinoma. Digestion. 96:81–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ueyama H, Yao T, Akazawa Y, Hayashi T,
Kurahara K, Oshiro Y, Yamada M, Oda I, Fujioka S, Kusumoto C, et
al: Gastric epithelial neoplasm of fundic-gland mucosa lineage:
Proposal for a new classification in association with gastric
adenocarcinoma of fundic-gland type. J Gastroenterol. 56:814–828.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bhaskar Rao D, Panneerpandian P,
Balakrishnan K and Ganesan K: YY1 regulated transcription-based
stratification of gastric tumors and identification of potential
therapeutic candidates. J Cell Commun Signal. 15:251–267.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Peng X, Shi J, Zhao Z, Tong R, Zhang X and
Zhong L: Emetine, a small molecule natural product, displays potent
anti-gastric cancer activity via regulation of multiple signaling
pathways. Cancer Chemother Pharmacol. 91:303–315. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun WW, Zhang L, Gu MM, Zhang YQ, Qiu CM
and Da Q: Gastric adenocarcinoma of the fundic gland type:
Clinicopathological analysis of six cases. Zhonghua Bing Li Xue Za
Zhi. 49:343–347. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
34
|
Jing F, Xudan Y, Juan L, Lei W, Xiao H,
Xiang L, Hong Z and Gang X: Two cases of adenocarcinoma of the
gastric fundus and literature review. J Clin Exp Pathol.
36:455–457. 2020.(In Chinese).
|
|
35
|
Matsubara A, Sekine S, Kushima R, Ogawa R,
Taniguchi H, Tsuda H and Kanai Y: Frequent GNAS and KRAS mutations
in pyloric gland adenoma of the stomach and duodenum. J Pathol.
229:579–587. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou H, Tan S, Li H and Lin X: Expression
and significance of EBV, ARID1A and PIK3CA in gastric carcinoma.
Mol Med Rep. 19:2125–2136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ratti M, Lampis A, Hahne JC, Passalacqua R
and Valeri N: Microsatellite instability in gastric cancer:
Molecular bases, clinical perspectives, and new treatment
approaches. Cell Mol Life Sci. 75:4151–4162. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang G: Microsatellite
instability/mismatch repair deficiency and activation of the
Wnt/β-catenin signaling pathway in gastric adenocarcinoma of the
fundic gland: A case report. Medicine (Baltimore).
101(e30311)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lei YY, Huang JY, Zhao QR, Jiang N, Xu HM,
Wang ZN, Li HQ, Zhang SB and Sun Z: The clinicopathological
parameters and prognostic significance of HER2 expression in
gastric cancer patients: A meta-analysis of literature. World J
Surg Oncol. 15(68)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Batistatou A, Doukas M, Baltogiannis G,
Panelos J, Kamina S, Charalabopoulos K and Agnantis NJ: Early
gastric carcinoma with oncocytic features and extensive metastases.
Pathol Res Pract. 203:539–541. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mavroeidis VK, Gkegkes ID, Saffioti F,
Kandilaris K, Alexiou K, Horti M, Economou N and Demonakou M:
Parietal cell/oncocytic gastric carcinoma: Systematic review and
first-time assessment of HER2 status in two new cases. Ann R Coll
Surg Engl. 102:300–307. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ke B, Wang XN, Liu N, Li B, Wang XJ, Zhang
RP and Liang H: Sonic Hedgehog/Gli1 signaling pathway regulates
cell migration and invasion via induction of
epithelial-to-mesenchymal transition in gastric cancer. J Cancer.
11:3932–3943. 2020.PubMed/NCBI View Article : Google Scholar
|