Introduction
Most hematologic diseases tend to be
immunosuppressed, either by the disease itself or by treatment, but
comprehensive reports have demonstrated the positive effect of
vaccination in hematologic diseases. While the decision to
vaccinate is often left to the discretion of the attending
physician, there is a lack of scientific evidence to support the
decision making. One troubling study has revealed that the severity
of blood diseases caused by recent coronavirus disease 2019
(COVID-19) infections is 58.8% and that the mortality rate is 27%
(1). Furthermore, in a study
analyzing 3,377 cases of hematologic diseases, the mortality rate
was 34% in adults and 4% in children, which is a key aspect to bear
in mind when considering the prevention of COVID-19 infection and
avoidance of severe disease by vaccination (2). It should also be noted that these
results are significantly higher than the 11.8% mortality rate from
COVID-19 infection in adults reported in a study on 38,517 cases in
USA (3) and cannot be
overlooked.
On the other hand, the acquisition rate of
antibodies against COVID-19 in healthy individuals or in patients
with solid tumors is estimated to be >90% after two doses of the
COVID-19 vaccine, whereas the antibody acquisition rate for
patients with hematological diseases varies from 66-88% (4). Furthermore, the antibody acquisition
rate is reported to decrease to 20-50% when rituximab is used,
based on the results of 569 patients immunized with a recombinant
zoster vaccine (4). It was
hypothesized that B-cell lymphoma treated with anti-CD20 antibody
would have a lower rate of antibody acquisition after vaccination
and that, if so, it would be appropriate as a model of
immunodeficiency.
In the present study, the treatment and immune
status related to antibody acquisition after the vaccination of
patients with B-cell lymphoma treated with anti-CD20 antibody was
investigated.
Materials and methods
Patients
The acquisition of antibodies after COVID-19
vaccination in patients with B-cell lymphoma who were attending the
Department of Hematology, Hakodate Municipal Hospital (Hakodate,
Japan) and were being or had been treated with CD20 antibodies was
prospectively evaluated. Enrollment of patients began on June 2021.
The target number of patients for enrollment was set to 40, and
patients who did not meet the exclusion criteria were enrolled
sequentially until the target number of patients was reached.
The primary endpoint is the antibody-positive rate
after three doses of COVID-19 vaccine, and the secondary endpoints
are: i) The association with liquid and cellular immunity such as
CD4, CD 19, IgG, IgA and IgM in COVID-19 vaccine-positive and
-negative cases; ii) the association between COVID-19
vaccine-positive cases and the stage and treatment of B-cell
lymphoma; iii) a comparison of various factors between antibody
titers at the lower limit of detection and those at which the
vaccine is expected to be effective; and (iv) adverse events due to
vaccination [Common Terminology Criteria for Adverse Events (CTCAE)
version 4.0].
The present study included patients with
pathologically diagnosed B-cell lymphoma treated with
cyclophosphamide, doxorubicin, Oncovin (vincristine) and prednisone
(CHOP), a CHOP-like regimen, or bendamustine, plus rituximab or
obinutuzumab. Patients on maintenance therapy with rituximab or
obinutuzumab once every two to three months or after the completion
of therapy were also eligible for enrollment. Excluded from
enrollment were patients with a history of anaphylaxis of any kind,
thrombosis of any kind, pregnant women and patients who did not
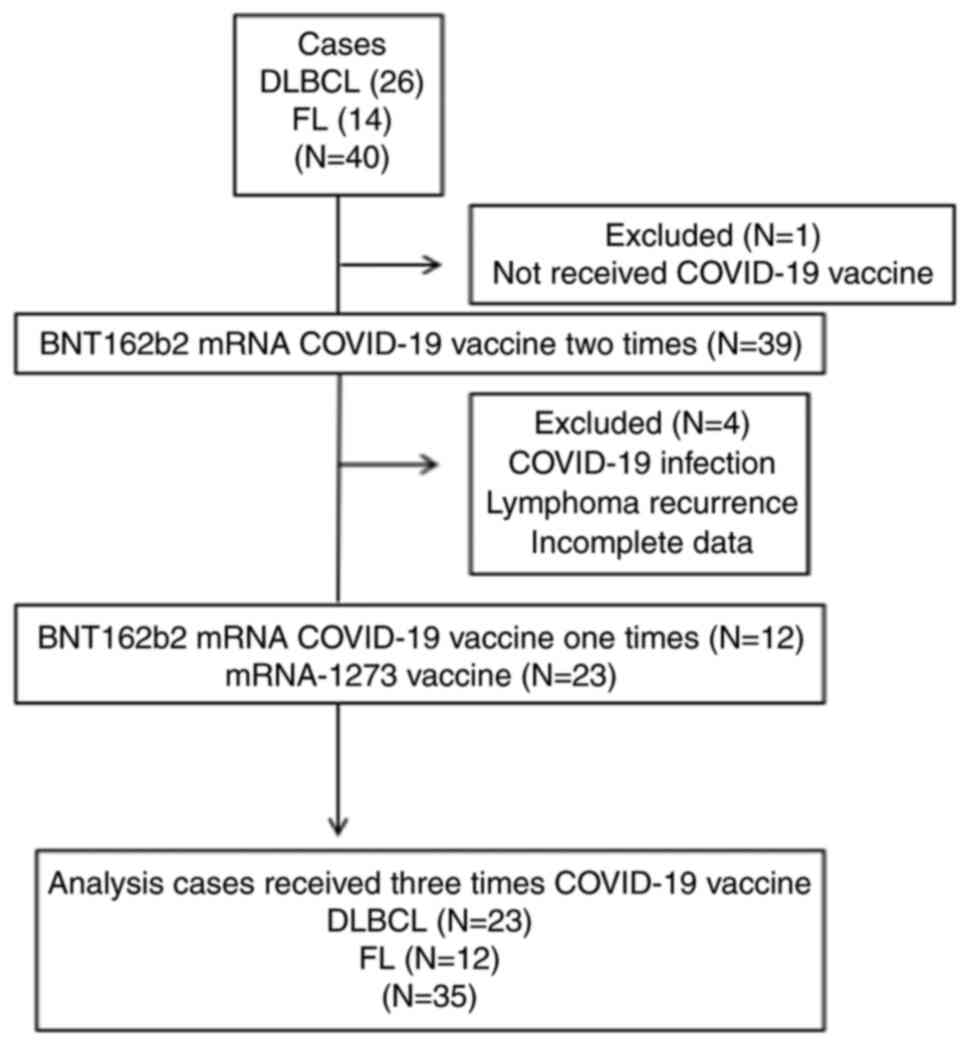
wish to receive the COVID-19 vaccine. A total of 26 patients with
diffuse large B-cell lymphoma (DLBCL) and 14 patients with
follicular lymphoma (FL) were enrolled. Excluded from the analysis
was one case who declined COVID-19 vaccination after declaring
participation in the trial, one case of recurrence requiring
treatment during the course of the trial and one case of COVID-19
infection that did not proceed to the third and subsequent
vaccination. Two additional cases were excluded from the analysis
due to a lack of laboratory data on immunity (Fig. 1). The protocol was approved
(approval no. 2021-61) by the local Ethics Committee of Hakodate
Municipal Hospital Institutional Review Board (Hakodate, Japan) on
21 June 2021, and patient enrollment began the following day.
Written informed consent based on The Declaration of Helsinki was
obtained from all patients.
Vaccination
Vaccinations were administered according to the
regulations of the Japanese vaccine program. Patients were
vaccinated twice with the BNT162b2 messenger RNA (mRNA) COVID-19
vaccine (Pfizer, Inc. and BioNTech SE) at 3-week intervals and then
again six months later with the same vaccine or the mRNA-1273
(Moderna, Inc.) initial vaccination.
Antibody testing
Antibody testing was performed ~30 days after the
third vaccination using the antibody titers to the anti-spike
(anti-S) immunoglobulin assay (Roche Diagnostics). In accordance
with the manufacturer's recommendations, the cut-off index value
for seropositivity was 0.8 U/ml and antibody titers higher than 264
U/ml were considered above the limit for a valid antibody (5-7).
Adverse event assessment
Adverse events due to vaccination were
self-evaluated by the patients by filling out a form describing the
issues and their severity in accordance with the CTCAE v.4.0. Their
responses were then tabulated.
Statistical analysis
Subgroups were compared using the Mann-Whitney U
test for continuous variables that do not follow normal
distribution and Fisher's exact tests for categorical variables.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed with EZR
(version 1.61; Saitama Medical Center, Jichi Medical University),
which is a graphical user interface for R (version 4.20; The R
Foundation). After collection, these data were analyzed by logistic
regression analysis. Conditional logistic regression analysis for
frequency-matched datasets was utilized to estimate the odds ratio
and its respective 95% confidence interval.
Results
Patients
After excluding five patients (as aforementioned),
analysis was performed on 35 cases, as listed in Table I. The median age was 69 years
(range: 46-83 years) and the number of men and women was almost
equal. In total, 23 cases were DLBCL, 12 were FL, 23 were stage
III-IV and 21 were undergoing treatment. No progression of
lymphomas was observed during treatment, and at the end of the
present study, 33 cases had obtained complete response and two
cases featured recurrence (one DLBCL and one FL). There were 21
cases under treatment at the time of vaccination and 14 cases after
completion of treatment. There were 20 cases with a CD4 count of
<400/µl at the time of the first vaccination, which was slightly
more common, but 18 cases (~50%) had a CD19 count of <100/µl.
Regarding immunoglobulin, IgM was detected to be below normal
values in numerous cases, but IgG and IgA were above normal values
in the majority of the cases.
 | Table ICharacteristics of patients at the
time of vaccination. |
Table I
Characteristics of patients at the
time of vaccination.
| Clinicopathological
characteristics | Value (%) |
|---|
| Total, n | 35 |
| Age, years [median,
(range)] | 69.00 (46-83) |
| Sex | |
|
Female | 17 (48.6) |
|
Male | 18 (51.4) |
| Disease | |
|
DLBCL | 23 (65.7) |
|
FL | 12 (34.3) |
| Clinical stage | |
|
I-II | 12 (34.3) |
|
III-IV | 23 (65.7) |
| sIL2R [median
(U/ml)] | 466.00
(229.00-1000.00) |
| Anti-CD20
antibody | |
|
Obinutuzumab | 4 (11.4) |
|
Rituximab | 31 (88.6) |
| Maintenance
therapy | |
|
Without | 23 (65.7) |
|
With | 12 (34.3) |
| Bendamustine | |
|
Without | 21 (60.0) |
|
With | 14 (40.0) |
| Under treatment | |
|
After | 14 (40.0) |
|
Ongoing | 21 (60.0) |
| Duration after
treatment (months) | |
|
<6 | 26 (74.3) |
|
>6 | 9 (25.7) |
| Third vaccine | |
|
mRNA1273 | 23 (65.7) |
|
BNT162b2 | 12 (34.3) |
| CD4 count (/µl) | |
|
<400 | 20 (57.1) |
|
>400 | 15 (42.9) |
| CD19 count (/µl) | |
|
<100 | 18 (51.4) |
|
>10 | 17 (48.6) |
| IgG (mg/dl) | |
|
<600 | 5 (14.3) |
|
>600 | 30 (85.7) |
| IgA (mg/dl) | |
|
<110 | 15 (42.9) |
|
>110 | 20 (57.1) |
| IgM (mg/dl) | |
|
<40 | 23 (65.7) |
|
>40 | 12 (34.3) |
| Adverse effect due to
vaccine | |
|
Grade
0-1 | 24 (68.6) |
|
>Grade
2 | 11 (31.4) |
| Outcome | |
|
CR | 33 (94.3) |
|
Relapse | 2 (5.7) |
Analysis of antibody titers and
related actors
Antibodies were detected in 19 cases (54.3%) when
the lower limit of detection was 0.8 U/ml of the antibodies
acquired with the COVID-19 vaccine. Factors associated with
antibody positivity were younger age (P=0.02), rituximab use
(P=0.035), no maintenance therapy by anti-CD20 antibody (P=0.03),
not on lymphoma therapy (P<0.001), after treatment duration of
more than six months (P=0.022), CD4 ≥400/µl (P=0.016), CD19 ≥100/µl
(P<0.001), both CD4 ≥400/µl and CD19 ≥100/µl (P<0.001), IgA
≥110 mg/dl (P=0.044) and IgM ≥40 mg/dl (P=0.03) (Table II).
 | Table IICharacteristics of patients (based on
antibody titer of 0.8 U/ml). |
Table II
Characteristics of patients (based on
antibody titer of 0.8 U/ml).
| Clinicopathological
characteristics | Antibody titer
<0.8 U/ml | Antibody titer
>0.8 U/ml | P-value |
|---|
| Total, n | 16 | 19 | |
| Age, years [median
(range)] | 71.00 (51-83) | 63.00 (46-79) | 0.02 |
| Sex | | | 0.738 |
|
Female, n
(%) | 7 (20.0) | 10 (28.6) | |
|
Male, n
(%) | 9 (25.7) | 9 (25.7) | |
| Disease | | | 0.09 |
|
DLBCL, n
(%) | 8 (22.9) | 15 (42.9) | |
|
FL, n
(%) | 8 (22.9) | 4 (11.4) | |
| Clinical stage | | | 0.152 |
|
I-II, n
(%) | 3 (8.6) | 9 (25.7) | |
|
III-IV, n
(%) | 13 (37.1) | 10 (28.6) | |
| sIL2R (median:
U/ml) | 499.50
(229-1000) | 466.00
(261-969) | 0.83 |
| Anti-CD20
antibody | | | 0.035 |
|
Obinutuzumab,
n (%) | 4 (11.4) | 0 (0.0) | |
|
Rituximab, n
(%) | 12 (34.3) | 19 (54.3) | |
| Maintenance therapy
by anti-CD20 | | | 0.03 |
|
Without, n
(%) | 7 (20.0) | 16 (45.7) | |
|
With, n
(%) | 9 (25.7) | 3 (8.6) | |
| Bendamustine | | | 0.094 |
|
Without, n
(%) | 7 (20.0) | 14 (40.0) | |
|
With, n
(%) | 9 (25.7) | 5 (14.3) | |
| Treatment of
lymphoma | | | <0.001 |
|
After, n
(%) | 1 (2.9) | 13 (37.1) | |
|
Ongoing, n
(%) | 15 (42.9) | 6 (17.1) | |
| Duration after
treatment (mo) | | | 0.022 |
|
<6, n
(%) | 15 (42.9) | 11 (31.4) | |
|
>6, n
(%) | 1 (2.9) | 8 (22.9) | |
| Third vaccine | | | 0.311 |
|
mRNA1273, n
(%) | 9 (25.7) | 14 (40.0) | |
|
BNT162b2, n
(%) | 7 (20.0) | 5 (14.3) | |
| CD4 count
(U/µl) | | | 0.016 |
|
<400, n
(%) | 13 (37.1) | 7 (20.0) | |
|
>400, n
(%) | 3 (8.6) | 12 (34.3) | |
| CD19 count
(U/µl) | | | <0.001 |
|
<100, n
(%) | 14 (40.0) | 4 (11.4) | |
|
>100, n
(%) | 2 (5.7) | 15 (42.9) | |
| CD4/CD19 (/µl) | | | <0.001 |
|
<400 or
100, n (%) | 16 (45.7) | 8 (22.9) | |
|
>400 and
100, n (%) | 0 (0.0) | 11 (31.4) | |
| IgG (mg/dl) | | | 0.642 |
|
<600, n
(%) | 3 (8.6) | 2 (5.7) | |
|
>600, n
(%) | 13 (37.1) | 17 (48.6) | |
| IgA (mg/dl) | | | 0.044 |
|
<110, n
(%) | 10 (28.6) | 5 (14.3) | |
|
>110, n
(%) | 6 (17.1) | 14 (40.0) | |
| IgM (mg/dl) | | | 0.03 |
|
<40, n
(%) | 14 (40.0) | 9 (25.7) | |
|
>40, n
(%) | 2 (5.7) | 10 (28.6) | |
| Adverse effect due
to vaccine | | | 1 |
|
Grade 0-1, n
(%) | 11 (31.4) | 13 (37.1) | |
|
>Grade 2,
n (%) | 5 (14.3) | 6 (17.1) | |
Interestingly, at the antibody titer at which the
COVID-19 vaccine was expected to be effective, namely, 264 U/ml,
effective antibody titers were only obtained in nine cases (25.7%).
The following criteria were utilized for antibody acquisition:
DLBCL (P=0.015), clinical stage I-II (P=0.003), no maintenance
therapy by anti-CD20 antibody (P=0.015), no bendamustine (P=0.005),
not on lymphoma therapy (P<0.001), after treatment duration of
more than six months (P<0.001), CD4 ≥400/µl (P=0.002), CD19
≥100/µl (P<0.001), and both CD4 ≥400/µl and CD19 ≥100/µl
(P<0.001). These were identified as the main factors for
obtaining effective antibody titers. However, age, type of
anti-CD20 antibody, IgG, IgA and IgM were not significantly
different, while no bendamustine treatment, DLBCL, and clinical
stage I-II were new factors that revealed significant differences
(Table III).
 | Table IIICharacteristics of patients (based on
antibody titer of 264 U/ml). |
Table III
Characteristics of patients (based on
antibody titer of 264 U/ml).
| Clinicopathological
characteristics | Antibody titer
<264 U/ml | Antibody titer
>264 U/ml | P-value |
|---|
| N | 26 | 9 | |
| Age, years [median
(range)] | 71.00 (46-83) | 62.00 (51-71) | 0.112 |
| Sex | | | 0.264 |
|
Female, n
(%) | 11 (31.4) | 6 (17.1) | |
|
Male, n
(%) | 15 (42.8) | 3 (8.5) | |
| Disease | | | 0.015 |
|
DLBCL, n
(%) | 14 (40.0) | 9 (25.7) | |
|
FL, n
(%) | 12 (34.2) | 0 (0.0) | |
| Clinical stage | | | 0.003 |
|
I-II, n
(%) | 5 (14.2) | 7 (20.0) | |
|
III-IV, n
(%) | 21 (60.0) | 2 (5.7) | |
| sIL2R (median:
U/ml) | 479.50
(229-1000) | 375.00
(261-816) | 0.086 |
| Anti-CD20
antibody | | | 0.553 |
|
Obinutuzumab,
n (n%) | 4 (11.4) | 0 (0.0) | |
|
Rituximab, n
(n%) | 22 (62.8) | 9 (25.7) | |
| Maintenance therapy
by anti-CD20 | | | 0.015 |
|
Without, n
(%) | 14 (0.40) | 9 (25.7) | |
|
With, n
(%) | 12 (34.2) | 0 (0.0) | |
| Bendamustine | | | 0.005 |
|
Without, n
(%) | 12 (34.2) | 9 (25.7) | |
|
With, n
(%) | 14 (40.0) | 0 (0.0) | |
| Treatment of
lymphoma | | | <0.001 |
|
After, n
(%) | 6 (17.1) | 8 (22.8) | |
|
Ongoing, n
(%) | 20 (57.1) | 1 (2.8) | |
| Duration after
treatment (mo) | | | <0.001 |
|
<6, n
(%) | 25 (60.0) | 1 (2.8) | |
|
>6, n
(%) | 1 (2.8) | 8 (22.8) | |
| Third vaccine | | | 0.121 |
|
mRNA1273, n
(%) | 15 (42.8) | 8 (22.8) | |
|
BNT162b2, n
(%) | 11 (31.4) | 1 (2.8) | |
| CD4 count
(U/µl) | | | 0.002 |
|
<400, n
(%) | 19 (54.2) | 1 (2.8) | |
|
>400, n
(%) | 7 (20.0) | 8 (22.8) | |
| CD19 count
(U/µl) | | | <0.001 |
|
<100, n
(%) | 18 (51.4) | 0 (0.0) | |
|
>100, n
(%) | 8 (22.8) | 9 (25.7) | |
| CD4/CD19
(U/µl) | | | <0.001 |
|
<400 or
100, n (%) | 23 (65.7) | 1 (2.8) | |
|
>400 and
100, n (%) | 3 (8.6) | 8 (22.8) | |
| IgG (mg/dl) | | | 0.297 |
|
<600, n
(%) | 5 (14.3) | 0 (0.0) | |
|
>600, n
(%) | 21 (60.0) | 9 (25.7) | |
| IgA (mg/dl) | | | 0.244 |
|
<110, n
(%) | 13 (37.1) | 2 (5.7) | |
|
>110, n
(%) | 13 (37.1) | 7 (20.0) | |
| IgM (mg/dl) | | | 0.22 |
|
<40, n
(%) | 19 (54.2) | 4 (11.4) | |
|
>40, n
(%) | 7 (20.0) | 5 (14.3) | |
| Adverse effect due
to vaccine | | | 1 |
|
Grade 0-1, n
(%) | 18 (51.4) | 6 (17.1) | |
|
>Grade 2,
n (%) | 8 (22.8) | 3 (8.5) | |
Multivariate analysis of the antibody
acquisition factors
Multivariate analysis of these factors was performed
using logistic regression analysis. After removing confounding
factors, three analysis factors for antibody acquisition by vaccine
were extracted: i) CD19 ≥100/µl, ii) CD4 ≥400/µl and iii) no
maintenance therapy. When antibody titers were set to 0.8 U/ml, the
significant factor was CD19 ≥100/µl (P=0.008). Although it was not
significant at 264 U/ml or higher, there was a tendency for it to
be associated with CD4 ≥400/µl (P=0.07) (Table IV).
 | Table IVLogistic regression analysis based on
antibody titers of 0.8 U/ml and based on antibody titer of 264
U/ml. |
Table IV
Logistic regression analysis based on
antibody titers of 0.8 U/ml and based on antibody titer of 264
U/ml.
| Antibody titer of
0.8 U/ml | Odds ratio | 95% confidence
interval | P-value |
|---|
|
CD 4
(>400 U/µl) | 3.8 | (0.547, 26.4) | 0.177 |
|
CD 19
(>100 U/µl) | 17.8 | (2.07, 152) | 0.008 |
|
Maintenance
therapy | 0.874 | (0.10, 37.4) | 0.901 |
| Antibody titer of
264 U/ml | | | |
|
CD 4
(>400 U/µl) | 2.367 | (1.307, 1.811) | 0.07 |
|
CD 19
(>100 U/µl) | 20.191 | (5727, 0.004) | 0.99 |
|
Maintenance
therapy | -17.184 | (6535, -0.003) | 0.99 |
Adverse events of Grade 2 or higher due to
vaccination included chill, fatigue, fever, headache, myalgia, pain
at injection point and rash (Table
V). Grade 3 rashes were observed in one case of BNT162b2
inoculation while every other case was Grade 2. There was no
significant difference in adverse events between BNT162b2 and
mRNA1273.
 | Table VComparison of adverse effects between
BNT162b2 and mRNA1273. |
Table V
Comparison of adverse effects between
BNT162b2 and mRNA1273.
| | BNT162b2 | mRNA1273 | |
|---|
| Symptoms | <Grade 1 | >Grade 2 | <Grade 1 | >Grade 2 | P-value |
|---|
| Chills | 12 | 0 | 21 | 2 | 0.536 |
| Fatigue | 12 | 0 | 22 | 1 | 1 |
| Fever | 12 | 0 | 20 | 3 | 0.536 |
| Headache | 11 | 1 | 21 | 2 | 1 |
| Myalgia | 11 | 1 | 20 | 3 | 1 |
| Pain (injection
point) | 10 | 2 | 20 | 3 | 1 |
| Rash | 12 | 0 | 18 | 5 | 0.141 |
Discussion
As stated in the introduction, the severity rate of
COVID-19 infection in hematologic diseases is 58.8% and the
fatality rate is ~27%. Risk factors for severe disease include age
>70 years, uncontrolled hematologic disease, ECOG 3-4,
neutropenia and CRP >20 mg/dl. Analysis of 3,377 patients with
hematologic malignancies in a previous study indicated a mortality
rate of 34%, especially in patients >60 years, while systemic
anticancer therapy did not affect mortality (1,2).
These mortality rates are higher than general mortality rates,
which suggests that protection against COVID-19 by vaccination is
important for hematologic disease cases (3). A previous study on vaccination
against hematologic diseases revealed that the antibody acquisition
rate of rituximab-treated patients was reduced to 20-50%, compared
with 80% for patients who were vaccinated with common hematologic
diseases, as a result of the recombinant zoster vaccine. On the
other hand, in a small number of cases, COVID-19 vaccination was
reported to result in an antibody acquisition rate of 88% in
leukemia cases, of 67% in chronic lymphocytic leukemia cases and of
66% in transplant and chimeric antigen receptor T-cell therapy
cases (4).
There have been several studies on the acquisition
of antibodies by COVID-19 vaccination in B-cell lymphomas,
including a study that antibodies were acquired in 89% of patients
before rituximab treatment, in 66.7% six months after the end of
treatment and in 7.3% during treatment (8). In patients treated with anti-CD20
antibodies, 16 of 17 failed to acquire antibodies. By contrast, 14
of 22 patients acquired antibodies with the COVID-19 vaccine 12
months after the end of treatment (9). It has been reported that during
maintenance therapy for B-cell lymphoma with anti-CD20 antibodies,
the acquisition rate of antibodies by COVID-19 vaccine was 29.8%,
and that it was particularly difficult to acquire antibodies within
six months after the last administration of anti-CD20 antibodies
(10). According to the findings
of the present study, the overall antibody acquisition rate was
54.3% based on an anti-S antibody titer of 0.8 U/ml. Factors
including age, no obinutuzumab use, no maintenance therapy for
anti-CD20 antibody, no lymphoma treatment, at least six months
since completion of treatment, CD4 and CD19 levels and normal
levels of IgA and IgM were all important for the acquisition of
antibodies. These indices suggested that immunity may be an
advantage in acquiring antibodies.
There have been various studies on the factors that
determine whether or not antibodies are acquired after COVID-19
vaccination, including CD19 level, CD4 level, time since anti-CD20
antibody treatment, age, CD19 percentage, CD4 percentage,
immunoglobulin level and CD4/CD8 ratio, with common factors
associated with the success of COVID-19 vaccination including CD19
(regardless of actual number, percentage of lymphocytes), CD4
(regardless of actual number, percentage of lymphocytes) and time
since anti-CD20 antibodies treatment (11-15).
Notably, a decrease in CD19 is common in all studies and is
considered to be a particularly important factor for the success of
COVID-19 vaccination.
On the other hand, it has been reported that the
antibody titers to the anti-S are important in COVID-19 vaccination
and that antibody titers of 264 U/ml or higher are required to
control COVID-19 (5-7).
The results of the present study were analyzed according to the
aforementioned parameters and it was found that there was no
difference in antibody acquisition between the BNT162b2 and
mRNA1273 vaccines. The acquisition of antibodies by COVID-19
vaccination was also observed to be more likely in patients with
CD19 counts of 100/µl or more, CD4 counts of 400/µl or more and six
months after treatment with anti-CD20 antibodies, whether based on
an anti-S antibody titer of 0.8 or 264 U/ml. Other factors, such as
immunoglobulin, IgA and IgM, age and the non-use of obinutuzumab,
were significant when the standard value of the anti-S antibody
titer was 0.8 U/ml, but not when it was 264 U/ml, which constituted
the limit for a valid antibody titer. On the other hand,
immunologically unfavorable factors such as the use of bendamustine
and the clinical stage resulted in insufficient antibody titers.
Multivariate analysis also exhibited that CD19 was a significant
factor for the anti-S antibody titer of 0.8 U/ml, but at 264 U/ml,
there was no significant factor; however, there is a possibility
that it was related to CD4. These findings demonstrated that immune
recovery may be important for obtaining antibody titers that enable
vaccines to be effective.
Numerous previous studies have utilized 0.8 U/ml as
the standard at which the anti-S antibody titer is detected
(8,12,16).
If an effective antibody titer of 264 U/ml is used, the factors
that lead to an effective antibody titer may change (i.e., they may
be limited or influenced by the treatment method). Further study
based on antibody titers may be necessary to interpret the previous
studies. In the present study, CD19 was considered a key factor
associated with the acquisition of an effective antibody titer,
with a highly significant difference between anti-S titers of 0.8
and 264 U/ml. Multivariate analysis also suggested an association
between the anti-S titers of 0.8 U/ml and CD19. This has also been
shown in studies where CD19 is associated with antibody acquisition
at the time of COVID-19 vaccination in CAR-T therapy, in which the
actual amount of CD19 is extremely reduced (16,17).
The importance of CD19 in COVID-19 vaccination is demonstrated in
the present study.
An association between CD4 naïve T cells, especially
memory T cells, and COVID-19 vaccination response in
immunocompromised and hemodialysis patients has also been reported
(18,19). If CD4-positive T cells could be
subdivided, the association between antibody titer and CD4-positive
T cells may have been further confirmed. However, CD4 >400/µl
and CD19 >100/µl were also examined in this analysis, and while
0.8 U/ml was a highly significant factor even when the anti-S
antibody titer was detected, the association was more robust when
the anti-S titer was 264 U/ml. The multivariate analysis conducted
also suggested a relationship between the anti-S titer of 264 U/ml
and CD4, but the difference did not reach significance, perhaps due
to the small number of cases.
Ishio et al (20) examined the acquisition of
antibodies after two doses of vaccine for B-cell lymphoma treated
with anti-CD20 antibody and observed that antibody production
differs depending on the interval between anti-CD20 antibody
treatments. They also detected that the antibody titer acquired was
lower in cases treated with bendamustine. Liebers et al
(21) reported that CD19 and CD4
are independent factors that acquire antibodies through vaccines,
which appears to agree with the findings of the present study.
However, they also revealed that T cell responses to COVID-19 were
observed even in cases that did not seroconvert. A previous study
conducted by Nishikubo et al (22) revealed that the third vaccine
booster can produce a T cell response to COVID-19 regardless of the
interval of anti-CD20 antibody treatment, and that regardless of
the antibody titer, it was revealed that prevention of COVID-19 can
be achieved. In conclusion, for the acquisition of antibodies by
vaccination of B-cell lymphomas during or after treatment with
anti-CD20 antibodies, a certain amount of CD19 and CD4 is
considered necessary (in the present study, CD19 at 100/µl or
higher and CD4 at 400/µl or higher, preferably both). In addition,
patients with a treatment interval of six months or longer, those
who have not received maintenance therapy with anti-CD20 antibodies
and those who have completed treatment are more likely to recover
from immunosuppression and are expected to acquire antibodies. The
antibody titer is also considered an important factor in
interpreting the results of vaccination, since the relevant index
and its degree differ when the criteria for antibody titer are
compared between 0.8 U/ml, the lower limit of detectable antibody
titer, and 264 U/ml, the level at which vaccine effect can be
expected. However, the present study features only a small number
of cases and does not examine the response of T cells to COVID-19,
so it is insufficient for a full evaluation of the effectiveness of
the vaccine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to personal
information included in the medical record but are available from
the corresponding author on reasonable request.
Authors' contributions
YT conceptualized the present study and developed
methodology. SI and FH performed software analysis. SI and AM
validated data. SI conducted formal analysis and investigation,
provided resources and curated data. YT wrote the original draft.
TT made substantial contributions to conception and design,
acquisition, analysis and interpretation of data wrote, reviewed
and edited the manuscript. All authors read and approved the final
version of the manuscript. YT and SI confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
2021-61) by the local Ethics Committee of Hakodate Municipal
Hospital Institutional Review Board (Hakodate, Japan). Written
informed consent was obtained from the patients who participated in
the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pinana JL, Martino R, Garcia-Garcia I,
Parody R, Morales MD, Benzo G, Gómez-Catalan I, Coll R, De La
Fuente I, Luna A, et al: Risk factors and outcome of COVID-19
inpatients with hematological malignancies. Exp Hematol Oncol.
9(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vijenthira A, Gong IY, Fox TA, Booth S,
Cook G, Fattizzo B, Martin-Moro F, Razanamahery J, Riches JC,
Zwicker J, et al: Outcomes of patients with hematologic
malignancies and COVID-19: A systematic review and meta-analysis of
3377 patients. Blood. 136:2881–2892. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Asch DA, Sheils NE, Islam MN, Chen Y,
Werner RM, Buresh J and Doshi JA: Variation in US hospital
mortality rates for patients admitted with COVID-19 during the
first 6 months of the pandemic. JAMA Intern Med. 181:471–478.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun C, Pleyer C and Wiestner A: COVID-19
vaccines for patients with hematological conditions. Lancet
Haematol. 8:e312–e314. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Riester E, Findeisen P, Hegel JK, Kabesch
M, Ambrosch A, Rank CM, Pessl F, Laengin T and Niederhauser C:
Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S
immunoassay. J Virol Methods. 297(114271)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Feng S, Phillips DJ, White T, Sayal H,
Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, et al:
Correlates of protection against symptomatic and asymptomatic
SARS-CoV-2 infection. Nat Med. 27:2032–2040. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Perkmann T, Perkmann-Nagele N, Koller T,
Mucher P, Radakovics A, Marculescu R, Wolzt M, Wagner OF, Binder CJ
and Haslacher H: Anti-Spike protein assays to determine SARS-CoV-2
antibody levels: A head-to-head comparison of five quantitative
assays. Microbiol Spectr. 9(e0024721)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Perry C, Luttwak E, Balaban R, Shefer G,
Morales MM, Aharon A, Tabib Y, Cohen C, Benyamini N, Beyar-Katz O,
et al: Efficacy of the BNT162b2 mRNA COVID-19 vaccine patients with
B-cell non-Hodgkin lymphoma. Blood Adv. 5:3053–3061.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Re D, Barriere J, Chamorey E, Delforge M,
Gastaud L, Petit E, Chaminade A, Verrriere B and Peyrade F: Low
rate of seroconversion after m RNA anti-SARS-CoV-2 vaccination in
patients with hematological malignancies. Leuk Lymphoma.
62:3308–3310. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Avivi I, Luttwak E, Saiang E, Halperin T,
Haberman S, Saring A, Levi S, Aharon A, Herishanu Y and Perry C:
BNT-162b2 m RNA COVID-19 vaccine booster induces seroconversion in
patients with B-cell non-Hodgkin lymphoma who failed to respond to
two prior vaccine doses. Br J Haematol. 196:1329–1333.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moor MB, Suter-Riniker F, Horn MP, Aeberli
D, Amsler J, Möller B, Njue LM, Medri C, Angelillo-Scherrer A,
Borradori L, et al: Humoral and cellular responses to mRNA vaccines
against SARS-CoV-2 in patients with a history of CD20
B-cell-depleting therapy (RituxiVac): An investigator-initiated,
single-centre, open-label study. Lancet Rheumatol. 3:e789–e797.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schubert L, Koblischke M, Schneider L,
Porpaczy E, Winkler F, Jaeger U, Blüml S, Haslacher H, Burgmann H,
Aberle JH, et al: Immunogenicity of COVID-19 vaccinations in
hematological patients: 6-month follow-up and evaluation of a 3rd
vaccination. Cancers (Basel). 14(1962)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tsushima T, Terao T, Narita K, Fukumoto A,
Ikeda D, Kamura Y, Kuzume A, Tabata R, Miura D, Takeuchi M and
Matsue K: Antibody response to COVID-19 vaccine in 130 recipients
of hematopoietic stem cell transplantation. Int J Hematol.
115:611–615. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Narita K, Nakaji S, Tabata R, Terao T,
Kuzume A, Tsushima T, Ikeda D, Fukumoto A, Miura D, Takeuchi M, et
al: Antibody response to COVID-19 vaccination in patients with
lymphoma. Int J Hematol. 115:728–736. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Antolí A, Rocamora-Blanch G, Framil M,
Mas-Bosch V, Navarro S, Bermudez C, Martinez-Yelamos S, Dopico E,
Calatayud L, Garcia-Muñoz N, et al: Evaluation of Humoral and
Cellular Immune Responses to the SARS-CoV-2 Vaccine in Patients
With Common Variable Immunodeficiency Phenotype and Patient
Receiving B-Cell Depletion Therapy. Front Immunol.
13(895209)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ram R, Hagin D, Kikozashvilli N, Freund T,
Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C,
et al: Safety and Immunogenicity of the BNT162b2 mRNA COVID-19
vaccine in patients after allogeneic HCT or CD19-based CART
therapy-A single-center prospective cohort study. Transplant Cell
Ther. 27:788–794. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jarisch A, Wiercinska E, Huenecke S, Bremm
M, Cappel C, Hauler J, Rettinger E, Soerensen J, Hellstern H,
Klusmann JH, et al: Immune Responses to SARS-CoV-2 vaccination in
young patients with Anti-CD19 chimeric antigen receptor T
cell-induced B Cell Aplasia. Transplant Cell Ther.
28:366.e1–366.e7. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Baron F, Canti L, Ariën KK, Kemlin D,
Desombere I, Gerbaux M, Pannus P, Beguin Y, Marchant A and
Humblet-Baron S: . Insights from early clinical trials assessing
response to mRNA SARS-CoV-2 vaccination in immunocompromised
patients. Front Immunol. 13(827242)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duni A, Markopoulos GS, Mallioras I,
Pappas H, Pappas E, Koutlas V, Tzalavra E, Baxevanos G, Priska S,
Gartzonika K, et al: The humoral immune response to BNT162b2
vaccine is associated with circulating CD19+ B lymphocytes and the
naïve CD45RA to Memory CD45RO CD4+ T helper cells ratio in
hemodialysis patients and kidney transplant recipients. Front
Immunol. 12(760249)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ishio T, Tsukamoto S, Yokoyama E,
Izumiyama K, Saito M, Muraki H, Kobayashi M, Mori A, Morioka M and
Kondo T: Anti-CD20 antibodies and bendamustine attenuate humoral
immunity to COVID-19 vaccination in patients with B-cell
non-Hodgkin lymphoma. Ann Hematol. 102:1421–1431. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liebers N, Speer C, Benning L, Bruch PM,
Kraemer I, Meissner J, Schnitzler P, Kräusslich HG, Dreger P,
Mueller-Tidow C, et al: Humoral and cellular responses after
COVID-19 vaccination in anti-CD20-treated lymphoma patients. Blood.
139:142–147. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nishikubo M, Shimomura Y, Yamamoto R,
Yoshioka S, Maruoka H, Nasu S, Nishioka T, Sakizono K, Mitsuyuki S,
Kubo T, et al: Humoral and cellular responses after COVID-19
booster vaccination in patients recently treated with anti-CD20
antibodies. Blood Cancer J. 13(17)2023.PubMed/NCBI View Article : Google Scholar
|