Introduction
Pancreatic cancer is one of the most aggressive
cancers, with an extremely low five-year survival rate of 10% in
the US and Japan, and its incidence continues to increase (1,2).
Diabetes (3), obesity (4,5),
smoking (6), heavy drinking
(7) and chronic pancreatitis
(8) are listed as risk factors for
the development of pancreatic cancer. Although increase in the
incidence of these lifestyle-related factors and aging have been
cited as causes of the increased incidence of pancreatic cancer,
not all the mechanisms of onset have been elucidated. In most
cases, pancreatic cancer is detected at an advanced stage,
representing the fourth leading cause of cancer-related mortality
in both the U.S. and Japan (1,2).
Surgical resection is the only potentially curative treatment, but
only 20% of cases are resectable at diagnosis, and most patients
have unresectable disease (1,2).
Furthermore, the recurrence rate is very high even in patients who
have undergone radical resection, and the 5-year survival rate does
not reach 30% (2).
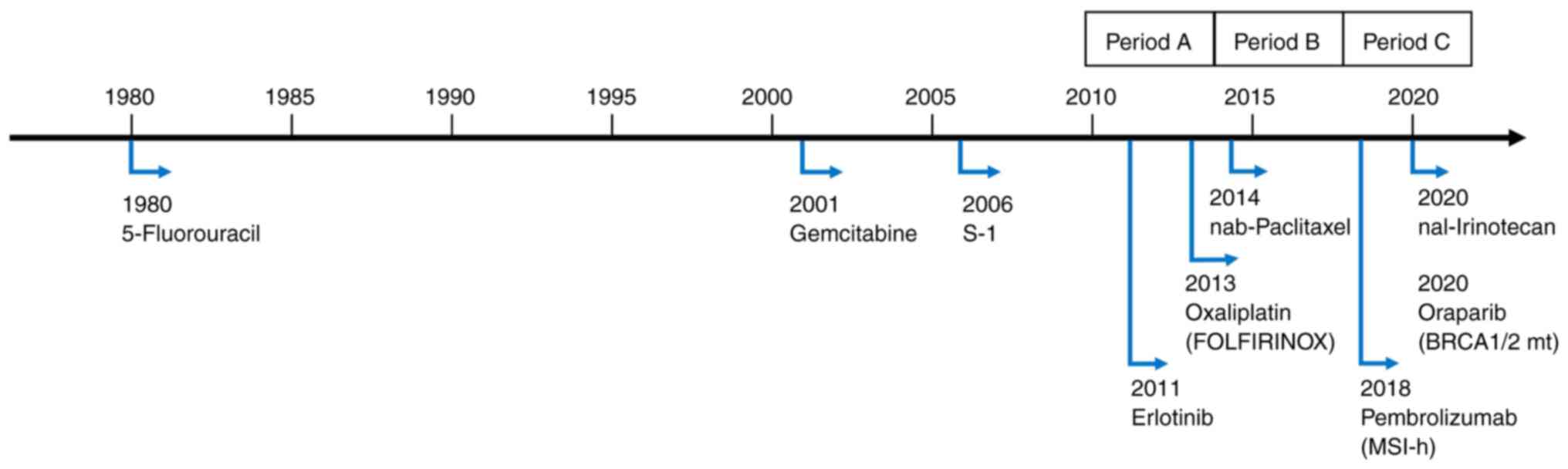
Before 2010, only 5-fluorouracil (5-FU),
gemcitabine, and tegafur/gimeracil/oteracil (S-1) regimens had been
approved for use in cases of advanced/recurrent pancreatic cancer
in Japan. Gemcitabine has been used worldwide for many years
because of its demonstrated improvement in quality of life and
prolongation of overall survival (OS) in a gold-standard phase III
trial (vs. 5-FU) (9) and was
approved for use in Japan in 2001. S-1 is an oral 5-FU derivative
developed in Japan and was approved for use in 2006 based on the
results of phase II studies in patients with advanced pancreatic
cancer (10,11). In 2007, the results of a phase III
trial examining the efficacy of gemcitabine in combination with
erlotinib were reported and showed a statistically significant
improvement in survival, but the difference was small and did not
have enough impact to change actual clinical practice (12).
Recently, some additional regimens for
chemotherapy-naïve pancreatic cancer, proven to be effective in
gold-standard clinical trials have been approved and are now in
widespread use, including fluorouracil, folinic acid, oxaliplatin,
and irinotecan (FOLFIRINOX) (approved in Japan since 2013)
(13-15)
and gemcitabine plus nab-paclitaxel (approved in Japan since 2014)
(16). In addition, a Japanese
phase III study in 2013 demonstrated the non-inferiority of S-1,
but not the superiority of gemcitabine plus S-1 therapy compared to
gemcitabine (17). Notably,
previous clinical trials have shown that each combination therapy
prolongs OS when compared with gemcitabine alone; NCCN guidelines
recommend FOLFIRINOX and gemcitabine plus nab-paclitaxel for
metastatic pancreatic cancer with good performance status (PS)
(18). Japanese guidelines for
pancreatic cancer also recommend the same two regimens (19), and they are widely used as standard
treatments for metastatic pancreatic cancer. Other approved
regimens include liposomal irinotecan plus 5-FU and leucovorin for
patients who have failed gemcitabine-based therapy (20) and olaparib as maintenance therapy
after platinum-based chemotherapy for BRCA mutation-positive
patients (21) (approved in Japan
since 2020). The history of approved agents in Japan is shown in
Fig. 1.
On the other hand, patients enrolled in clinical
trials are highly selected patients with good general condition and
organ functions. Therefore, there is often a discrepancy between
the treatment results shown in clinical trials and actual clinical
practice due to this selection bias (22). In recent years, cohort studies
based on large-scale databases have been conducted to fill these
gaps (23-26).
Fortunately, real-world data (RWD) regarding the health and
treatment status of patients receiving daily medical care are
collected within standard organizational processes (e.g.,
electronic medical records and hospitalization data) (27). To evaluate whether the results of
clinical trials are carried over to the real-world, we conducted an
exploratory cohort study using RWD to investigate temporal trends
in treatment patterns for metastatic pancreatic cancer as well as
treatment outcomes and prognostic factors that influence OS.
Materials and methods
Study overview
This nationwide retrospective cohort study was
conducted as part of the TREAD project, the outline of which has
been described elsewhere (28).
This project was approved by the Ethics Committee of the Tokushukai
Group in April 2020 (No. TGE01427-024) and was conducted following
the principles of the Declaration of Helsinki, and patients were
provided with information using opt-out methods. This study was
registered in the UMIN Clinical Trial Registry (http://www.umin.ac.jp/ctr/index.htm) and clinical
trial number was UMIN000050590.
Objective patients
We evaluated patients with pathologically or
radiologically confirmed metastatic pancreatic cancer who were
started on first-line chemotherapy at Tokushukai Medical Group
hospitals, which included 46 hospitals with 14829 beds, using the
same medical record system (e-Karte and Newtons2; Software Service
Inc., Osaka, Japan) and chemotherapy protocol system (srvApmDrop;
Software Service Inc., Osaka, Japan) between April 1, 2010, and
March 31, 2020.
All patients were administered gemcitabine, S-1,
gemcitabine plus S-1, gemcitabine plus nab-paclitaxel, or
FOLFIRINOX as first-line treatment. Pathological diagnoses
including adenocarcinoma, adenosquamous carcinoma, and
carcinoma/malignant neoplasms were included in the current study,
but patients with acinar and neuroendocrine carcinoma were excluded
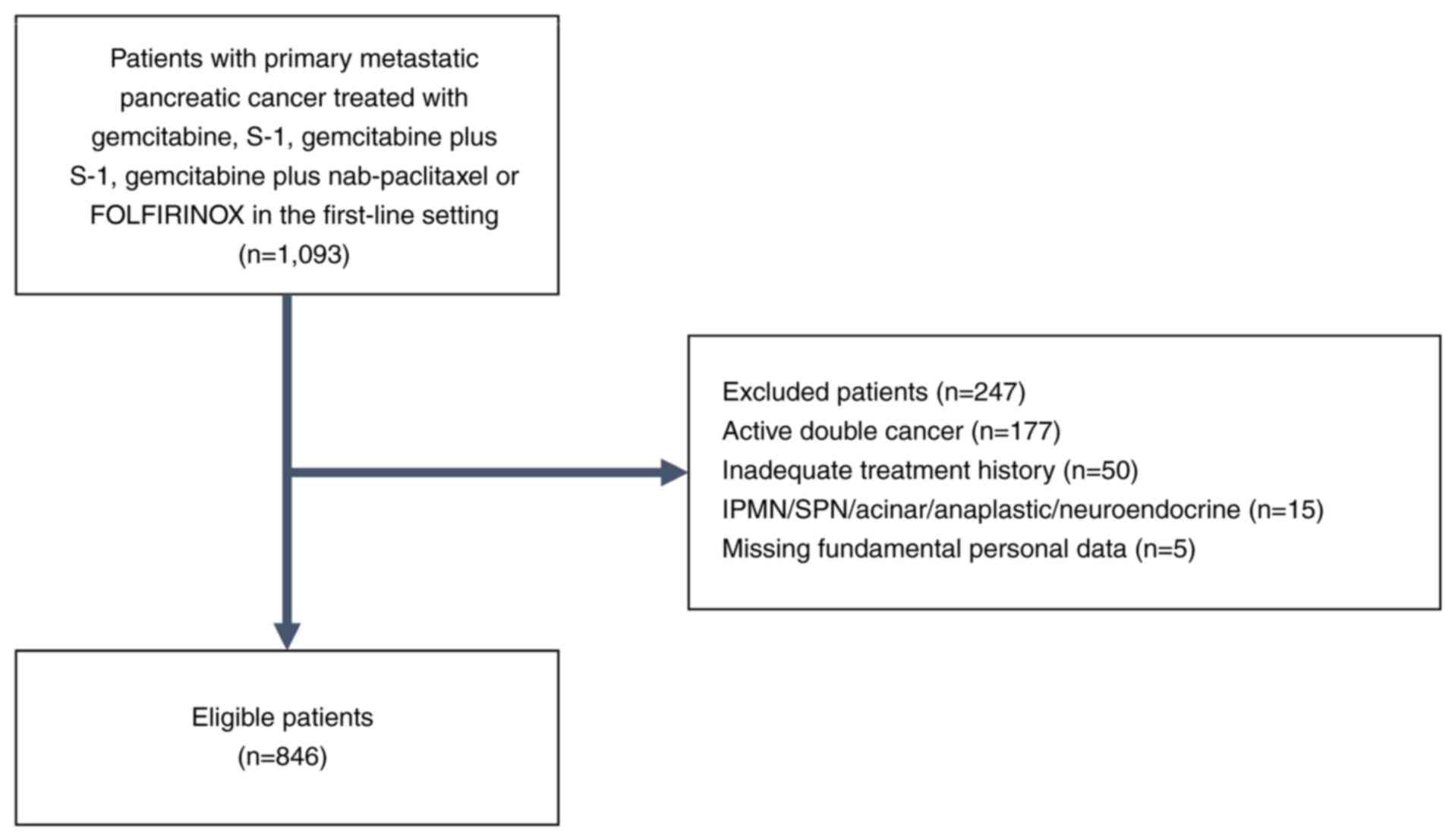
(see Fig. 2 for more information).
Additional key exclusion criteria were the presence of active
double cancer, inadequate treatment history, and missing
fundamental patient data, such as body weight and height.
Data collection
In the current study, we evaluated eligible patients
identified from electronic medical records. Patient information
such as age, sex, body mass index (BMI), the latest data on
survival confirmation, survival outcomes, and diagnosis on medical
receipt were extracted from the medical record system. Treatment
information related to chemotherapy regimens, start and end dates
of chemotherapy, and PS was extracted from the chemotherapy
protocol system. The linked cancer registry information including
diagnostic information (site, pathology, stage), treatment details
(surgery, endoscopic procedure, radiotherapy, chemotherapy), and
prognosis (final date of survival confirmation, date of death,
cause of death) was extracted from the National Cancer Registry
Data in Japan (29). Hospital
volume and hospital type (government designated cancer hospital,
prefectural designated cooperative cancer hospital, or
non-designated general hospital) were also noted.
The treatment history was organized based on the
extracted chemotherapy information, and when discrepancies or
missing information were detected, the missing data were
investigated by directly checking the medical records at Tokushukai
Information Inc. (Osaka, Japan). Patients with inadequate treatment
history (i.e., previous or subsequent cancer treatment outside of
Tokushukai Medical Group hospitals or no detailed treatment
information available) were excluded from the study. The study was
divided into three periods for the elucidation of secular trends
(A, 2010-2013; B, 2014-2016; C, 2017-2020).
Statistical analysis
The primary endpoint evaluated in the current study
was OS, which is defined as the time from the start date of initial
palliative chemotherapy to the date of death or final survival
confirmation. The secondary endpoint was time to treatment failure
(TTF), which is defined as the time from the start date of the
first-line chemotherapy treatment to discontinuation of the
treatment for any reason.
Basic statistics (absolute and relative frequencies
for categorical variables; quartiles, maximum values, minimum
values, and means for continuous variables; and quartiles and
relative frequencies for discrete variables) were obtained to
summarize the distribution of variables related to patient
background factors, complications, other prognostic factors, and
primary and secondary endpoints. Survival analyses were performed
using OS as the primary endpoint. The start date of the study was
April 1, 2010, and the study end date was March 31, 2020. The time
variable represents the number of days from the start date of the
first-line chemotherapy treatment to the date of death. The
censored cases included patients who were alive at the study end
date or who dropped out of the study for any reason.
Kaplan-Meier curves (univariate analyses) and
log-rank test were applied for each stratum, defined according to
the patient background and prognostic factors (age at the start of
first-line chemotherapy, sex, PS, BMI, smoking status, pathology,
primary disease site, study period, hospital volume, hospital type,
and first-line chemotherapy regimen) for the occurrence of events
associated with study endpoints (OS, TTF).
In addition, several hierarchical predictive models
were constructed by combining explanatory variables that were
expected to contribute to the evaluated endpoints, and single- and
multi-tiered proportional hazard models were established by
incorporating each predictive model. Stratified/conventional Cox
multiple regression analyses were performed. Conventional Cox
regression was applied when the proportional hazards hypothesis was
valid; otherwise, a stratified Cox regression was applied.
The Akaike Information Criterion (AIC), based on
partial likelihood, was used to explore the optimal model in the
current study (i.e., when the number of eligible cases differed
between models, the average AIC per case was substituted). Hazard
ratios (HRs) and 95% confidence intervals (CIs) were obtained for
each category of OS-related prognostic factors selected in the
optimal model, and the impact of prognostic factors in the optimal
model was examined using likelihood tests with associated p-values
for each item. All analyses were performed using R, version 4.2.2
(R Foundation for Statistical Computing, Vienna, Austria). All
statistical analyses were two-sided, and probability values of
<0.05 were considered statistically significant.
Results
Patient flow
A total of 1,093 patients were detected using the
procedures described above, and 846 patients were found to be
eligible according to the study inclusion and exclusion criteria
specified above, as shown in Fig.
2.
Patient characteristics
Patient medical and demographic characteristics were
typical for metastatic pancreatic cancer (Table I). Approximately 30% of the
patients were over 75 years of age. Over 90% of the included
patients had an Eastern Cooperative Oncology Group PS of 0 or 1.
Most patients had pathologically proven disease. Patient
characteristics by treatment regimen are shown in Table II. There was a trend toward fewer
patients over 75 years of age and PS 2 or higher for combination
chemotherapy. Patient backgrounds were generally similar among the
three time periods, but the proportion of patients receiving
combination therapy increased over time to 11.2, 37.8, and 67.8% in
study periods A, B, and C, respectively (Table III). The patient background
between hospital volume and type is also presented in Table SI.
 | Table IPatient medical and demographic
characteristics (n=846). |
Table I
Patient medical and demographic
characteristics (n=846).
|
Characteristics | Value |
|---|
| Age (at start of
first-line treatment) | |
|
Median age,
years (quantile) | 70 (36, 64, 70, 76,
90) |
|
≥75 years, n
(%) | 266 (31.4) |
| Sex, n (%) | |
|
Male | 503 (59.5) |
|
Female | 343 (40.5) |
| PS, n (%) | |
|
0 | 232 (27.4) |
|
1 | 290 (34.3) |
|
≥2 | 53 (6.3) |
|
Not
available | 271 (32.0) |
| Median BMI,
kg/m2 (quantile) | 19.7 (11.2, 17.4,
19.7, 21.9, 35.4) |
| Smoking status, n
(%) | |
|
Current or
former (BI>0) | 217 (25.7) |
|
Never smoked
(BI=0) | 562 (66.4) |
|
Not
available | 67 (7.9) |
| Diagnosis, n
(%) | |
|
Pathologically
confirmed | 745 (88.1) |
|
Adenocarcinoma | 418 |
|
Adenosquamous | 7 |
|
Carcinoma/malignant
neoplasm | 320 |
|
Radiological
diagnosis only | 101 (11.9) |
| Primary disease
site, n (%) | |
|
Pancreas
head | 359 (42.4) |
|
Pancreas
body | 232 (27.4) |
|
Pancreas
tail | 220 (26.0) |
|
Not
evaluable | 35 (4.1) |
| Previous
proceduresa, n
(%) | |
|
Surgery | 123 (14.5) |
|
Endoscopic
procedure | 44 (5.2) |
|
Radiotherapy | 47 (5.6) |
|
None of the
above | 678 (80.1) |
| Study period, n
(%) | |
|
Period A
(2010-2013) | 268 (31.7) |
|
Period B
(2014-2016) | 251 (29.7) |
|
Period C
(2017-2020) | 327 (38.7) |
| Hospital volume, n
(%) | |
|
High volume
(n≥50) | 509 (60.2) |
|
Low volume
(n<50) | 337 (39.8) |
| Hospital type, n
(%) | |
|
Government
designated cancer hospital | 218 (25.7) |
|
Prefectural
designated cooperative cancer hospital | 316 (37.4) |
|
General
hospital | 312 (36.9) |
| First-line systemic
therapy, n (%) | |
|
Gemcitabine
monotherapy | 302 (35.7) |
|
S-1
monotherapy | 197 (23.3) |
|
Gemcitabine
plus S-1 | 66 (7.8) |
|
Gemcitabine
plus nab-paclitaxel | 229 (27.1) |
|
FOLFIRINOX | 52 (6.1) |
 | Table IIPatient medical and demographic
characteristics by regimen. |
Table II
Patient medical and demographic
characteristics by regimen.
|
Characteristics | Gem (n=302) | S-1 (n=197) | GS (n=66) | GnP (n=229) | FOLFIRINOX
(n=52) |
|---|
| Age (at start of
first-line treatment) | | | | | |
|
Median age,
years (quantile) | 71 (36, 65, 71, 77,
89) | 71 (45, 66, 71, 78,
90) | 67 (39, 61, 67, 73,
84) | 70 (37, 64, 70, 75,
86) | 65 (42, 57, 65, 69,
82) |
|
≥75 years, n
(%) | 113 (37.4) | 75 (38.1) | 14 (21.1) | 60 (26.2) | 4 (6.2) |
| Sex, n (%) | | | | | |
|
Male | 162 (53.6) | 122 (61.9) | 42 (63.6) | 141 (61.6) | 36 (69.2) |
|
Female | 140 (46.4) | 75 (38.1) | 24 (36.4) | 88 (38.4) | 16 (30.3) |
| Performance status,
n (%) | | | | | |
|
0 | 100 (33.1) | 19 (9.6)) | 25 (37.9) | 69 (30.1) | 19 (36.5) |
|
1 | 124 (41.1) | 20 (10.2)) | 12 (18.2) | 112 (48.9) | 22 (42.3) |
|
≥2 | 28 (9.2) | 8 (4.1)) | 1 (1.5) | 16 (7.0) | 11 (21.2) |
|
Not
available | 50 (16.6) | 150 (76.1) | 28 (42.4) | 32 (14.0) | 0 (0.0) |
| Median body mass
index, kg/m2 (quantile) | 19.7 (12.0, 17.5,
19.7, 22.0, 34.8) | 19.3 (11.6, 17.3,
19.3, 21.5, 35.4) | 19.4 (13.6, 17.3,
19.4, 21.8, 29.9) | 20.1 (11.2, 17.5,
20.1, 22.1, 34.8) | 20.2 (13.3, 17.4,
20.2, 22.6, 34.5) |
| Smoking status, n
(%) | | | | | |
|
Current or
former (BI>0) | 71 (23.5) | 49 (24.9) | 17 (25.8) | 62 (27.0) | 18 (34.6) |
|
Never smoked
(BI=0) | 198 (65.6) | 132 (67.0) | 46 (69.7) | 157 (68.6) | 29 (55.8) |
|
Not
available | 33 (10.9) | 16 (8.1) | 3 (4.5) | 10 (4.4) | 5 (9.6) |
| Diagnosis, n
(%) | | | | | |
|
Pathologically
confirmed | 241 (79.8) | 181 (91.9) | 55 (83.3) | 221 (96.5) | 47 (90.4) |
|
Adenocarcinoma | 129 (42.7) | 87 (44.2) | 22 (33.3) | 147 (64.2) | 33 (63.5) |
|
Adenosquamous | 1 (0.3) | 1 (0.5) | 0 (0.0) | 4 (1.7) | 1 (1.9) |
|
Carcinoma/malignant
neoplasm | 111 (36.8) | 93 (47.2) | 33 (50.0) | 70 (30.6) | 13 (25.0) |
|
Radiological
diagnosis only | 61 (20.2) | 16 (8.1) | 11 (16.7) | 8 (3.5) | 5 (9.6) |
| Primary site of
disease, n (%) | | | | | |
|
Pancreas
head | 130 (43.0) | 81 (41.1) | 25 (37.9) | 100 (43.7) | 23 (44.2) |
|
Pancreas
body | 84 (27.8) | 55 (27.9) | 20 (30.3) | 59 (25.8) | 14 (26.9) |
|
Pancreas
tail | 78 (25.8) | 54 (27.4) | 20 (30.3) | 54 (23.5) | 14 (26.9) |
|
Not
evaluable | 10 (3.3) | 7 (3.5) | 1 (1.5) | 16 (7.0) | 1 (1.9) |
| Previous
procedurea, n
(%) | | | | | |
|
Surgery | 51 (16.9) | 39 (19.8) | 13 (19.7) | 18 (7.9) | 2 (3.8) |
|
Endoscopic
procedure | 21 (7.0) | 13 (6.6) | 5 (7.6) | 5 (2.2) | 0 (0.0) |
|
Radiotherapy | 12 (4.0) | 17 (8.6) | 6 (9.1) | 8 (3.5) | 4 (7.7) |
|
None of the
above | 240 (79.5) | 141 (71.6) | 47 (71.2) | 203 (88.6) | 47 (90.4) |
| Study period, n
(%) | | | | | |
|
Period A
(2010-2013) | 179 (59.3) | 59 (29.9) | 30 (45.5) | 0 (0.0) | 0 (0.0) |
|
Period B
(2014-2016) | 79 (26.1) | 77 (39.1) | 22 (33.3) | 54 (23.6) | 19 (36.5) |
|
Period C
(2017-2020) | 44 (14.6) | 61 (31.0) | 14 (21.2) | 175 (76.4) | 33 (63.5) |
| Hospital volume, n
(%) | | | | | |
|
High-volume
hospital (≥50) | 184 (60.9) | 115 (58.4) | 30 (45.5) | 156 (68.1) | 24 (46.2) |
|
Low-volume
hospital (<50) | 118 (39.1) | 82 (41.6) | 36 (54.4) | 73 (31.9) | 28 (53.8) |
| Hospital type, n
(%) | | | | | |
|
Government
designated cancer hospital | 94 (31.1) | 39 (19.8) | 0 (0.0) | 77 (33.6) | 8 (15.4) |
|
Prefectural
designated cooperative cancer hospital | 102 (33.8) | 76 (38.6) | 25 (37.9) | 94 (41.0) | 19 (36.5) |
|
General
hospital | 106 (35.1) | 82 (41.6) | 41 (62.1) | 58 (25.3) | 25 (48.1) |
 | Table IIIPatient medical and demographic
characteristics by study period. |
Table III
Patient medical and demographic
characteristics by study period.
|
Characteristics | Period A
(2010-2013) (n=268) | Period B
(2014-2016) (n=251) | Period C
(2017-2020) (n=327) |
|---|
| Age (at start of
first-line treatment) | | | |
|
Median age,
years (quantile) | 69 (36, 63, 69, 76,
90) | 69 (37, 62, 69, 75,
89) | 71 (44, 66, 71, 76,
86) |
|
≥75 years, n
(%) | 80 (29.9) | 69 (27.5) | 117 (35.8) |
| Sex, n (%) | | | |
|
Male | 157 (58.6) | 153 (61.0) | 193 (59.0) |
|
Female | 111 (41.4) | 98 (39.0) | 134 (41.0) |
| PS, n (%) | | | |
|
0 | 89 (33.2) | 63 (25.1) | 80 (24.5) |
|
1 | 83 (30.0) | 78 (31.1) | 129 (39.4) |
|
≥2 | 19 (7.1) | 15 (6.0) | 19 (5.8) |
|
Not
available | 77 (28.7) | 95 (37.8) | 99 (30.3) |
| Median BMI,
kg/m2 (quantile) | 19.5 (12.0, 17.6,
19.5, 21.9, 30.6) | 19.7 (11.6, 17.5,
19.7, 21.9, 35.4) | 19.8 (11.2, 17.2,
19.8, 22.1, 34.8) |
| Smoking status, n
(%) | | | |
|
Current or
former (BI>0) | 63 (23.5) | 57 (22.7) | 97 (29.7) |
|
Never smoked
(BI=0) | 173 (64.6) | 173 (68.9) | 216 (66.1) |
|
Not
available | 32 (11.9) | 21 (8.4) | 14 (4.2) |
| Diagnosis, n
(%) | | | |
|
Pathologically
confirmed | 204 (76.1) | 232 (92.4) | 309 (94.5) |
|
Adenocarcinoma | 106 (39.6) | 157 (62.5) | 174 (53.2) |
|
Adenosquamous | 1 (0.3) | 3 (1.2) | 3 (0.9) |
|
Carcinoma/malignant
neoplasm | 97 (36.2) | 72 (28.7) | 132 (40.4) |
|
Radiological
diagnosis only | 64 (23.9) | 19 (7.6) | 18 (5.5) |
| Primary disease
site, n (%) | | | |
|
Pancreas
head | 127 (47.4) | 89 (35.5) | 143 (43.7) |
|
Pancreas
body | 61 (22.8) | 79 (31.5) | 89 (27.2) |
|
Pancreas
tail | 68 (25.4) | 72 (28.7) | 80 (24.5) |
|
Not
evaluable | 9 (3.4) | 11 (4.3) | 15 (4.6) |
| Previous
proceduresa, n
(%) | | | |
|
Surgery | 67 (25.0) | 37 (12.0) | 19 (5.8) |
|
Endoscopic
procedure | 24 (9.0) | 18 (7.2) | 2 (0.6) |
|
Radiotherapy | 5 (1.9) | 22 (8.8) | 20 (6.1) |
|
None of the
above | 197 (73.5) | 192 (76.5) | 289 (88.4) |
| Hospital volume, n
(%) | | | |
|
High volume
(n≥50) | 173 (64.6) | 149 (59.4) | 187 (57.2) |
|
Low volume
(n<50) | 95 (35.4) | 102 (40.6) | 140 (42.8) |
| Hospital type, n
(%) | | | |
|
Government
designated cancer hospital | 63 (23.5) | 69 (27.4) | 86 (26.3) |
|
Prefectural
designated cooperative cancer hospital | 112 (41.8) | 89 (35.5) | 115 (35.2) |
|
General
hospital | 93 (34.7) | 93 (37.1) | 126 (38.5) |
| First-line systemic
therapy, n (%) | | | |
|
Gemcitabine
monotherapy | 179 (66.8) | 79 (31.5) | 44 (13.5) |
|
S-1
monotherapy | 59 (22.0) | 77 (30.7) | 61 (18.7) |
|
Gemcitabine
plus S-1 | 30 (11.2) | 22 (8.8) | 14 (4.2) |
|
Gemcitabine
plus nab-paclitaxel | 0 (0.0) | 54 (21.5) | 175 (53.5) |
|
FOLFIRINOX | 0 (0.0) | 19 (7.5) | 33 (10.1) |
Trends in the implementation of
chemotherapy regimens
Trends in the implementation of first-line
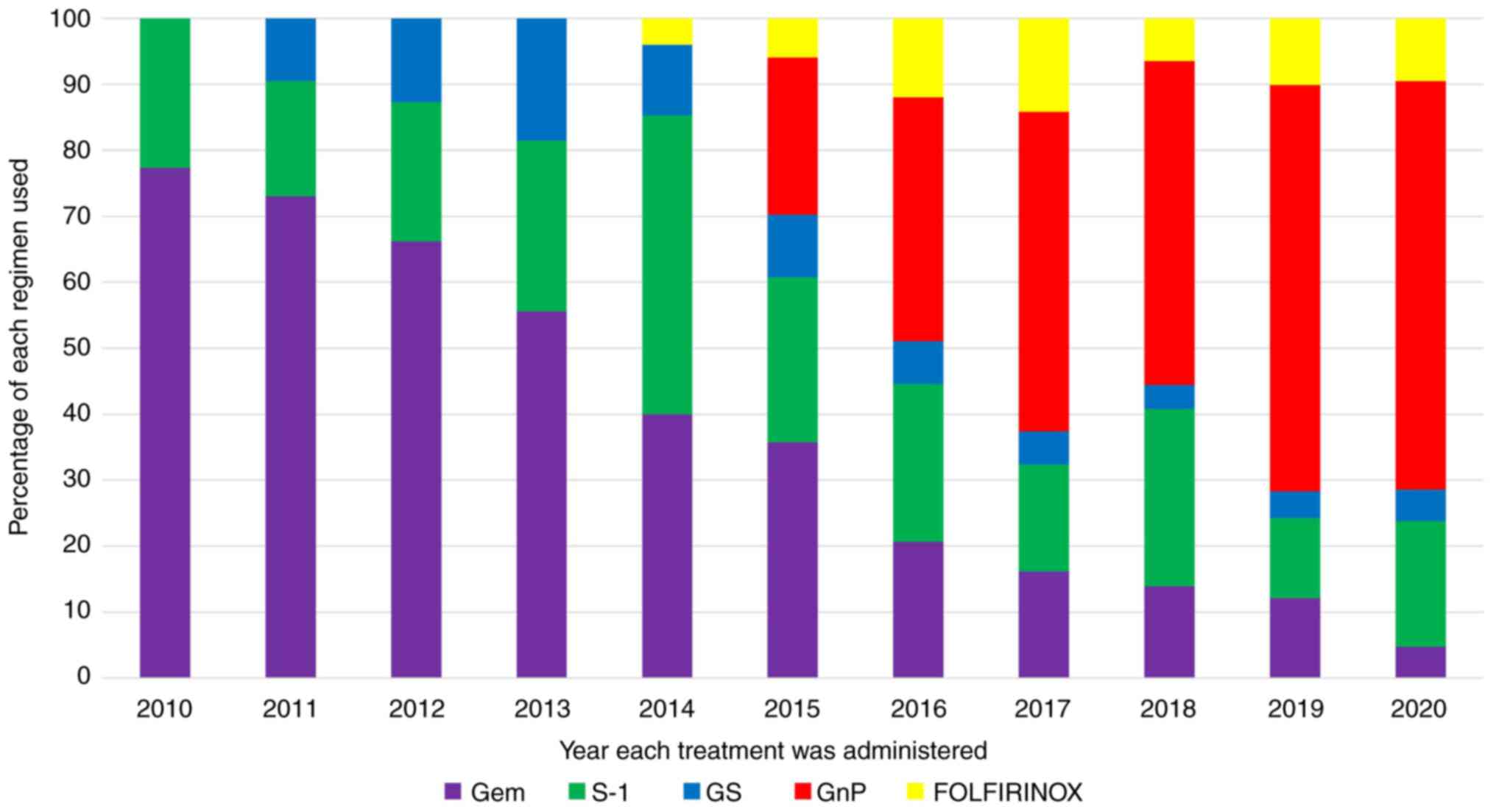
chemotherapy are shown in Fig. 3.
In 2010, when the study began, gemcitabine was the most commonly
used drug, but its percentage gradually decreased, while that of
nab-paclitaxel increased after 2014, when nab-paclitaxel was
approved. However, the use of FOLFIRINOX remained consistently low
during the study period, even after its approval in 2013.
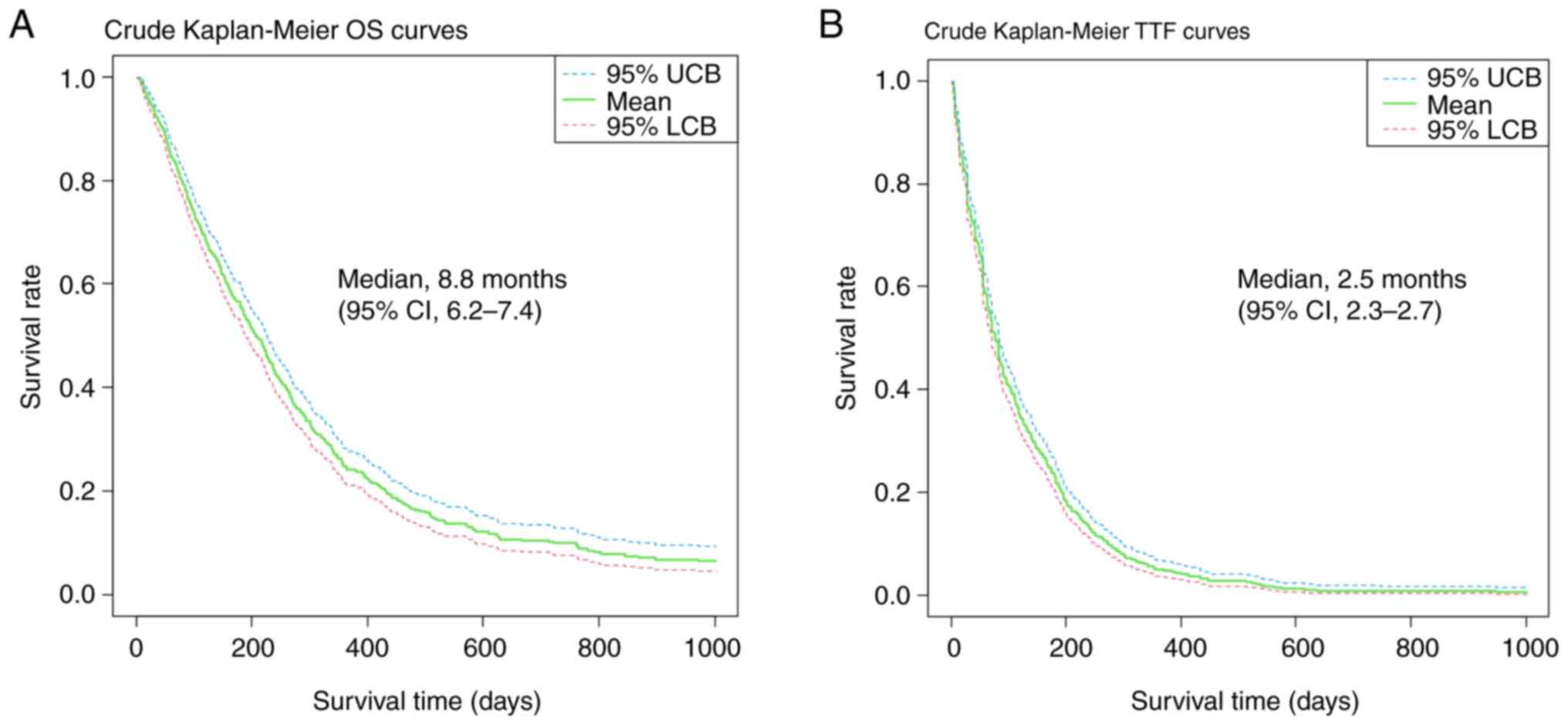
Kaplan-Meier survival curves
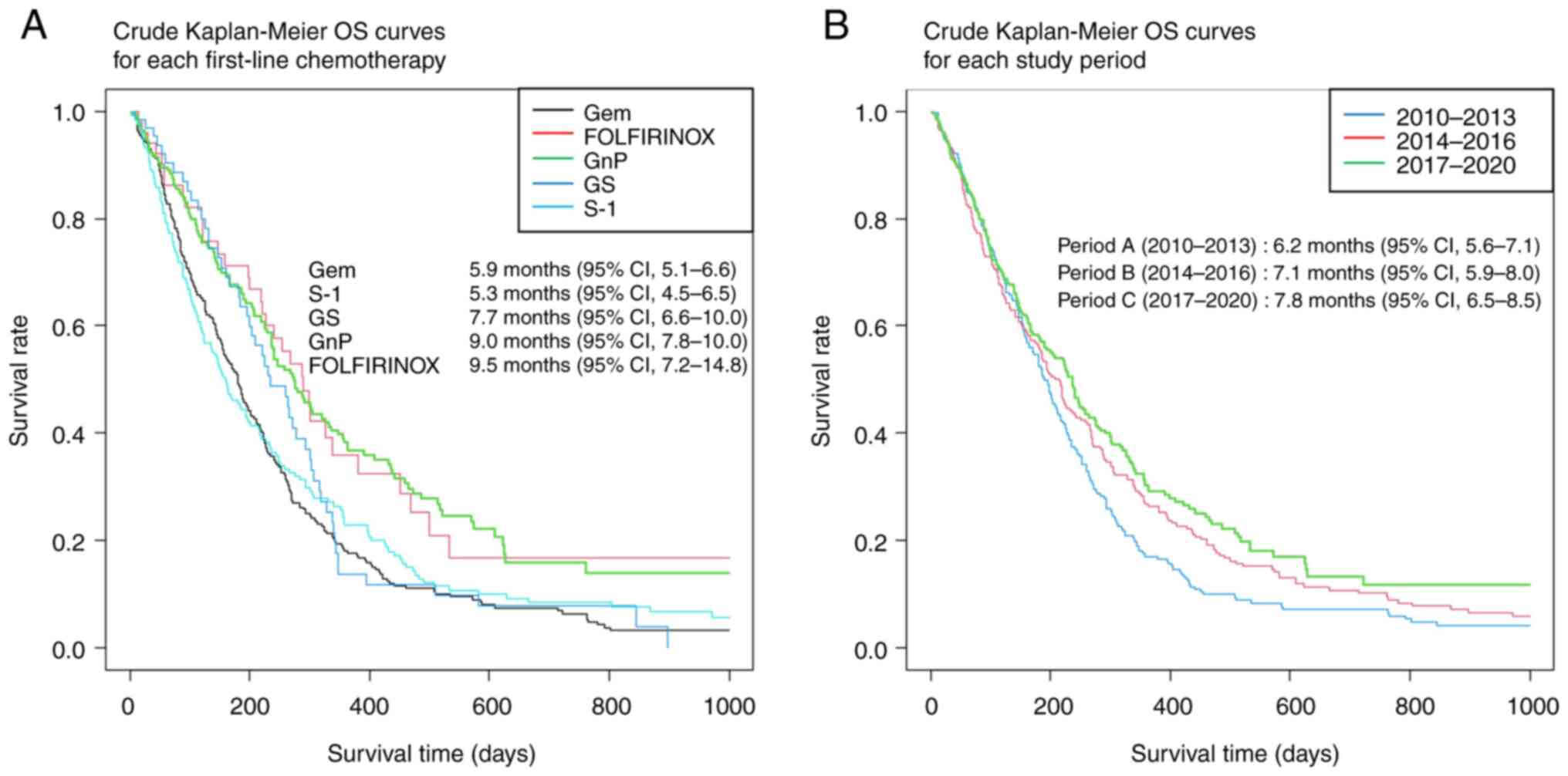
The crude (before adjusting for background factors)
survival curves evaluating OS and TTF using the Kaplan–Meier method
are shown in Fig. 4. The median
follow-up duration was 5.4 months (95% confidence interval [CI],
4.8-6.0). The median OS of all included patients was 6.8 months
(95% CI, 6.3-7.4), and the median TTF was 2.5 months (95% CI,
2.3-2.7). In addition, crude Kaplan-Meier OS curves according to
the first-line chemotherapy regimen and study period are shown in
Fig. 5. The median OS for
gemcitabine, S-1, gemcitabine plus S-1, gemcitabine plus
nab-paclitaxel, and FOLFIRINOX was 5.9, 5.3, 7.7, 9.0, and 9.5
months, respectively, and the median OS according to study period
(A, B, C) was 6.2, 7.1, and 7.8 months, respectively.
Cox regression analyses
Cox regression analyses evaluating prognostic
factors for OS are presented in Table
IV. In a univariate analysis, age, PS, BMI, study period, and
first-line systemic therapy regimens were found to affect OS.
However, a multivariate analysis showed that study period did not
affect OS (P=0.989). Based on first-line systemic therapy, patients
who received gemcitabine plus nab-paclitaxel or FOLFIRINOX
demonstrated significantly longer survival times (with HRs of 0.622
and 0.608, respectively) than those who received gemcitabine
monotherapy.
 | Table IVStratified Cox regression analyses
evaluating prognostic factors for OS. |
Table IV
Stratified Cox regression analyses
evaluating prognostic factors for OS.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex | | | | |
|
Male
(Ref.) | 1.000 | [0.444] | 1.000 | [0.140] |
|
Female | 0.940
(0.802-1.102) | 0.444 | 0.883
(0.748-1.042) | 0.140 |
| Age, years | | | | |
|
<75
(Ref.) | 1.000 | [0.010] | 1.000 | [0.249] |
|
≥75 | 1.243
(1.053-1.466) | 0.010 | 1.109
(0.931-1.321) | 0.249 |
| PS | | | | |
|
0
(Ref.) | 1.000 | [<0.001] | 1.000 | [<0.001] |
|
1 | 1.195
(0.976-1.462) | 0.084 | 1.268
(1.031-1.559) | 0.025 |
|
≥2 | 2.154
(1.540-3.011) | <0.001 | 2.159
(1.526-3.054) | <0.001 |
|
Unknown | 1.523
(1.245-1.862) | <0.001 | 1.527
(1.218-1.915) | <0.001 |
| BMI,
kg/m2 | | | | |
|
≥18.5 and
<25.0 (Ref.) | 1.000 | [0.050] | 1.000 | [0.018] |
|
<18.5 | 0.866
(0.733-1.024) | 0.092 | 0.839
(0.707-0.996) | 0.045 |
|
≥25.0 | 1.205
(0.916-1.586) | 0.182 | 1.250
(0.942-1.657) | 0.122 |
| Primary disease
site | | | | |
|
Pancreas
head (Ref.) | 1.000 | [0.500] | 1.000 | [0.736] |
|
Pancreas
body | 0.885
(0.731-1.071) | 0.209 | 0.905
(0.743-1.101) | 0.318 |
|
Pancreas
tail | 1.032
(0.851-1.252) | 0.747 | 1.047
(0.859-1.277) | 0.647 |
|
Not
evaluable | 0.951
(0.626-1.444) | 0.813 | 0.961
(0.627-1.472) | 0.855 |
| Smoking status | | | | |
|
Never smoked
(Ref.) | 1.000 | [0.900] | 1.000 | [0.926] |
|
Current or
former | 1.046
(0.875-1.250) | 0.622 | 1.036
(0.850-1.261) | 0.729 |
|
Unknown | 1.046
(0.756-1.447) | 0.786 | 0.978
(0.704-1.360) | 0.896 |
| Study period | | | | |
|
Period A
(2010-2013) (Ref.) | 1.000 | [0.001] | 1.000 | [0.989] |
|
Period B
(2014-2016) | 0.858
(0.710-1.037) | 0.114 | 0.928
(0.754-1.142) | 0.482 |
|
Period C
(2017-2020) | 0.754
(0.623-0.912) | 0.004 | 0.948
(0.746-1.204) | 0.659 |
| Hospital
volume | | | | |
|
High volume
(n≥50) | 1.000 | [0.610] | 1.000 | [0.132] |
|
Low volume
(n<50) | 1.040
(0.889-1.221) | 0.610 | 0.872
(0.729-1.042) | 0.132 |
| Hospital type | | | | |
|
Government
designated cancer hospital (Ref.) | 1.000 | [0.522] | 1.000 | [0.187] |
|
Prefectural
designated cooperative cancer hospital | 1.118
(0.915-1.367) | 0.277 | 1.134
(0.922-1.394) | 0.235 |
|
General
hospital | 1.097
(0.897-1.342) | 0.367 | 0.952
(0.765-1.185) | 0.659 |
| First-line systemic
therapy | | | | |
|
Gemcitabine
(Ref.) | 1.000 | [<0.001] | 1.000 | [0.006] |
|
S-1 | 0.929
(0.762-1.133) | 0.466 | 0.839
(0.669-1.053) | 0.130 |
|
Gemcitabine
+ S-1 | 0.826
(0.613-1.113) | 0.209 | 0.829
(0.609-1.128) | 0.232 |
|
Gemcitabine
+ nab-paclitaxel | 0.592
(0.489-0.729) | <0.001 | 0.622
(0.480-0.806) | <0.001 |
|
FOLFIRINOX | 0.556
(0.387-0.799) | 0.002 | 0.608
(0.410-0.902) | 0.013 |
The details of treatment regimens with gemcitabine
plus nab-paclitaxel and FOLFIRINOX, are shown in Table V. Both regimens are recommended as
first-line therapy in the Japanese and NCCN guidelines (18,19).
In total, 11% of patients treated with gemcitabine plus
nab-paclitaxel crossed over to FOLFIRINOX, and 46% of patients
treated with FOLFIRINOX crossed over to a gemcitabine plus
nab-paclitaxel regimen. During the study period, erlotinib,
nal-irinotecan, pembrolizumab, and olaparib were not used for
subsequent systemic therapy in this population. The adjusted
Kaplan-Meier OS curves for each prognostic factor are shown in
Fig. 6, based on the results of
the stratified Cox regression analyses provided in Table IV.
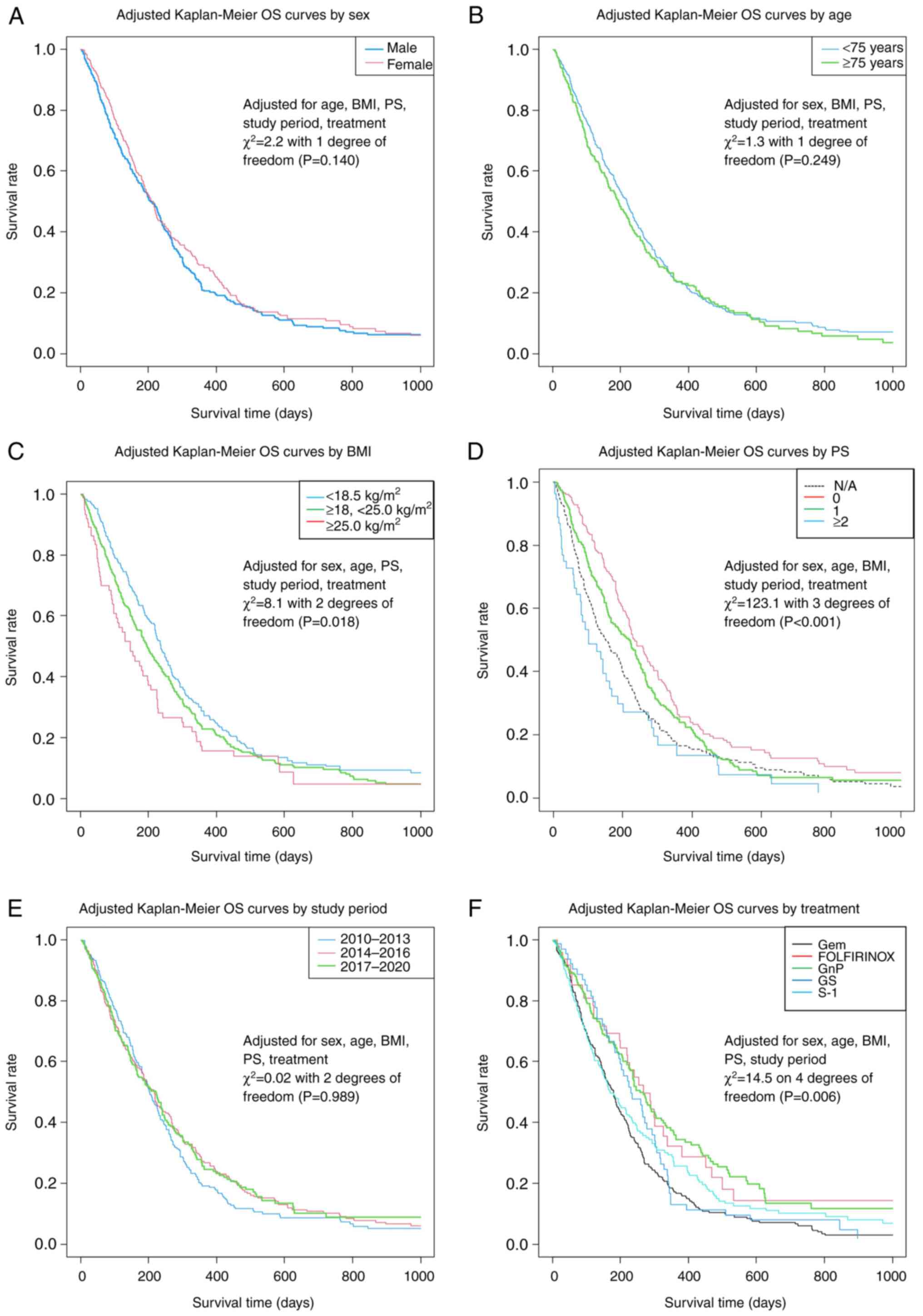 | Figure 6Adjusted OS curves based on
stratified Cox multiple regression analyses for patient groups
based on (A) sex, (B) age, (C) BMI, (D) PS, (E) study period and
(F) treatment. BMI, body mass index; FOLFIRINOX, fluorouracil,
folic acid, oxaliplatin and irinotecan; Gem, gemcitabine; GnP,
gemcitabine plus nanoparticle albumin-bounded-paclitaxel; GS,
gemcitabine + S-1; N/A, not available; OS, overall survival; PS,
performance status; S-1, tegafur/gimeracil/oteracil. |
 | Table VTreatment data for first-line
gemcitabine plus nab-paclitaxel and FOLFIRINOX regimens. |
Table V
Treatment data for first-line
gemcitabine plus nab-paclitaxel and FOLFIRINOX regimens.
|
Characteristics | Gemcitabine +
nab-paclitaxel (n=229) | FOLFIRINOX
(n=52) |
|---|
| Duration of
chemotherapy | | |
|
Median no.
of cycles (range) | 4 (1-28) | 6 (1-22) |
|
Median
duration, days (range) | 90 (2-748) | 76 (6-391) |
| Sequential surgical
procedure, n | | |
|
Yes | 2 | 0 |
| Sequential
radiotherapy, n | | |
|
Yes | 3 | 1 |
| Sequential systemic
therapy | | |
|
Median no.
(range) | 0 (0-4) | 1 (0-6) |
|
0, n
(%) | 123 (53.7) | 16 (30.8) |
|
1, n
(%) | 75 (32.8) | 19 (36.5) |
|
≥2, n
(%) | 31 (13.5) | 17 (32.7) |
| Sequential
regimens, n (%) | | |
|
Gemcitabine | 23 (10.0) | 5 (9.6) |
|
S-1 | 46 (20.1) | 6 (11.5) |
|
Gemcitabine
+ S-1 | 12 (5.2) | 1 (1.9) |
|
Gemcitabine
+ nab-paclitaxel | - | 24 (46.2) |
|
FOLFIRINOX | 25 (10.9) | - |
Discussion
In this large retrospective cohort study of patients
with metastatic pancreatic cancer, we clarified the actual state of
treatment in clinical practice in a large representative hospital
system. Although there is concern that treatment outcomes for the
portion of the patient population that fulfills the eligibility
criteria for clinical trials do not apply to older adults or the
clinical population of patients experiencing complications
(22), this RWD study demonstrated
that most patients could be administered standard treatment and
obtained survival benefits.
As of 2010, only gemcitabine and S-1 had been
approved for use in Japan, and consistent with previous Japanese
RWD study (30), nearly 80% of our
study population received gemcitabine. Although FOLFIRINOX became
available in 2013, it was not frequently used in our study
population; the use of this regimen remained at approximately 10%
through 2020. One of the reasons why FOLFIRINOX therapy is not
widely used is its serious adverse events, including
myelosuppression. A phase II study of FOLFIRINOX in Japan showed
that 77.8% of patients had Grade 3 or higher neutropenia and 22.2%
of patients had febrile neutropenia, which was much higher than the
45.7 and 5.4% in the global Phase III study (13). Therefore, it is recommended only
for selected patients in good general condition (19). On the contrary, since less toxic
gemcitabine plus nab-paclitaxel regimen became available in 2014,
the frequency of its use has increased rapidly, and the use of this
regimen in our study population exceeded 60% as of 2020. According
to a previous paper published by Terashima et al (31), as of 2015, the frequency of the use
of gemcitabine plus nab-paclitaxel was approximately 25%. According
to the latest clinical practice guidelines (18,19),
both gemcitabine plus nab-paclitaxel and FOLFIRINOX regimens were
recommended as first-line treatments for metastatic pancreatic
cancer. However, gemcitabine and S-1 were given weak
recommendations; these regimens were recommended only for patients
who are unsuitable for the aforementioned treatment regimens.
Moreover, gemcitabine plus S-1 was only recommended in the
neoadjuvant setting (19). Our
work provides a timely follow-up to previously reported data and
suggests that the treatment strategies selected by physicians in
actual clinical practice adhere closely to these guidelines.
Univariate and multivariate analyses in our study
showed a greater survival benefit of gemcitabine plus
nab-paclitaxel and FOLFIRINOX than that of gemcitabine alone. On
the other hand, no survival benefits of S-1 monotherapy or S-1 +
gemcitabine over gemcitabine monotherapy was demonstrated. These
results are consistent with the results of previous clinical trials
and RWD studies. The results of previous clinical trials and RWD
studies are shown in Table V. In
addition, our univariate analyses revealed a prolongation of OS in
the late study period. However, this effect was not confirmed by
multivariate analyses after adjusting for other prognostic factors,
such as treatment regimen. Furthermore, our univariate analyses did
not suggest a hospital volume-outcome relationship, unlike a
previous report from the Netherlands accounting for patients
diagnosed with metastatic pancreatic cancer between 2007 and
2011(30). Indeed, in our study,
the variances in chemotherapy regimens administered categorized by
hospital volume and type exhibited uniformity, with combination
therapy consistently accounting for approximately 40% (Table SI). These results suggested that
improvement in survival over time may be primarily due to the
approval of new treatment regimens, even though other factors such
as advances in diagnostic imaging and supportive care could also
have improved survival, and the widespread use of more effective
standard treatments may have reduced differences by hospital volume
and type.
In two prior pivotal phase III studies, the median
survival following the administration of FOLFIRINOX was 11.1 months
(13), while the median survival
following the administration of gemcitabine plus nab-paclitaxel was
8.5 months (16). A phase II study
on modified FOLFIRINOX, in which irinotecan was reduced from 180 to
150 mg/m2 and bolus 5-FU was omitted, showed favorable
results with a median survival of 11.2 months in 2018, and it is
now widely used in Japan (15). In
the present study, the median survival times following the
administration of gemcitabine plus nab-paclitaxel and FOLFIRINOX
were 9.0 and 9.5 months, respectively, which are comparable to the
median survival associated with gemcitabine plus nab-paclitaxel
reported in prior clinical trials but approximately 1.6 months
shorter than that associated with FOLFIRINOX (13,16).
No data were extracted on the dose of FOLFIRINOX in this study;
hence, there are no data on whether FOLFIRINOX or modified
FOLFIRINOX was administered or how withdrawal or dose reduction was
performed. In several recent RWD studies on gemcitabine plus
nab-paclitaxel and FOLFIRINOX in metastatic pancreatic cancer, OS
varied; moreover, OS tended to be shorter with FOLFIRINOX treatment
in RWD studies compared to that in clinical trials (31-37)
(Table VI). As mentioned above,
actual analysis of dose intensity is needed, but it is possible
that FOLFIRINOX dose reduction may have led to the shorter OS. We
await the results of the trials currently underway in Japan
comparing gemcitabine plus nab-paclitaxel, modified FOLFIRINOX, and
S-1 plus irinotecan and oxaliplatin for final conclusions (38).
 | Table VIOverall survival data from recent
clinical trials and RWD investigations evaluating treatment
regimens for advanced/metastatic pancreatic cancer. |
Table VI
Overall survival data from recent
clinical trials and RWD investigations evaluating treatment
regimens for advanced/metastatic pancreatic cancer.
| | Overall survival,
months | |
|---|
| First author/s,
year | No. of
patients | Disease status | Study design | 5-FU | S-1 | Gem | GE | GS | GnP | FOLFIRINOX |
P-valuea | (Refs.) |
|---|
| Burris et
al, 1997 | 126 | laPC; mPC | Phase III | 4.5 | - | 5.7 | - | - | - | - | 0.0025 | (9) |
| Ueno et al,
2005 | 19 | mPC | Phase II | - | 5.6 | - | - | - | - | - | - | (10) |
| Moore et al,
2007 | 569 | laPC; mPC | Phase III | - | - | 5.9 | 6.2 | - | - | - | 0.038 | (12) |
| Okusaka et
al, 2008 | 40 | mPC | Phase II | - | 9.2 | - | - | - | - | - | - | (11) |
| Conroy et
al, 2011 | 342 | mPC | Phase III | - | - | 6.8 | - | - | - | 11.1 | <0.001 | (13) |
| Ueno et al,
2013 | 835 | laPC; mPC | Phase III | - | 13.8 | 12.7 | - | 15.9 | - | - | 0.15b, <0.001c | (17) |
| Von Hoff et
al, 2013 | 861 | mPC | Phase III | - | - | 6.7 | - | - | 8.5 | - | <0.001 | (16) |
| Okusaka et
al, 2014 | 36 | mPC | Phase II | - | - | - | - | - | 10.7 | - | - | (14) |
| Ozaka et al,
2018 | 69 | mPC | Phase II | - | - | - | - | - | 11.2 | - | - | (15) |
| Terashima et
al, 2018 | 1,085 | laPC; mPC | RWD | - | 8.5 | 7.5 | 8.2 | 10.3 | 9.9 | 10.3 | - | (31) |
| Sasaki et
al, 2019 | 321 | mPC | RWD | - | - | - | - | - | 11.5 | 17.1 | - | (32) |
| Javed et al,
2019 | 1,056 | mPC | RWD | - | - | 4.9 | - | - | 7.9 | 9.9 | - | (33) |
| Cho et al,
2020 | 167 | mPC | RWD | - | - | - | - | - | 12.1 | 10.7 | 0.157 | (34) |
| Chan et al,
2020 | 1,130 | mPC | RWD | - | - | - | - | - | 6.1 | 8.2 | <0.0001 | (35) |
| Franco et
al, 2021 | 119 | mPC | RWD | - | - | - | - | - | 10.2 | 12.7 | 0.912 | (36) |
| Pijnappel et
al, 2021 | 1,586 | mPC | RWD | - | - | 2.9 | - | - | 4.7 | 6.6 | - | (37) |
| Present study | 846 | mPC | RWD | - | 5.3 | 5.9 | - | 7.7 | 9.0 | 9.5 | - | - |
The present study had several limitations. First,
this study had a retrospective design, and hence, the choice of the
first-line treatment regimen administered to each patient was left
to the attending physician, and no clear criteria have been
established yet. As shown in Table
II, the background of each regimen differs greatly. Therefore,
it is possible that FOLFIRINOX was selected by the attending
physician for patients with large tumors and extensive metastases,
resulting in a shorter OS. Second, as discussed before, although
dose intensity is a factor that may contribute significantly to OS,
data on dose intensity were not extracted in this analysis. Third,
several prognostic factors, such as metastatic sites, tumor
markers, prognostic scores based on laboratory data, comorbidities,
and complications, were not examined. Finally, in the current
study, treatment data were extracted from the protocol system;
hence, only data on treatment duration could be extracted, and it
was not possible to distinguish between treatment discontinuation
due to disease progression or adverse events (28). As this is an RWD study, periodic
imaging assessment were not performed, and descriptions of disease
progression were not mandatory. In addition, it is not possible to
extract data on subjective adverse events, because physicians are
not required to state their judgment of intolerance.
Allowing for these limitations, however, the
strength of this study is that we included a large population and
the evaluation of RWD for many treatment regimens that reflect
current population trends.
In conclusion, our RWD analyses demonstrated that a
standard care for metastatic pancreatic cancer was largely
available in hospitals across Japan and verified the survival
benefits of gemcitabine plus nab-paclitaxel and FOLFIRINOX regimens
observed in previous clinical trials. As such, our findings provide
important information for future research directions, policy
initiatives, medical guidelines, and clinical decision-making.
Supplementary Material
First-line chemotherapy by hospital
scale and type.
Acknowledgements
The authors acknowledge the dedication of Dr.
Hisaaki Afuso (chair of Medical Corporation Tokushukai, Tokyo,
Japan and General Incorporated Association Tokushukai, Tokyo,
Japan) for their support in conducting clinical research within the
Tokushukai Group, and Ms. Megumi Shiragami (software development
department, Tokushukai Information System, Inc., Osaka, Japan) and
Mr. Katsuhiko Ozaki (President of Tokushukai Information System,
Inc., Osaka, Japan) for their assistance in using the medical
database.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS, YI and MO made substantial contributions to the
study design and conception. RS, YF and MH were responsible for
data acquisition. RS and YI interpreted the data and drafted the
manuscript. KU, TM, KO, NS and HM provided advice on research
design and aided in the critical interpretation of this research
for critical content. RS and YI confirm the authenticity of all the
raw data. NS and HM comprehensively reviewed and approved the final
version of this manuscript. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
This project was approved by the Ethics Committee of
the Tokushukai Group (Tokyo, Japan) in April 2020 (no.
TGE01427-024) and was conducted following the principles of the
Declaration of Helsinki. Patients were provided with information
using opt-out methods and no patient declared not to
participate.
Patient consent for publication
Patient consent for publication was obtained through
opt-out methods.
Competing interests
Some of the authors have received research funding,
honoraria, or scholarship donations from various pharmaceutical
companies and other organizations outside of the submitted work,
none of which construe actual or potential conflicts of interest.
RS received speakers' bureau fees/honoraria from Daiichi-Sankyo,
Ono Pharm, Taiho Pharma and Chugai outside of the submitted work.
YI received speakers' bureau fees/honoraria from Bayer,
Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer and Ono Pharm outside
of the submitted work. HM has received speakers' bureau
fees/honoraria from Daiichi-Sankyo and Ono Pharm, research funding
from Astelas-Amgen Biopharma, Bayer, Bristol Myers Squibb, Chugai,
Daiichi-Sankyo, Incite, Novartis, Ono Pharm, Pfizer and
Rakuten-Medical, and scholarship donations from Bayer, Chugai,
Daiichi-Sankyo, Eisai, Kyowa-Kirin, Lilly, Ono Pharmaceutical,
Pfizer, Taiho Pharma and Takeda outside of the submitted work.
These organizations had no role in the design, conduct, or
reporting of this work. All other authors declare that they have no
competing interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cancer statistics. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html.
Accessed June 30 2022 Cancer Information Service, Natl Cancer Cntr,
Japan. Vital Statistics of Japan Ministry of Health, Labour and
Welfare.
|
|
3
|
Ben Q, Xu M, Ning X, Liu J, Hong S, Huang
W, Zhang H and Li Z: Diabetes mellitus and risk of pancreatic
cancer: A meta-analysis of cohort studies. Eur J Cancer.
47:1928–1937. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stolzenberg-Solomon RZ, Adams K, Leitzmann
M, Schairer C, Michaud DS, Hollenbeck A, Schatzkin A and Silverman
DT: Adiposity, physical activity, and pancreatic cancer in the
National Institutes of Health-AARP Diet and Health Cohort. Am J
Epidemiol. 167:586–597. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aune D, Greenwood DC, Chan DS, Vieira R,
Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ and Norat T:
Body mass index, abdominal fatness and pancreatic cancer risk: A
systematic review and non-linear dose-response meta-analysis of
prospective studies. Ann Oncol. 23:843–852. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Matsuo K, Ito H, Wakai K, Nagata C, Mizoue
T, Tanaka K, Tsuji I, Tamakoshi A, Sasazuki S, Inoue M, et al:
Cigarette smoking and pancreas cancer risk: An evaluation based on
a systematic review of epidemiologic evidence in the Japanese
population. Jpn J Clin Oncol. 41:1292–1302. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tramacere I, Scotti L, Jenab M, Bagnardi
V, Bellocco R, Rota M, Corrao G, Bravi F, Boffetta P and La Vecchia
C: Alcohol drinking and pancreatic cancer risk: A meta-analysis of
the dose-risk relation. Int J Cancer. 126:1474–1486.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Raimondi S, Lowenfels AB, Morselli-Labate
AM, Maisonneuve P and Pezzilli R: Pancreatic cancer in chronic
pancreatitis; aetiology, incidence, and early detection. Best Pract
Res Clin Gastroenterol. 24:349–358. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ueno H, Okusaka T, Ikeda M, Takezako Y and
Morizane C: An early phase II study of S-1 in patients with
metastatic pancreatic cancer. Oncology. 68:171–178. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Okusaka T, Funakoshi A, Furuse J, Boku N,
Yamao K, Ohkawa S and Saito H: A late phase II study of S-1 for
metastatic pancreatic cancer. Cancer Chemother Pharmacol.
61:615–621. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: Folfirinox versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Okusaka T, Ikeda M, Fukutomi A, Ioka T,
Furuse J, Ohkawa S, Isayama H and Boku N: Phase II study of
FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic
pancreatic cancer. Cancer Sci. 105:1321–1326. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M,
Uesugi K, Sata N, Miyashita K, Mizuno N, Tsuji K, et al: A phase II
study of modified folfirinox for chemotherapy-naïve patients with
metastatic pancreatic cancer. Cancer Chemother Pharmacol.
81:1017–1023. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
National Comprehensive Cancer Network
(NCCN): NCCN clinical practice guidelines in oncology pancreatic
adenocarcinoma. Version 1.2022. https://www.nccn.org/guidelines/category_1. Accessed
June 30, 2022.
|
|
19
|
Okusaka T, Nakamura M, Yoshida M, Kitano
M, Uesaka K, Ito Y, Furuse J, Hanada K and Okazaki K: Committee for
Revision of Clinical Guidelines for Pancreatic Cancer of the Japan
Pancreas Society. Clinical practice guidelines for pancreatic
cancer 2019 from the Japan Pancreas Society: A synopsis. Pancreas.
49:326–335. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang-Gillam A, Hubner RA, Siveke JT, Von
Hoff DD, Belanger B, de Jong FA, Mirakhur B and Chen LT: NAPOLI-1
phase 3 study of liposomal irinotecan in metastatic pancreatic
cancer: Final overall survival analysis and characteristics of
long-term survivors. Eur J Cancer. 108:78–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance olaparib for germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Averitt AJ, Weng C, Ryan P and Perotte A:
Translating evidence into practice: Eligibility criteria fail to
eliminate clinically significant differences between real-world and
study populations. NPJ Digit Med. 3(67)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hilgers RD, König F, Molenberghs G and
Senn S: Design and analysis of clinical trials for small rare
disease populations. J Rare Dis Res Treat. 1:53–60. 2016.
|
|
24
|
Latimer NR: Survival analysis for economic
evaluations alongside clinical trials-extrapolation with
patient-level data: Inconsistencies, limitations, and a practical
guide. Med Decis Making. 33:743–754. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Corrigan-Curay J, Sacks L and Woodcock J:
Real-world evidence and real-world data for evaluating drug safety
and effectiveness. JAMA. 320:867–868. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Katkade VB, Sanders KN and Zou KH: Real
world data: An opportunity to supplement existing evidence for the
use of long-established medicines in health care decision making. J
Multidiscip Healthc. 11:295–304. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Makady A, de Boer A, Hillege H, Klungel O
and Goettsch W: (on behalf of GetReal Work Package 1). What is
real-world data? A review of definitions based on literature and
stakeholder interviews. Value Health. 20:858–865. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shimoyama R, Imamura Y, Uryu K, Mase T,
Fujimura Y, Hayashi M, Ohtaki M, Ohtani K, Shinozaki N and Minami
H: Real-world outcomes of systemic therapy in Japanese patients
with cancer (Tokushukai REAl-world Data project: TREAD): Study
protocol for a nationwide cohort study. Healthcare (Basel).
10(2146)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
National Cancer Registry (Ministry of
Health, Labour and Welfare) tabulated by Cancer Information
Service, National Cancer Center, Japan. https://ganjoho.jp/med_pro/cancer_control/can_reg/index.html.
Accessed June 30, 2022.
|
|
30
|
Haj Mohammad N, Bernards N, Besselink MG,
Busch OR, Wilmink JW, Creemers GJ, De Hingh IH, Lemmens VE and van
Laarhoven HW: Volume matters in the systemic treatment of
metastatic pancreatic cancer: A population-based study in the
Netherlands. J Cancer Res Clin Oncol. 142:1353–1360.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Terashima T, Yamashita T, Sakai A, Ohta H,
Hinoue Y, Toya D, Kawai H, Yonejima M, Urabe T, Noda Y, et al:
Treatment patterns and outcomes of unresectable pancreatic cancer
patients in real-life practice: A region-wide analysis. Jpn J Clin
Oncol. 48:966–973. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sasaki T, Kanata R, Yamada I, Matsuyama M,
Ozaka M and Sasahira N: Improvement of treatment outcomes for
metastatic pancreatic cancer: A real-world data analysis. In Vivo.
33:271–276. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Javed MA, Beyer G, Le N, Vinci A, Wong H,
Palmer D, Morgan RD, Lamarca A, Hubner RA, Valle JW, et al: Impact
of intensified chemotherapy in metastatic pancreatic ductal
adenocarcinoma (PDAC) in clinical routine in Europe. Pancreatology.
19:97–104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cho IR, Kang H, Jo JH, Lee HS, Chung MJ,
Park JY, Park SW, Song SY, An C, Park MS and Bang S: Folfirinox vs
gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic
cancer: Single-center cohort study. World J Gastrointest Oncol.
12:182–194. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chan KKW, Guo H, Cheng S, Beca JM,
Redmond-Misner R, Isaranuwatchai W, Qiao L, Earle C, Berry SR,
Biagi JJ, et al: Real-world outcomes of FOLFIRINOX vs gemcitabine
and nab-paclitaxel in advanced pancreatic cancer: A
population-based propensity score-weighted analysis. Cancer Med.
9:160–169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Franco F, Camara JC, Martín-Valadés JI,
López-Alfonso A, Marrupe D, Gutiérrez-Abad D, Martínez-Amores B,
León A, Juez I, Pérez M, et al: Clinical outcomes of folfirinox and
gemcitabine-nab paclitaxel for metastatic pancreatic cancer in the
real world setting. Clin Transl Oncol. 23:812–819. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pijnappel EN, Dijksterhuis WPM, van der
Geest LG, de Vos-Geelen J, de Groot JWB, Homs MYV, Creemers GJ,
Mohammad NH, Besselink MG, van Laarhoven HWM, et al: First- and
second-line palliative systemic treatment outcomes in a real-world
metastatic pancreatic cancer cohort. J Natl Compr Canc Netw.
20:443–450.e3. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mizusawa J, Ohba A, Ozaka M, Katayama H,
Okusaka T, Kobayashi S, Ikeda M, Terashima T, Sasahira N, Okano N,
et al: Protocol of a randomized phase II/III study of gemcitabine
plus nab-paclitaxel combination therapy versus modified FOLFIRINOX
versus S-IROX for metastatic or recurrent pancreatic cancer:
JCOG1611 (GENERATE). Jpn J Clin Oncol. 53:80–84. 2023.
|