Introduction
Uveal melanoma (UM) is the most common primary
intraocular malignancy in adults (1,2), but
it is still considered a rare cancer with ~5-6 new cases per
million individuals per year worldwide (3,4).
According to the Collaborative Ocular Melanoma Study (COMS), the
precision of UM diagnosis has increased markedly from ~20 to
>99% in recent years (5).
Originating in the uveal tract of the eye, which comprises the
iris, ciliary body and choroid, this neoplasm poses not only a
significant risk to vision, but also a considerable metastatic
potential. The 5-year overall survival rate for metastatic UM is
80.9% (6,7). Despite advancements in diagnostic
methods and treatment modalities, survival rates have not
significantly improved over the past few decades.
The most common treatment options are surgical
interventions (such as local tumor resection and enucleation of the
eye) and radiation therapy (RT), including proton beam radiotherapy
(PBRT), photon (RT) radiotherapy (RT), stereotactic body radiation
therapy (SBRT), stereotactic radiosurgery and brachytherapy. These
treatments are often associated with varying degrees of success and
complications. Local recurrence is linked to shorter life
expectancy, highlighting the importance of the initial choice of
treatment (8). The treatment
choice depends on the extent of the primary process, the
availability and experience of treatment methods and patient
preferences.
Although plaque brachytherapy using ophthalmic
applicators and eye enucleation are the most available treatment
options for UM worldwide (9), PBRT
and SBRT are being increasingly employed. These methods have been
proven to be both effective and safe. Radiotherapy is the most
common eye globe-conserving therapy for UM. The COMS demonstrated
that radiation therapy with iodine-125 (125I) is as
effective as enucleation in preventing metastases (10). With SBRT, the 5-year local tumor
control (LC) rate was 92.2% and progression-free survival (PFS) was
77.0% (11). With CyberKnife
radiosurgical systems, the 5-year LC and PFS rate was 73.0 and
57.0%, respectively (12). PBRT
was determined to have a 5-year LC rate of 90% (13).
Numerous studies have shown that photon irradiation
delivers an adequate dose to the target area, similar to PBRT
(14,15), while achieving satisfactory
treatment results (16-18).
Long-term follow-up results for SBRT are more limited than those
for other radiotherapy modalities, although available studies
indicate similar rates of local control and distant metastatic
disease (19-21).
Case report
A 79-year-old female patient was regularly followed
up for macular degeneration and cataract of the left eye at an
external hospital. The patient had been diagnosed with a choroidal
nevus in 2016. The patient's medical history included a pigmented
nevus located nasally from the optic disc in the left eye and right
breast cancer (in 2015), which had been in remission for six years.
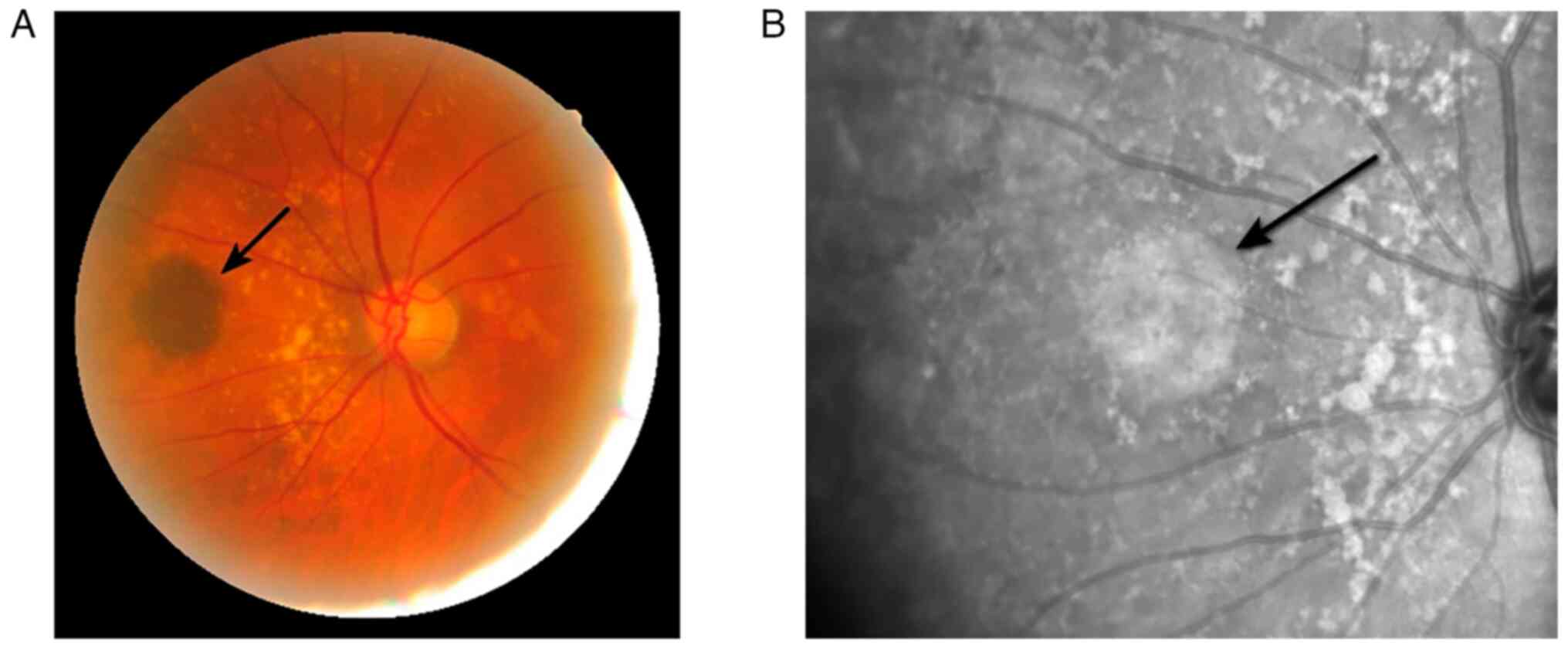
During ophthalmoscopy in 2016, a protruding lesion with sharp edges
measuring 7x9 mm and hyperpigmentation was discovered nasally from
the optic disc in the left eye (Fig.
1).
In October 2021, the patient came to our hospital
(European Medical Center, Moscow, Russia), and magnetic resonance
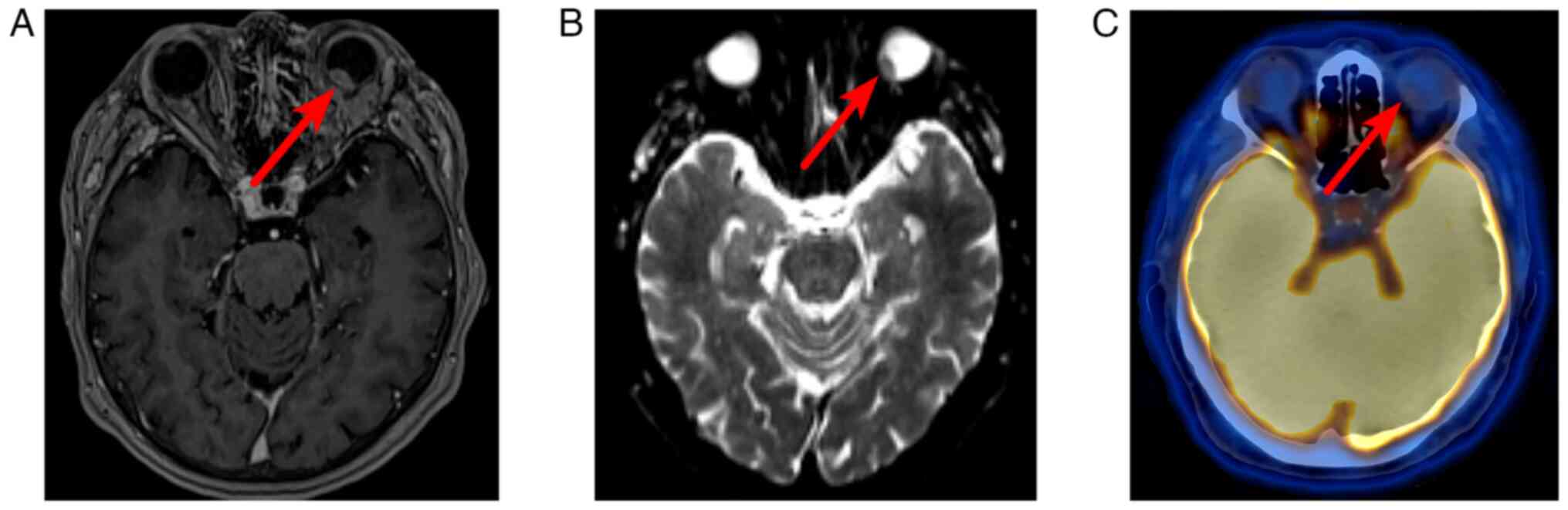
imaging (MRI) of the orbits revealed a tumor in the posterior part
of the left eyeball, adjacent to a wide base to the membranes of
the eye, measuring 12x7x9 mm, accumulating a contrast agent with
limited MR diffusion indicators (Fig.
2A and B).
Taking into account the history of right breast
cancer, whole-body positron emission tomography (PET/CT) with
18F-fluorodeoxyglucose (18FDG) was performed
to exclude distant metastatic lesions. A neoplasm was detected on
the posteromedial surface of the left eyeball with low accumulation
of radiopharmaceuticals (maximum standardized uptake volume, 2.52),
as shown in Fig. 2C.
Thus, based on ophthalmoscopy, MRI data, ultrasound,
18FDG PET/CT and the presence of a pigmented nevus in
the anamnesis, a diagnosis of choroidal melanoma cT2aN0M0 without
histological verification was established.
All treatment options and possible side effects were
discussed. The patient refused surgery and agreed to undergo RT.
Considering the diameter and thickness of the tumor, the patient's
refusal to undergo surgery and the unavailability of proton
therapy, an interdisciplinary meeting consisting of a medical
oncologist, radiation oncologist and ophthalmologist the decision
was held and it was decided to perform SBRT on the linear
accelerator Varian EDGE Radiosurgery system (Varian Medical System)
using the image-guided volumetric modulated arc therapy (IG-VMAT)
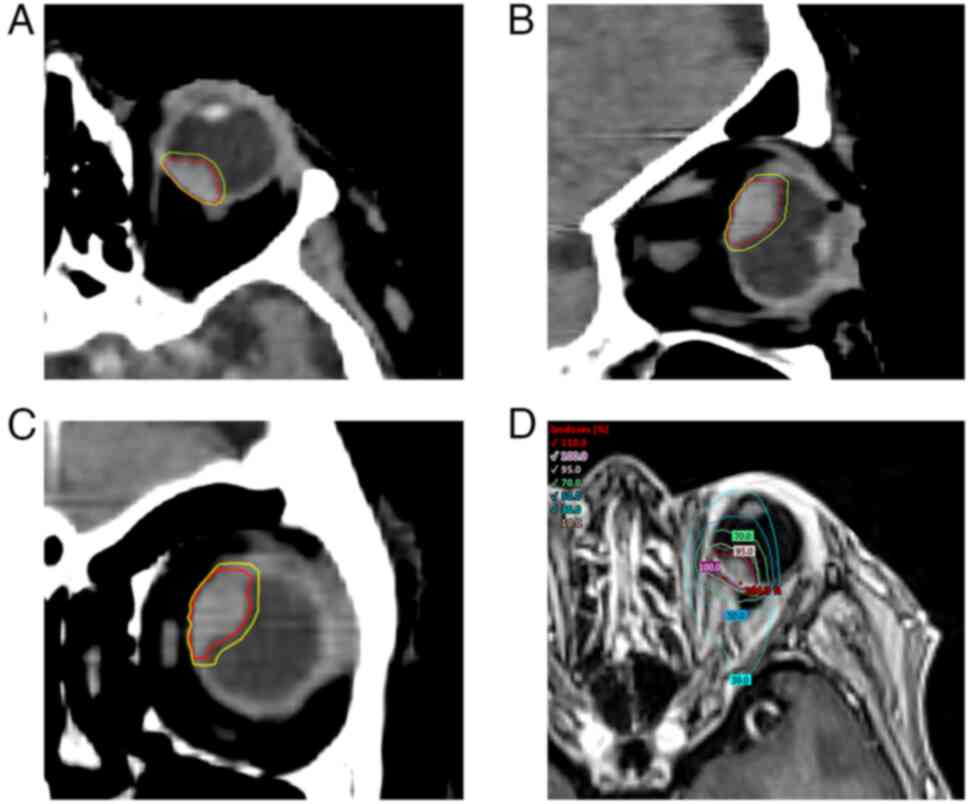
method. The delineation of the tumor in the three projections and
the dose distribution are shown in Fig. 3.
In January 2022, radiation therapy of the posterior
choroid of the left eye was administered with a single dose of 10.0
Gray (Gy) x5 fractions, adding up to a total dose of 50.0 Gy. The
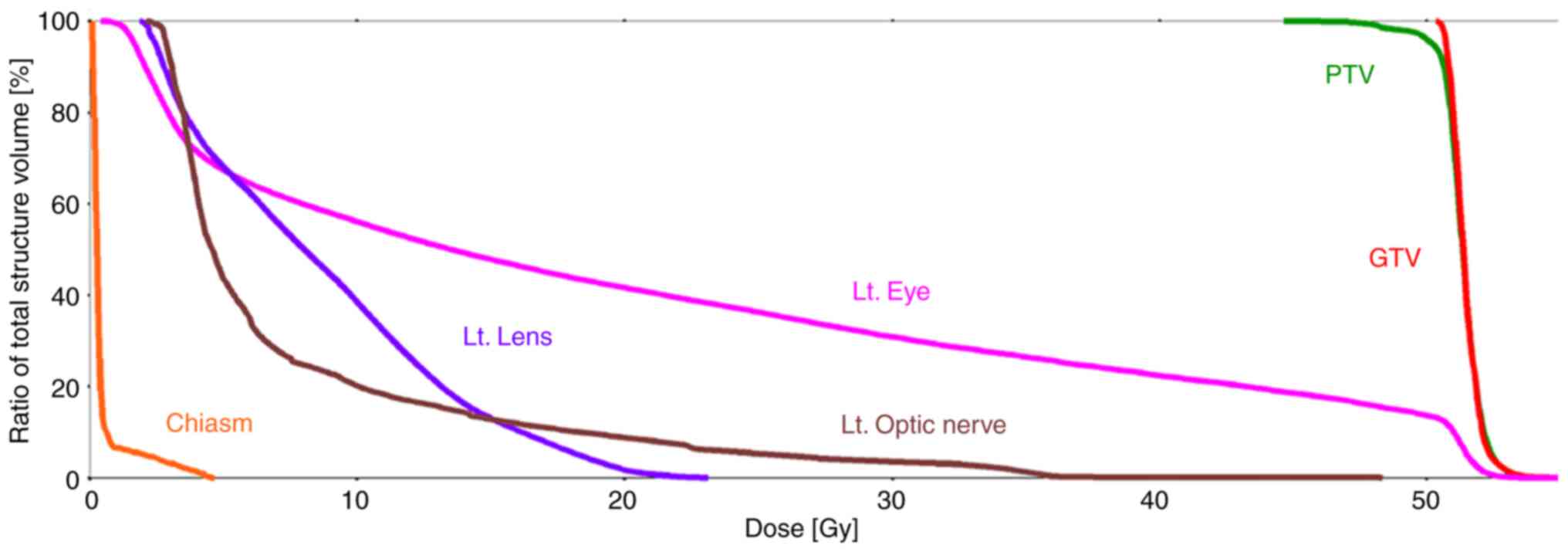
dose-volume histograms and dose distribution in the organs at risk
(OARs) plan evaluations are shown in Fig. 4 and Table I. The patient's head was then fixed
using a thermoplastic mask. By performing several CT simulations
with different views of the patient, the internal target volume was
formed, taking into account the possible amplitude of tumor
movement during treatment.
 | Table IDose distribution in the organs at
risk, GTV and PTV. |
Table I
Dose distribution in the organs at
risk, GTV and PTV.
| Item | Volume,
cm3 | Maximum dose
(<0.035 cm3), Gy | Mean dose, Gy |
|---|
| PTV | 1.0 | 52.3 | 51.3 |
| GTV | 0.6 | 52.3 | 51.4 |
| Lt. optic
nerve | 0.5 | 22.3 | 7.8 |
| Lt. lens | 0.2 | 23.1 | 8.7 |
| Lt. eye | 7.5 | 52.3 | 20.4 |
| Rt. optic
nerve | 0.4 | 0.2 | 0.1 |
| Rt. eye | 6.3 | 0.1 | 0.1 |
| Chiasm | 0.5 | 4.7 | 0.5 |
The main radiobiological effects of radiosurgery are
damage to the vascular endothelium and the subsequent apoptosis of
endothelial cells (22). This
radiobiological effect simultaneously reduces the activity of the
subretinal neovascular membrane. The next administration of an
anti-VEGF treatment (brolucizumab), which the patient had
previously taken regularly for the treatment of retinal dystrophy,
was required 10 months after irradiation. Ophthalmological
examination showed an improvement in visual acuity from 0.3 in
October 2021 to 0.8 in September 2022.
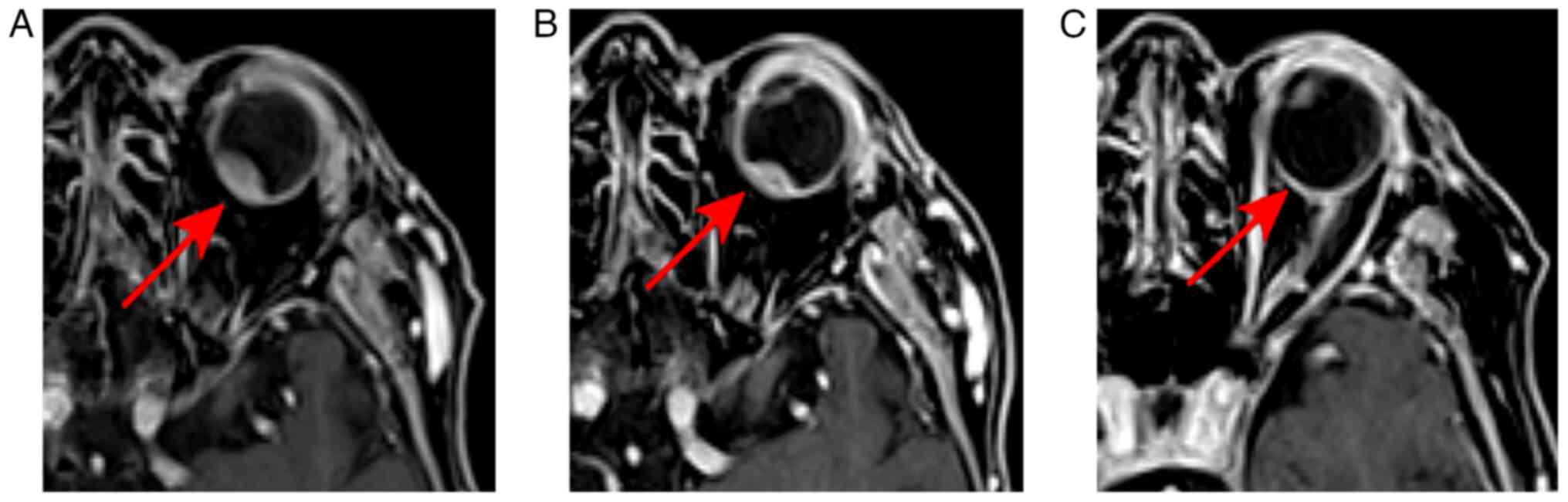
On control MRIs, a gradual decrease in tumor size
was observed, as shown in Fig. 5A.
A complete response was achieved 1 year after treatment (Fig. 5B).
No early or late radiation complications were noted
over the period of 1.5 years. No evidence of disease progression or
relapse was observed according to control MRI data from June 2023.
Currently, the patient is being actively monitored.
Discussion
The genetic profile of UM distinguishes it from
other tumor types, making the selection of systemic therapy
difficult. Although UM tumors exhibit a relatively low mutational
burden, they are characterized by certain recurrent mutations.
Typically, UM tumors possess an initiating mutation in either
guanine nucleotide-binding protein G(q) (GNAQ) or G protein subunit
alpha 11 (GNA11), followed by secondary mutations in genes such as
eukaryotic translation initiation factor 1A X-linked, splicing
factor 3b subunit 1, serine and arginine rich splicing factor 2 or
BRCA1 associated protein 1, which are the focus of most research
(23-25).
In particular, mutations in GNAQ and GNA11, present in >80% of
UM cases, have been the focus of targeted therapy research, with
inhibitors such as protein kinase C (PKC), mitogen-activated
protein kinase kinase inhibitors, and mesenchymal-epithelial
transition factor (MET) inhibitors. Most studies have shown either
limited effectiveness or ineffectiveness of therapies targeting
these inhibitors (26-28).
Crizotinib, an inhibitor of MET that is highly expressed in the UM,
has shown encouraging results in preclinical models. However, its
use as an adjuvant in patients with high-risk UM did not reduce the
relapse rates in a phase II trial and had numerous side effects
(29). Park et al (30) concluded in their study that UM
cells have complex, PKC-independent signaling pathways that
contribute to their survival and resistance to targeted therapies.
In addition, the preferentially expressed antigen in melanoma and
epigenetic modifications have emerged as novel biomarkers that can
potentially guide personalized treatment strategies (31). However, the heterogeneity within UM
subpopulations necessitates further investigation to determine the
efficacy of therapies that target these molecular aberrations. Of
note, there is no specific systemic therapy regimen for UM and most
studies have focused on metastatic disease.
According to the National Comprehensive Cancer
Network Guidelines v.1.2023, which divide UM according to tumor
size, the patient of the present study belongs to the second
category (32). The main treatment
methods for the second category of the disease are brachytherapy
and radiation therapy with protons or photons. The COMS randomized
trial noted no significant difference in survival rates in patients
with medium-sized UM after enucleation compared with
125I brachytherapy after a 15-year follow-up period
(10). PBRT is associated with a
minimal risk of local tumor recurrence in cases of UM. A
significant number of PBRT studies have demonstrated high LC rates
after treatment (33-35).
Despite the success of PBRT in the treatment of UM, there is a
problem with the availability of proton therapy for patients
worldwide. At the beginning of 2023, according to the Particle
Therapy Co-Operative Group data (36), 89 proton centers were used for the
treatment of diseases worldwide, including facilities in scientific
research institutes. Most of these are located in the USA-49,
Japan-19 and Germany-5. In this context, the advantage of SBRT is
its wide accessibility, based on linear accelerators commonly used
in the majority of RT departments.
Weber et al (14) conducted a comparative study of PBRT
and SBRT using the intensity-modulated radiation therapy (IMRT)
technique in the treatment of UM. The results showed that PBRT and
SBRT had similar dose distributions in the treatment of UM.
However, to achieve the treatment planning goal and dose
constraints of OARs organs at risk, the SBRT method requires a
large number of non-coplanar beams. Furthermore, the modern
radiotherapy-IMRT method did not improve the dose distribution
compared with single-field non-IMRT (14). VMAT is an improved version of IMRT,
which represents a sophisticated iteration of IMRT that involves
rotating one or more beams of radiation around the patient
(37).
IG-VMAT delivers a high-power, targeted dose of
radiation with minimal damage to the surrounding tissue (38). SBRT using VMAT has proven to be an
effective and safe method for treating both solid tumors and
metastases, regardless of their location in the body (39).
In SBRT, multiple photon beams converge on the tumor
from different directions, delivering a concentrated radiation dose
to the tumor and minimizing collateral damage to surrounding
healthy tissue. Jager et al (40) also consider photon radiation
therapy as the most acceptable treatment option for UM, taking into
account both the effectiveness and availability of the method. In a
study by Akbaba et al (41), the authors concluded that SBRT is
an effective treatment method for UM with a high level of local
control and a 2-year vision retention rate comparable to
brachytherapy or PBRT, even available in numerous radiation
oncology departments and easy to implement.
It is also worth noting that several adverse
reactions may occur during radiation therapy. The development of
cataracts is a common eye complication resulting from radiotherapy,
with risk factors including an overall dose exceeding 12 Gy and the
presence of anterior tumors; there is a 65-90% risk of cataract
development. After radiotherapy, maculopathy and optic nerve
neuropathy manifest in ~25 and 8-14% of patients, respectively, and
significantly impair visual acuity (42). It is crucial to recognize that
adverse events are associated with any treatment approach used for
UM (43,44). Tumor location, size, volume and
total radiation dose were the primary risk factors for these
adverse events. In the present case, we did no adverse events
related to early or late RT were observed.
In conclusion, the management of UM continues to
evolve, with an array of therapeutic modalities. Used for primacy.
SBRT using IG-VMAT has emerged as a potent, reliable and
efficacious method for treating UM, which was used in the patient
of the present study. Its flexibility, precision and capacity to
deliver high doses of targeted radiation while sparing surrounding
healthy tissues accentuate its prominence. In addition, SBRT's
broad accessibility due to the prevalence of linear accelerators in
most RT departments ensures that a larger patient demographic can
benefit from this state-of-the-art treatment. The results from our
case, coupled with the growing body of supportive literature,
suggest that SBRT may be an alternative to PBRT. As healthcare
professionals continue to prioritize both treatment efficacy and
patient quality of life, SBRT stands out as a viable,
eye-conserving and commonly available treatment for UM. Future
research with a larger cohort is essential to validate these
preliminary observations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All authors (NS, IL, AS, OL, IP and KT) contributed
to the study conception and design. Material preparation, data
collection and analysis were performed by NS, IL, AS and KT. The
first draft of the manuscript was written by IL and AS, and all
authors commented on previous versions of the manuscript. Images
were prepared by IL. NS and IL confirm the authenticity of all the
raw data. The final version of the manuscript was completed by KТ.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval for the study was obtained from the
Local Research Ethics Committee (European Medical Center, Moscow,
Russia) dated April 16, 2019.
Patient consent for publication
Written consent for publication of this case report
including all images was obtained from the patient.
Competing interests
The authors have no competing interests to
disclose.
References
|
1
|
Tarlan B and Kiratli H: Uveal melanoma:
Current trends in diagnosis and management. Turk J Ophthalmol.
46:123–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li Y, Shi J, Yang J, Ge S, Zhang J, Jia R
and Fan X: Uveal melanoma: Progress in molecular biology and
therapeutics. Ther Adv Med Oncol.
12(1758835920965852)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu Y, Lou L, Wang Y, Miao Q, Jin K, Chen M
and Ye J: Epidemiological Study of Uveal Melanoma from US
Surveillance, Epidemiology, and End Results Program (2010-2015). J
Ophthalmol. 2020(3614039)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Singh AD, Turell ME and Topham AK: Uveal
melanoma: Trends in incidence, treatment, and survival.
Ophthalmology. 118:1881–1885. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Collaborative Ocular Melanoma Study Group.
Histopathologic characteristics of uveal melanomas in eyes
enucleated from the collaborative ocular melanoma study. COMS
report No 6. Am J Ophthalmol. 125:745–766. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aronow ME, Topham AK and Singh AD: Uveal
melanoma: 5-Year update on incidence, treatment, and survival (SEER
1973-2013). Ocul Oncol Pathol. 4:145–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
American Cancer Society. Ocular melanoma.
Collaborative Ocular Melanoma Study (COMS) staging of melanoma of
the eye, 2018. Available online: https://www.cancer.org/cancer/types/eye-cancer/detection-diagnosis-staging/staging.html.
|
|
8
|
Egan KM, Ryan LM and Gragoudas ES:
Survival implications of enucleation after definitive radiotherapy
for choroidal melanoma: An example of regression on time-dependent
covariates. Arch Ophthalmol. 116:366–370. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Malouff TD and Trifiletti DM (eds):
Principles and practice of particle therapy. Wiley.
(pp204)2022.
|
|
10
|
Collaborative Ocular Melanoma Study Group.
The COMS randomized trial of iodine 125 brachytherapy for choroidal
melanoma: V. Twelve-year mortality rates and prognostic factors:
COMS report no. 28. Arch Ophthalmol. 124:1684–1693. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Beek JGM, van Rij CM, Baart SJ,
Yavuzyigitoglu S, Bergmann MJ, Paridaens D, Naus NC and Kiliç E:
Fractionated stereotactic radiotherapy for uveal melanoma:
Long-term outcome and control rates. Acta Ophthalmol. 100:511–519.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yazici G, Kiratli H, Ozyigit G, Sari SY,
Cengiz M, Tarlan B, Mocan BO and Zorlu F: Stereotactic radiosurgery
and fractionated stereotactic radiation therapy for the treatment
of uveal melanoma. Int J Radiat Oncol Biol Phys. 98:152–158.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Verma V and Mehta MP: Clinical outcomes of
proton radiotherapy for uveal melanoma. Clin Oncol (R Coll Radiol).
28:e17–e27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Weber D, Bogner J, Verwey J, Georg D,
Dieckmann K, Escudé L, Caro M, Pötter R, Goitein G, Lomax AJ and
Miralbell R: Proton beam radiotherapy versus fractionated
stereotactic radiotherapy for uveal melanomas: A comparative study.
Int J Radiat Oncol Biol Phys. 63:373–384. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sikuade MJ, Salvi S, Rundle PA, Errington
DG, Kacperek A and Rennie IG: Outcomes of treatment with
stereotactic radiosurgery or proton beam therapy for choroidal
melanoma. Eye (Lond). 29:1194–1198. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Muller K, Naus N, Nowak PJ, Schmitz PI, de
Pan C, van Santen CA, Marijnissen JP, Paridaens DA, Levendag PC and
Luyten GP: Fractionated stereotactic radiotherapy for uveal
melanoma, late clinical results. Radiother Oncol. 102:219–224.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dunavoelgyi R, Zehetmayer M, Gleiss A,
Geitzenauer W, Kircher K, Georg D, Schmidt-Erfurth U, Poetter R and
Dieckmann K: Hypofractionated stereotactic photon radiotherapy of
posteriorly located choroidal melanoma with five fractions at ten
Gy-clinical results after six years of experience. Radiother Oncol.
108:342–347. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Somani S, Sahgal A, Krema H, Heydarian M,
McGowan H, Payne D, Xu W, Michaels H, Laperriere N and Simpson ER:
Stereotactic radiotherapy in the treatment of juxtapapillary
choroidal melanoma: 2-Year follow-up. Can J Ophthalmol. 44:61–65.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Krema H, Heydarian M, Beiki-Ardakani A,
Weisbrod D, Xu W, Simpson ER and Sahgal A: A comparison between
125Iodine brachytherapy and stereotactic radiotherapy in
the management of juxtapapillary choroidal melanoma. Br J
Ophthalmol. 97:327–332. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Krema H, Heydarian M, Beiki-Ardakani A,
Weisbrod D, Xu W, Laperriere NJ and Sahgal A: Dosimetric and late
radiation toxicity comparison between iodine-125 brachytherapy and
stereotactic radiation therapy for juxtapapillary choroidal
melanoma. Int J Radiat Oncol Biol Phys. 86:510–515. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
van Beek JGM, Ramdas WD, Angi M, van Rij
CM, Naus NC, Kacperek A, Errington RD, Damato B, Heimann H and
Kiliç E: Local tumour control and radiation side effects for
fractionated stereotactic photon beam radiotherapy compared to
proton beam radiotherapy in uveal melanoma. Radiother Oncol.
157:219–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wijerathne H, Langston JC, Yang Q, Sun S,
Miyamoto C, Kilpatrick LE and Kiani MF: Mechanisms of
radiation-induced endothelium damage: Emerging models and
technologies. Radiother Oncol. 158:21–32. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Silva-Rodríguez P, Fernández-Díaz D, Bande
M, Pardo M, Loidi L and Blanco-Teijeiro MJ: GNAQ and GNA11 genes: A
comprehensive review on oncogenesis, prognosis and therapeutic
opportunities in uveal melanoma. Cancers (Basel).
14(3066)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu L, Zhou D, Zhang G, Ren Z, Luo X, Liu
P, Plouffe SW, Meng Z, Moroishi T, Li Y, et al: Co-occurrence of
BAP1 and SF3B1 mutations in uveal melanoma induces cellular
senescence. Mol Oncol. 16:607–629. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Decatur CL, Ong E, Garg N, Anbunathan H,
Bowcock AM, Field MG and Harbour JW: Driver mutations in uveal
melanoma: associations with gene expression profile and patient
outcomes. JAMA Ophthalmol. 134:728–733. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mergener S, Siveke JT and Peña-Llopis S:
Monosomy 3 is linked to resistance to MEK inhibitors in uveal
melanoma. Int J Mol Sci. 22(6727)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Khalili JS, Yu X, Wang J, Hayes BC, Davies
MA, Lizee G, Esmaeli B and Woodman SE: Combination small molecule
MEK and PI3K inhibition enhances uveal melanoma cell death in a
mutant GNAQ- and GNA11-dependent manner. Clin Cancer Res.
18:4345–4355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Leyvraz S, Konietschke F, Peuker C,
Schütte M, Kessler T, Ochsenreither S, Ditzhaus M, Sprünken ED,
Dörpholz G, Lamping M, et al: Biomarker-driven therapies for
metastatic uveal melanoma: A prospective precision oncology
feasibility study. Eur J Cancer. 169:146–155. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khan S, Lutzky J, Shoushtari AN, Jeter J,
Chiuzan C, Sender N, Blumberg LE, Nesson A, Singh-Kandah SV,
Hernandez S, et al: Adjuvant crizotinib in high-risk uveal melanoma
following definitive therapy. J Clin Oncol. 38 (15
Suppl)(S10075)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park JJ, Stewart A, Irvine M, Pedersen B,
Ming Z, Carlino MS, Diefenbach RJ and Rizos H: Protein kinase
inhibitor responses in uveal melanoma reflects a diminished
dependency on PKC-MAPK signaling. Cancer Gene Ther. 29:1384–1393.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ahmadian SS, Dryden IJ, Naranjo A, Toland
A, Cayrol RA, Born DE, Egbert PS, Brown RA, Mruthyunjaya P and Lin
JH: Preferentially expressed antigen in melanoma
immunohistochemistry labeling in uveal melanomas. Ocul Oncol
Pathol. 8:133–140. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
National Comprehensive Cancer Network
(NCCN) Guidelines Version 1.2023 Melanoma: Uveal. Available online:
https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1488.
|
|
33
|
Damato B, Kacperek A, Chopra M, Campbell
IR and Errington RD: Proton beam radiotherapy of choroidal
melanoma: The liverpool-clatterbridge experience. Int J Radiat
Oncol Biol Phys. 62:1405–1411. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hrbacek J, Mishra K, Kacperek A, Dendale
R, Nauraye C, Auger M, Herault J, Daftari IK, Trofimov AV, Shih HA,
et al: Practice patterns analysis of ocular proton therapy centers:
The international optic survey. Int J Radiat Oncol Biol Phys.
95:336–343. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Z, Nabhan M, Schild SE, Stafford SL,
Petersen IA, Foote RL and Murad MH: Charged particle radiation
therapy for uveal melanoma: A systematic review and meta-analysis.
Int J Radiat Oncol Biol Phys. 86:18–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Particle therapy facilities in clinical
operation. Particle Therapy Co-Operative Group (PTCOG). Available
online: https://www.ptcog.site/index.php/facilities-in-operation-public.
|
|
37
|
Otto K: Volumetric modulated arc therapy:
IMRT in a single gantry arc. Med Phys. 35:310–317. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Teoh M, Clark CH, Wood K, Whitaker S and
Nisbet A: Volumetric modulated arc therapy: A review of current
literature and clinical use in practice. Br J Radiol. 84:967–996.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Macchia G, Deodato F, Cilla S, Cammelli S,
Guido A, Ferioli M, Siepe G, Valentini V, Morganti AG and
Ferrandina G: Volumetric modulated arc therapy for treatment of
solid tumors: Current insights. Onco Targets Ther. 10:3755–3772.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jager M, Shields C..Cebulla C,
Abdel-Rahman MH, Grossniklaus HE, Stern MH, Carvajal RD, Belfort
RN, Jia R, Shields JA and Damato BE: Uveal melanoma. Nat Rev Dis
Primers. 6(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Akbaba S, Foerster R, Nicolay NH, Arians
N, Bostel T, Debus J and Hauswald H: Linear accelerator-based
stereotactic fractionated photon radiotherapy as an eye-conserving
treatment for uveal melanoma. Radiat Oncol. 13(140)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ataides FG, Silva SFBR and Baldin JJCMC:
Radiation-induced optic neuropathy: Literature review.
Neuroophthalmology. 45:172–180. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mishra KK, Daftari IK, Weinberg V, Cole T,
Quivey JM, Castro JR, Phillips TL and Char DH: Risk factors for
neovascular glaucoma after proton beam therapy of uveal melanoma: A
detailed analysis of tumor and dose-volume parameters. Int J Radiat
Oncol Biol Phys. 87:330–336. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pagliara MM, Tagliaferri L, Azario L,
Lenkowicz J, Lanza A, Autorino R, Caputo CG, Gambacorta MA,
Valentini V and Blasi MA: Ruthenium brachytherapy for uveal
melanomas: Factors affecting the development of radiation
complications. Brachytherapy. 17:432–438. 2018.PubMed/NCBI View Article : Google Scholar
|