Introduction
Immune checkpoint inhibitors (ICIs) have become a
standard of care for patients with metastatic non-small cell lung
cancer (NSCLC) (1). Immunotherapy
may be utilized alone in the first-line treatment if the NSCLC has
a high level of PD-L1 expression (2). Single-agent ICIs can also be used in
second and subsequent lines of NSCLC treatment, with different
drugs having different requirements for programmed cell
death-ligand 1 (PD-L1) status (3).
In addition, ICIs can be administered upfront in combination with
chemotherapy in both PD-L1-positive and -negative patients in the
first-line settings (2). However,
only a total of 20% of patients with NSCLC treated with
immunotherapy demonstrate a durable response (1,2).
There is an intensive search for reliable predictive markers
allowing clinically meaningful selection of patients for the
treatment with anti-PD-1/PD-L1 antibodies, thus avoiding
unnecessary adverse events and decreasing financial burden
(3).
Immunohistochemical (IHC) analysis of PD-L1
expression on tumor cells and/or tumor-infiltrating immune cells is
the only companion test approved for ICI therapy of metastatic
NSCLC (2). The indication was
based on the results of the study KEYNOTE-024(4), which showed improved progression-free
survival (PFS) and overall survival (OS) using anti-PD-1
monotherapy compared with standard chemotherapy in previously
untreated metastatic NSCLC expressing PD-L1 ≥50% (4). However, the predictive value of PD-L1
expression is controversial, as both positive and negative
predictive value of this marker is relatively low (5). Other biomarkers, such as tumor
mutation burden (TMB) or various gene expression signatures, have
shown promise, however their use requires sophisticated laboratory
tests and they have not been rigorously validated for
reproducibility (6,7). Therefore, there is an unmet need for
robust predictive markers for ICI efficacy in metastatic NSCLC.
Cancer-associated inflammation was suggested as one
of the crucial factors interacting with immune surveillance
(8,9). High neutrophil-to-lymphocyte ratio
(NLR) is a well-known peripheral blood marker of systemic
inflammation (10). Changes of the
NLR may be an indicator of immune response in patients with various
cancers, including metastatic NSCLC (10). Several studies have revealed that
increased NLR has a negative predictive value in patients with
metastatic NSCLC treated with ICIs (11,12).
Baseline and on-treatment clinical parameters, such as smoking
status, Eastern Cooperative Oncology Group performance status (ECOG
PS) and immune-related adverse events (irAEs), may also serve as
non-invasive and feasible predictive markers for ICIs. The use of
these indicators alone proved to have insufficient reliability for
personalizing ICI treatment (8).
The accuracy of the prediction of the ICI response may be increased
if other relevant parameters are taken into consideration (13). In the present study, the predictive
role of clinical and laboratory characteristics of patients with
NSCLC were evaluated and it was demonstrated that these are
predictive for a long-term outcome of the ICI therapy.
Materials and methods
Patients and data collection
The present retrospective study included 181
patients with metastatic NSCLC without epidermal growth factor
receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK)
rearrangements, who received single-agent ICI in the second or
subsequent lines of therapy. The median age at diagnosis was 65
years, with a range of 34-86 years. The ICI cohort consisted of 129
males (71.3%) and 52 females (28.7%). Patients were treated
according to standard clinical practice. These patients received at
least two doses of standard therapeutic regimens (pembrolizumab,
200 mg intravenously once every 3 weeks; nivolumab, 3 mg/kg every 2
weeks or 480 mg every 4 weeks; atezolizumab, 1,200 mg intravenously
once every 3 weeks). The therapy was performed until disease
progression, unacceptable toxicity, or for up to two years. ICI
predictive factors were also analyzed in the comparison group,
composed of 63 patients with metastatic NSCLC without activating
mutations in EGFR/ALK genes, who were treated by the first-line
standard platinum-based chemotherapy and did not receive ICIs in
subsequent treatment lines (comparison cohort). The median age was
63 years (range, 41-80 years). The latter cohort was collected to
determine whether the analyzed predictive factors are
ICI-specific.
All patients were treated between February 2017 and
September 2022 at the Pavlov First Saint Petersburg State Medical
University or at the N.P. Napalkov City Cancer Center (both at
Saint Petersburg, Russia). The investigation was approved by the
local Ethics Committee of Pavlov First Saint Petersburg State
Medical University (approval no. 312-2022). The study protocol
conformed to the ethical guidelines of the Declaration of Helsinki
and the ethical guidelines for medical and health research
involving human subjects. All participants signed informed consent
forms.
Inclusion criteria for both cohorts were as follows:
Age, 18 years and older; ECOG PS, 0-3(12); histologically confirmed NSCLC;
absence of EGFR mutations and ALK translocations; and stage IV. The
stage was classified based on the tumor node metastasis staging and
the American Joint Committee on Cancer (8th edition, 2017)
(14). Exclusion criteria for the
ICI group were as follows: Previous immunotherapy; concomitant
infection including human immunodeficiency virus or hepatitis;
therapy by systemic steroids; and previous or ongoing autoimmune
disease. Exclusion criteria for the chemotherapy cohort was the
presence of ICIs in subsequent lines of therapy.
Baseline demographics (age, sex, body mass index and
smoking status), clinical (ECOG PS, number of metastases and their
sites), morphological (histology and PD-L1 expression) and
treatment data (previous and subsequent type of treatment, line of
therapy and type of anti-PD-1/PD-L1 agents) were assessed. The main
characteristics of the patients are summarized in Table I. The tumor PD-L1 expression was
assessed as part of standard clinical practice and was evaluated in
114 patients with NSCLC in the ICI cohort by two IHC kits: Dako
PD-L1 clone 22C3 pharmDx (Agilent Technologies, Inc.) and Ventana
PD-L1 clone SP142 (Ventana Medical Systems, Inc.).
 | Table IMain baseline characteristics of
patients receiving ICIs and chemotherapy. |
Table I
Main baseline characteristics of
patients receiving ICIs and chemotherapy.
|
Characteristics | ICIs cohort
(n=181) | Comparison cohort
(n=63) |
|---|
| Sex, n (%) | | |
|
Male | 129 (71.3) | 43 (68.3) |
|
Female | 52 (28.7) | 20 (31.7) |
| Age (years), median
(IQR), n (%) | 65 (57-72) | 63 (54-70) |
|
<65 | 98 (54.1) | 37 (58.7) |
|
≥ 65 | 83 (45.9) | 26 (41.3) |
| ECOG PS | | |
|
0-1 | 147 (81.2) | 53 (84.1) |
|
≥2 | 34 (18.8) | 10 (15.9) |
| Smoking status | | |
|
Ever
smoking | 116 (64.1) | 38 (60.3) |
|
Never
smoking | 65 (35.9) | 25 (39.7) |
| Histological type,
n (%) | | |
|
Adenocarcinoma | 95 (52.5) | 26 (41.3) |
|
Squamous
cell carcinoma | 82 (45.3) | 36 (57.1) |
|
Other
NSCLCs | 4 (2.2) | 1 (1.6) |
| PD-L1 IHC
expression | | |
|
<1% | 30 (16.6) | 0 (0.0) |
|
≥1-49% | 66 (36.5) | 0 (0.0) |
|
≥50% | 18 (9.9) | 63 (100.0) |
|
No data | 67 (37.0) | |
| Line of therapy, n
(%) | | |
|
1 | 0 (0.0) | 63 (100.0) |
|
2 | 137 (74.7) | 0 (0.0) |
|
≥3 | 44 (24.3) | 0 (0.0) |
| Type of therapy, n
(%) | | |
|
Nivolumab | 92 (50.8) | 0 (0.0) |
|
Pembrolizumab | 76 (42.0) | 0 (0.0) |
|
Atezolizumab | 13 (7.2) | 0 (0.0) |
|
Platinum-based
doublet | 0 (0.0) | 63 (100.0) |
| ORR, n (%) | 41(23) | 17(27) |
| Median PFS, months
(95% CI) | 4.9 (4.2-5.6) | 4.6 (4.0-5.9) |
| Median OS, months
(95% CI) | 13.7
(11.5-17.2) | 10.4
(8.9-14.2) |
| Any irAEs, n
(%) | 71(39) | 0 (0.0) |
|
Any grade ≥3
irAE, n (% of total irAEs) | 12(17) | |
|
Thyroid
dysfunction | 25(35) | |
|
Skin
reactions | 17(24) | |
|
Pneumonitis | 8(11) | |
|
Hepatitis | 6(8) | |
The routine blood tests, including neutrophil and
lymphocyte count for subsequent calculation of the ratio, were
performed within one week before the start of therapy and after two
cycles of treatment.
Tumor response was defined using the Immune Response
Evaluation Criteria in Solid Tumors (iRECIST) (for ICIs cohort) and
RECIST 1.1 (for comparison cohort) (15). irAEs, which occurred during the
period of ICI administration, were also recorded. The severity of
irAEs was graded using the criteria of the National Cancer
Institute Common Terminology Criteria for Adverse Events version
5.0(16).
Statistical analysis
The endpoints of the study were objective response
rate (ORR), PFS and OS. ORR was defined as a proportion of patients
with complete and partial response according to iRECIST (for the
ICI cohort) or RECIST 1.1 (for the comparison cohort). PFS was
defined as the time from the start of ICI therapy to the
progression of disease or censored at the date of the last patient
contact or at the follow-up date. OS was defined as the time from
the first cycle of ICI treatment until death or the date of last
patient contact or the follow-up date.
Cut-off values were calculated using the receiver
operating characteristic (ROC) analysis. Fisher's exact test and
chi-squared test were used to compare qualitative parameters and
Mann-Whitney U test was applied to quantitative characteristics.
PFS and OS were compared between the groups using a log-rank test
with HR analysis and subsequent graphical visualization was
performed using the Kaplan-Meier method. Multivariate Cox
proportional hazard regression analysis was used to determine the
relationship between survival and different potential predictive
variables.
All statistical analyses were performed using
GraphPad Prism (v.9.3.1; GraphPad Software, Inc.; Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
Results
The median follow-up for patients receiving ICI was
13.6 months (95% CI, 11.5-16.1 months). The ORR was 41/181 (23%)
(Table I). Median PFS in patients
with NSCLC receiving ICI in the second or subsequent line of
therapy approached 4.9 months (95% CI, 4.2-5.6 months) and median
OS was 13.7 months (95% CI, 11.5-17.2 months) (Table I).
No significant association was observed between age,
sex, ECOG PS, smoking history, histological tumor type, PD-L1
expression or line of the therapy and ORR (Table II) However, some clinical
characteristics of the patients demonstrated statistically
significant associations with PFS or OS. Median PFS was
significantly longer in patients with good performance status (ECOG
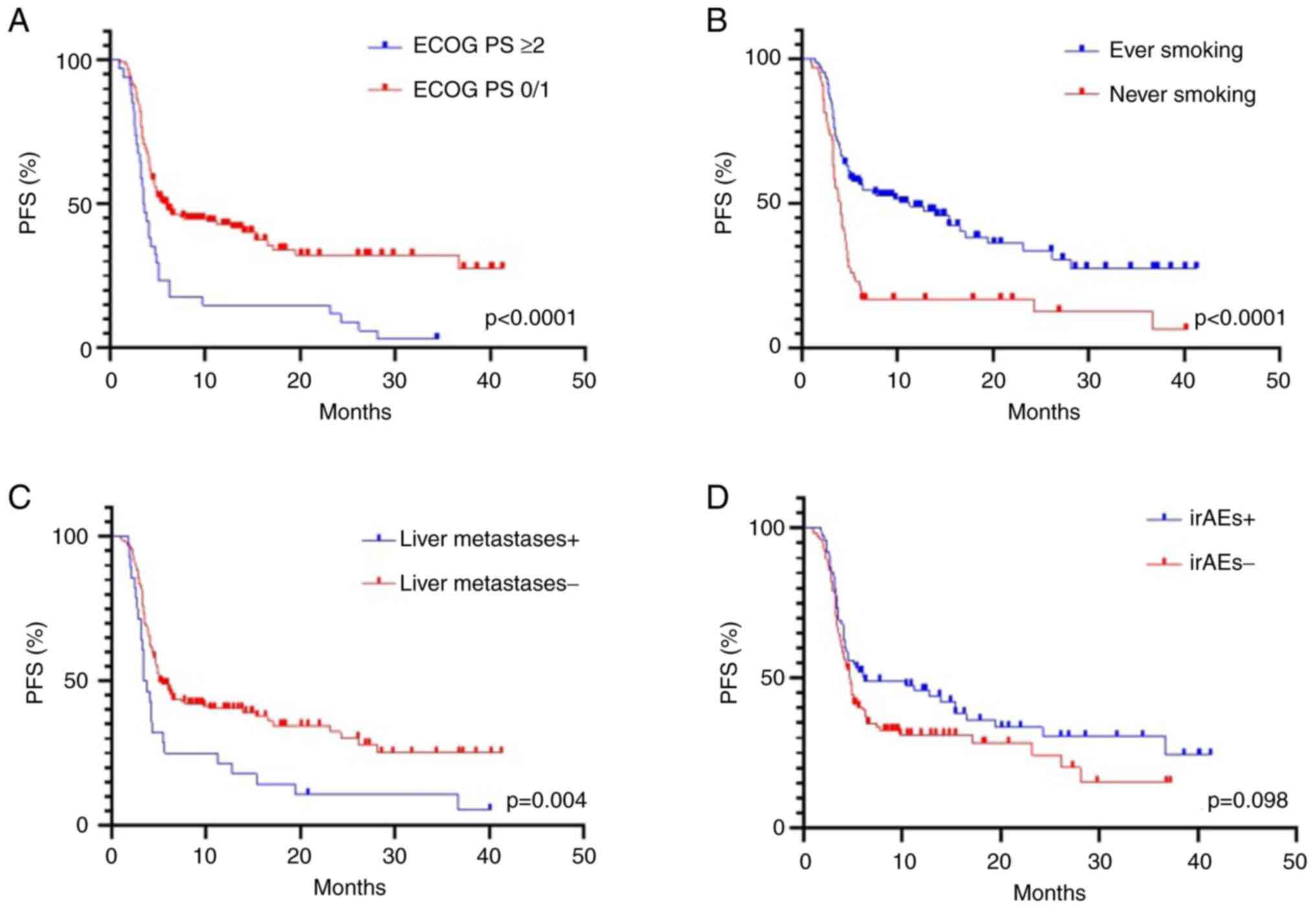
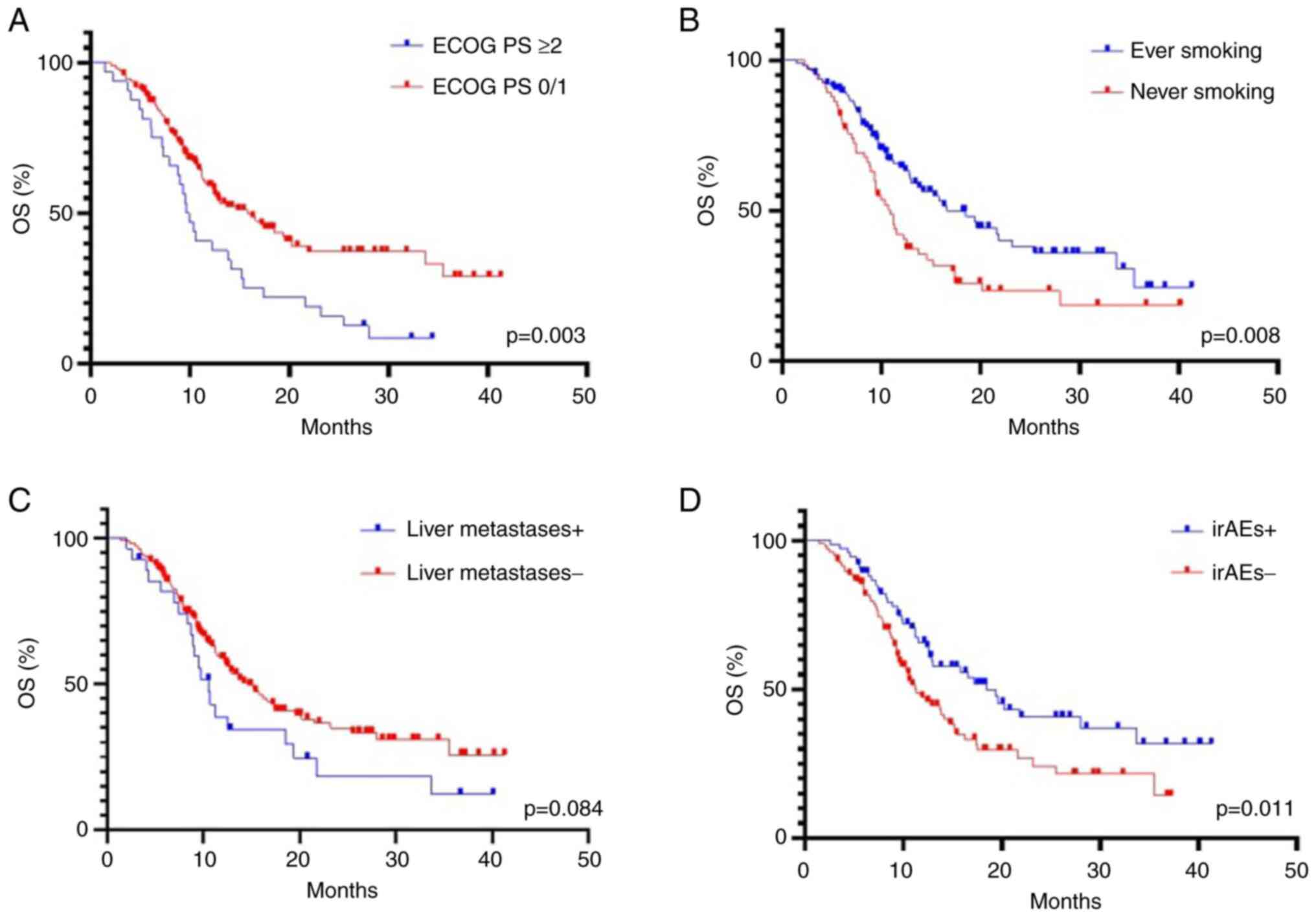
0/1 vs. 2/3: 6.0 vs. 3.6 months, respectively; P<0.0001)
(Fig. 1A). This association was
also maintained for OS (15.8 vs. 9.8 months, P=0.003) (Fig. 2A). The median PFS and OS were
significantly longer in ever-smokers compared with never-smokers
[PFS: 11.3 vs. 4.0 months, P<0.0001 (Fig. 1B); OS: 16.6 vs. 9.9 months, P=0.008
(Fig. 2B)]. Presence of liver
metastases was associated with shorter PFS [3.6 vs. 5.1 months,
P=0.004 (Fig. 1C)], however this
trend did not reach statistical significance for OS (P=0.084)
(Fig. 2C). In addition, no
relationship was observed between histological tumor type (squamous
cell carcinoma vs. non-squamous cell carcinoma) and PFS (P=0.239)
and OS (P=0.378) (data not shown).
 | Table IIORRs according to main
characteristics in patients receiving ICIs. |
Table II
ORRs according to main
characteristics in patients receiving ICIs.
|
Characteristics | ORR, % (N) | P-value |
|---|
| Age (<65 vs. ≥
65) | 24 (24/98) vs. 20
(17/83) | 0.594 |
| Sex (male vs.
female) | 24 (31/129) vs. 19
(10/52) | 0.560 |
| ECOG PS (0/1 vs.
≥2) | 25 (37/147) vs. 12
(4/34) | 0.113 |
| Smoking
(former/active vs. never) | 26 (30/116) vs. 17
(11/65) | 0.198 |
| Liver metastasis
(no vs. yes) | 24 (36/153) vs. 14
(4/28) | 0.329 |
| Histology
(non-squamous vs. squamous) | 26 (25/95) vs. 19
(16/86) | 0.286 |
| irAEs (yes vs.
no) | 28 (20/71) vs. 19
(21/110) | 0.203 |
| NLR (<4.3 vs.
≥4.3) | 27 (34/124) vs. 12
(7/57) | 0.034 |
| NLR after two
cycles (<4.5 vs. ≥4.5) | 27 (31/116) vs. 15
(10/65) | 0.098 |
| NLR dynamic changes
(<24% vs. ≥24%) | 26 (28/106) vs. 19
(14/75) | 0.284 |
| NSE score | | 0.002 |
|
Group 1 | 35 (27/77) | |
|
Group 2 | 17 (11/66) | |
|
Group 3 | 8 (3/38) | |
| ORR, objective
response rate; ICIs, immune checkpoint inhibitors; ECOG PS, Eastern
Cooperative Oncology Group performance status; irAEs,
immune-related adverse events; NLR, neutrophil-to-lymphocyte ratio;
NSE, NLR, smoking status, ECOG. | | |
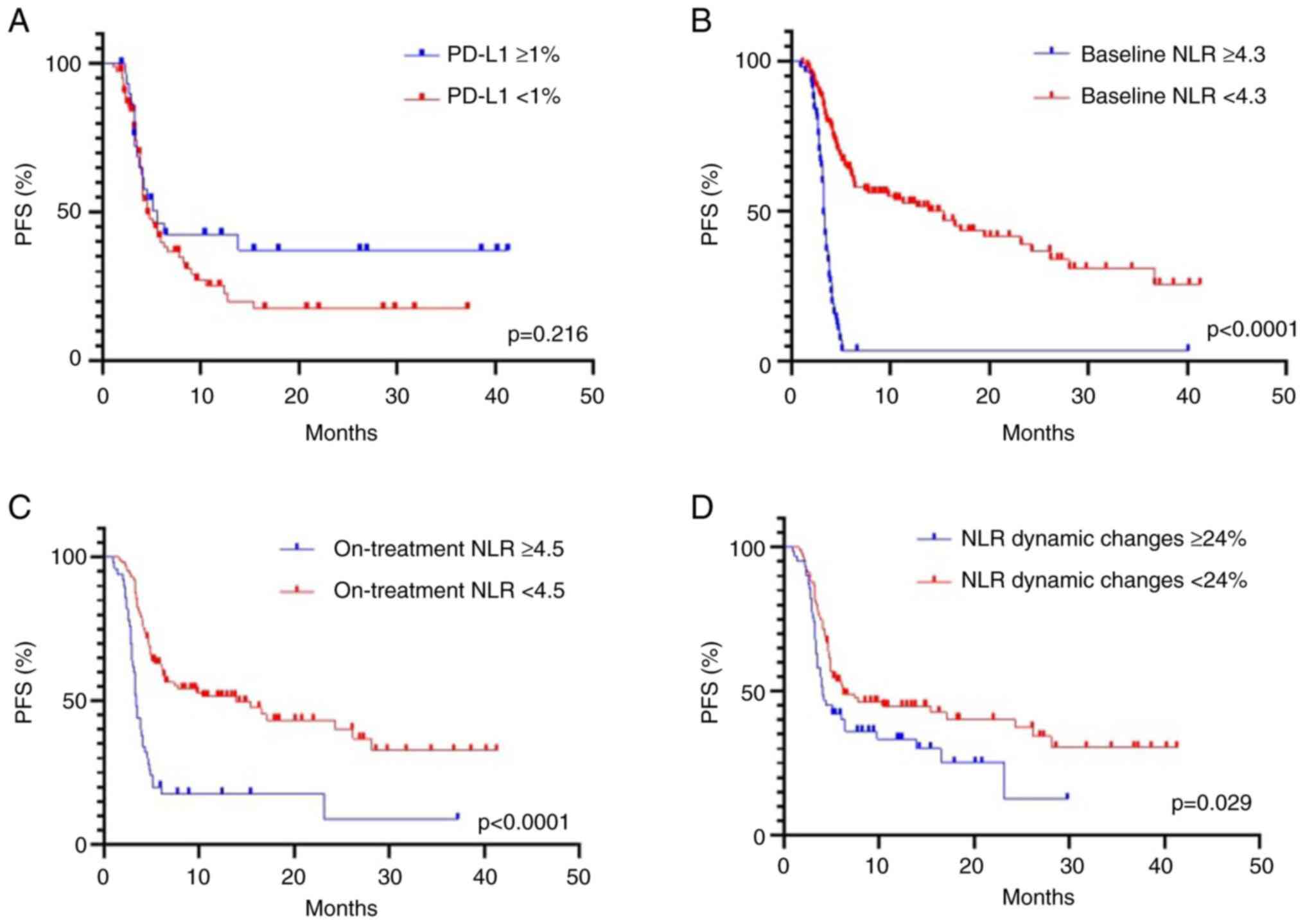
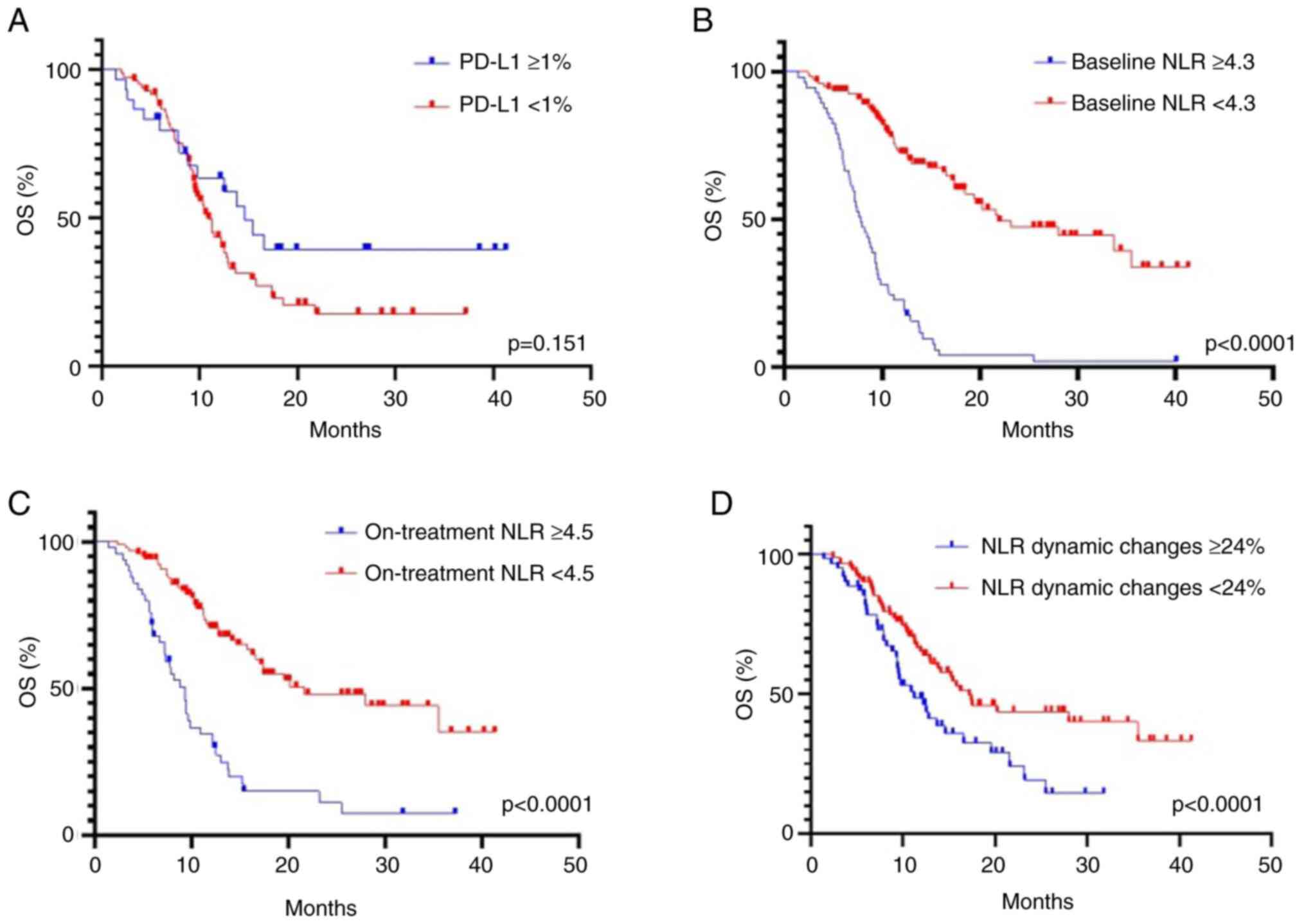
PD-L1 expression status was known for 114 patients
with patients with NSCLC treated by ICI therapy. The optimal
cut-off value for tumor PD-L1 expression was determined as ≥1% for
both PFS and OS. There was no statistically significant
relationship between PD-L1 expression ≥1% and longer PFS (5.6 vs.
4.1 months, HR 1.25; 95% CI, 0.89-1.83; P=0.216) (Fig. 3A) and OS (14.6 vs. 10.9 months; HR
1.33; 95% CI, 0.90-1.99; P=0.151) (Fig. 4A). Furthermore, stratification into
three categories of PD-L1 expression (<1%, ≥1-49% and ≥50%) did
not reveal a significant association with PFS (P=0.354) and OS
(P=0.413) (data not shown).
irAEs were observed in 71/181 (39%) patients with
NSCLC receiving second or subsequent lines of ICI (Table I). Grade ≥3 toxicity was detected
in 12/71 patients (17%) (Table I).
The most common irAEs were thyroid dysfunction [25/71 (35%)], skin
reactions [17/71 (24%)], pneumonitis [8/71 (11%)] and hepatitis
[6/71 (8%)] (Table I). The
presence of irAEs was associated with prolonged OS (18.5 vs. 11.3
months, P=0.011) (Fig. 2D), but
not with increased PFS (P=0.098) (Fig.
1D). In addition, no significant association was observed
between irAEs and ORR (Table
II).
NLR was significantly associated with the outcome of
ICI therapy. The highest predictive value was observed for the
threshold equal to 4.3, as determined by the ROC analysis. PFS was
significantly shorter in patients with baseline NLR ≥4.3 compared
with patients with NLR <4.3: (3.2 vs. 15.4 months, P<0.0001)
(Fig. 3B). The median of OS was
also evidently shorter in patients with the NLR ≥4.3 compared with
the NLR <4.3 group (7.8 vs. 21.8 months, P<0.0001) (Fig. 4B). The ORR was significantly higher
in patients with baseline NLR <4.3 compared with patients with
NLR ≥4.3: 27 and 12%, respectively (P=0.034) (Table II).
The cut-off value for NLR after two cycles was
determined as ≥4.5 for monitoring ICI efficacy. The NLR ≥4.5 after
two cycles was a predictor of worse PFS (3.4 vs. 13.9 months,
P<0.0001) (Fig. 3C) and OS (9.3
vs. 21.6 months, P<0.0001) (Fig.
4C). It was further investigated whether the dynamic change of
NLR (the difference between the ratio after two cycles and baseline
value) observed during ICI therapy had a predictive value. The
optimal cut-off level of the dynamic change of NLR was an increase
of ≥24%. The NLR dynamic changes of ≥24% were associated with
shorter PFS (4.0 vs. 6.3 months, P=0.029) (Fig. 3D) and OS (11.2 vs. 17.2 months,
P<0.0001) (Fig. 4D). The
improvement of NLR during ICI exposure tended to indicate a better
outcome, however none of the thresholds determined by ROC analysis
produced statistically significant associations. In addition, no
significant association was observed between NLR after two cycles,
NLR dynamic changes and the ORR (Table II). Univariate analysis of the
patient data demonstrated that ECOG PS ≥2 (HR, 1.98; 95% CI,
1.30-2.93; P=0.001), never smoking (HR, 1.91; 95% CI, 1.38-2.82;
P=0.001), presence of liver metastasis (HR, 1.42; 95% CI,
1.07-2.31; P=0.026), NLR ≥4.3 (HR, 4.79; 95% CI, 3.24-7.07;
P<0.0001) and NLR after two cycles of ICI (HR, 2.09; 95% CI,
1.43-3.01; P=0.0001) were significantly associated with worse PFS
(Table III). In multivariate
analysis, only ECOG PS ≥2 (HR, 2.09; 95% CI, 1.09-4.07; P=0.028),
never smoking status (HR, 3.53; 95% CI, 2.07-9.29; P=0.007) and
baseline NLR ≥4.3 (HR, 4.34; 95% CI, 2.65-7.03; P<0.0001)
retained significance for decreased PFS (Table III).
 | Table IIIUnivariate and multivariate analyses
for PFS using Cox proportional hazards regression model in ICIs
cohort. |
Table III
Univariate and multivariate analyses
for PFS using Cox proportional hazards regression model in ICIs
cohort.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| PFS | | | | |
| Age | 1.00
(0.98-1.02) | 0.962 | - | - |
| Sex (male vs.
female) | 0.73
(0.49-1.19) | 0.249 | - | - |
| BMI | 0.99
(0.94-1.03) | 0.605 | | |
| ECOG PS (≥2 vs.
0/1) | 1.98
(1.30-2.93) | 0.001 | 2.09
(1.09-4.07) | 0.028 |
| Smoking (never vs.
former/active) | 1.91
(1.38-2.82) | 0.001 | 3.53
(2.07-9.29) | 0.007 |
| Liver metastasis
(yes vs. no) | 1.42
(1.07-2.31) | 0.026 | 1.55
(0.92-2.54) | 0.083 |
| Brain metastasis
(yes vs. no) | 0.89
(0.44-1.62) | 0.731 | - | - |
| Bone metastasis (no
vs. yes) | 0.91
(0.58-1.50) | 0.709 | - | - |
| Metastatic sites (≥
2 vs. <2) | 1.12
(0.76-1.65) | 0.419 | - | - |
| Histology (squamous
vs. adenocarcinoma) | 1.19
(0.84-1.72) | 0.333 | - | - |
| Previous
radiotherapy (no vs. yes) | 1.22
(0.69-2.34) | 0.519 | - | - |
| Immunotherapy
[anti-PD-1 (pembrolizumab/nivolumab) vs. anti-PD-L1
(atezolizumab)] | 1.09
(0.66-1.71) | 0.718 | - | - |
| Line of therapy (2L
vs. 3L+) | 1.28
(0.80-1.92) | 0.305 | - | - |
| NLR (≥4.3 vs.
<4.3) | 4.79
(3.24-7.07) | <0.0001 | 4.34
(2.65-7.03) | <0.0001 |
| NLR after two
cycles (≥4.5 vs. <4.5) | 2.09
(1.43-3.01) | 0.0001 | 1.27
(0.84-1.91) | 0.252 |
| NLR dynamic changes
(≥24% vs. <24%) | 1.23
(0.85-1.76) | 0.259 | - | - |
| irAEs (no vs.
yes) | 1.36
(0.95-1.97) | 0.099 | - | - |
The univariate analysis for OS demonstrated that
ECOG PS ≥2 (HR, 2.09; 95% CI, 1.35-3.16; P=0.001), never smoking
(HR, 1.82; 95% CI, 1.23-2.69; P=0.003), absence of irAEs (HR, 1.69;
95% CI, 1.14-2.54; P=0.011), baseline NLR ≥4.3 (HR, 5.07; 95% CI
3.58-8.09; P<0.0001), NLR after two cycles of the therapy (HR
2.67; 95% CI, 1.78-3.96; P<0.0001) and NLR dynamic changes ≥24%
(HR, 1.66; 95% CI, 1.11-2.46; P=0.012) were negative predictive
factors (Table IV). However, in
multivariate analysis only ECOG PS ≥2 (HR, 2.02; 95% CI, 1.06-3.91;
P=0.035), never smoking (HR, 1.80; 95% CI, 1.21-2.68; P=0.004) and
baseline NLR ≥4.3 (HR, 4.89; 95% CI, 3.16-7.62; P<0.0001)
retained significance for OS (Table
IV).
 | Table IVUnivariate and multivariate analyses
for OS using Cox proportional hazards regression model in ICIs
cohort. |
Table IV
Univariate and multivariate analyses
for OS using Cox proportional hazards regression model in ICIs
cohort.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| OS | | | | |
| Age | 1.01
(0.99-1.03) | 0.417 | - | - |
| Sex (male vs.
female) | 0.78
(0.52-1.18) | 0.234 | - | - |
| BMI | 0.97
(0.92-1.02) | 0.218 | - | - |
| ECOG PS (≥2 vs.
0/1) | 2.09
(1.35-3.16) | 0.001 | 2.02
(1.06-3.91) | 0.035 |
| Smoking (never vs.
former/active) | 1.82
(1.23-2.69) | 0.003 | 1.80
(1.21-2.68) | 0.004 |
| Liver metastasis
(yes vs. no) | 1.48
(0.88-2.36) | 0.119 | - | - |
| Brain metastasis
(yes vs. no) | 0.91
(0.43-1.69) | 0.780 | - | - |
| Bone metastasis (no
vs. yes) | 0.81
(0.50-1.37) | 0.407 | - | - |
| Metastatic sites
(≥2 vs. <2) | 1.12
(0.76-1.65) | 0.559 | - | - |
| Histology (squamous
vs. adenocarcinoma) | 1.30
(0.73-2.59) | 0.409 | - | - |
| Previous
radiotherapy (no vs. yes) | 1.45
(0.79-2.98) | 0.266 | - | - |
| Immunotherapy
[anti-PD-1 (pembrolizumab/nivolumab) vs. anti PD-L1
(atezolizumab)] | 0.89
(0.49-1.50) | 0.629 | - | - |
| Line of therapy (2L
vs. 3L+) | 1.23
(0.72-1.98) | 0.419 | - | - |
| NLR baseline (≥4.3
vs. <4.3) | 5.07
(3.58-8.09) | <0.0001 | 4.89
(3.16-7.62) | <0.0001 |
| NLR after two
cycles (≥4.5 vs. <4.5) | 2.67
(1.78-3.96) | <0.0001 | 1.41
(0.58-2.93) | 0.398 |
| NLR dynamic changes
(≥24% vs. <24%) | 1.66
(1.11-2.46) | 0.012 | 1.59
(0.97-2.63) | 0.064 |
| irAEs (no vs.
yes) | 1.69
(1.14-2.54) | 0.011 | 1.63
(0.91-2.82) | 0.088 |
Baseline prognostic score
In multivariate analysis, the independent predictors
of both worse PFS and OS were pretreatment NLR ≥4.3, non-smoking
status and ECOG PS≥2. Based on the data, a baseline prognostic NLR,
smoking status, ECOG (NSE) score named after the first letters of
the included markers was developed (NLR at baseline ≥4.3, 2 points;
non-smoking status, 1 point; and ECOG ≥2, 1 point) (Table V). NSE score categorized three
groups of patients with NSCLC depending on the outcome of ICI
therapy: Good (group 1, 0 points); intermediate (group 2, 1-2
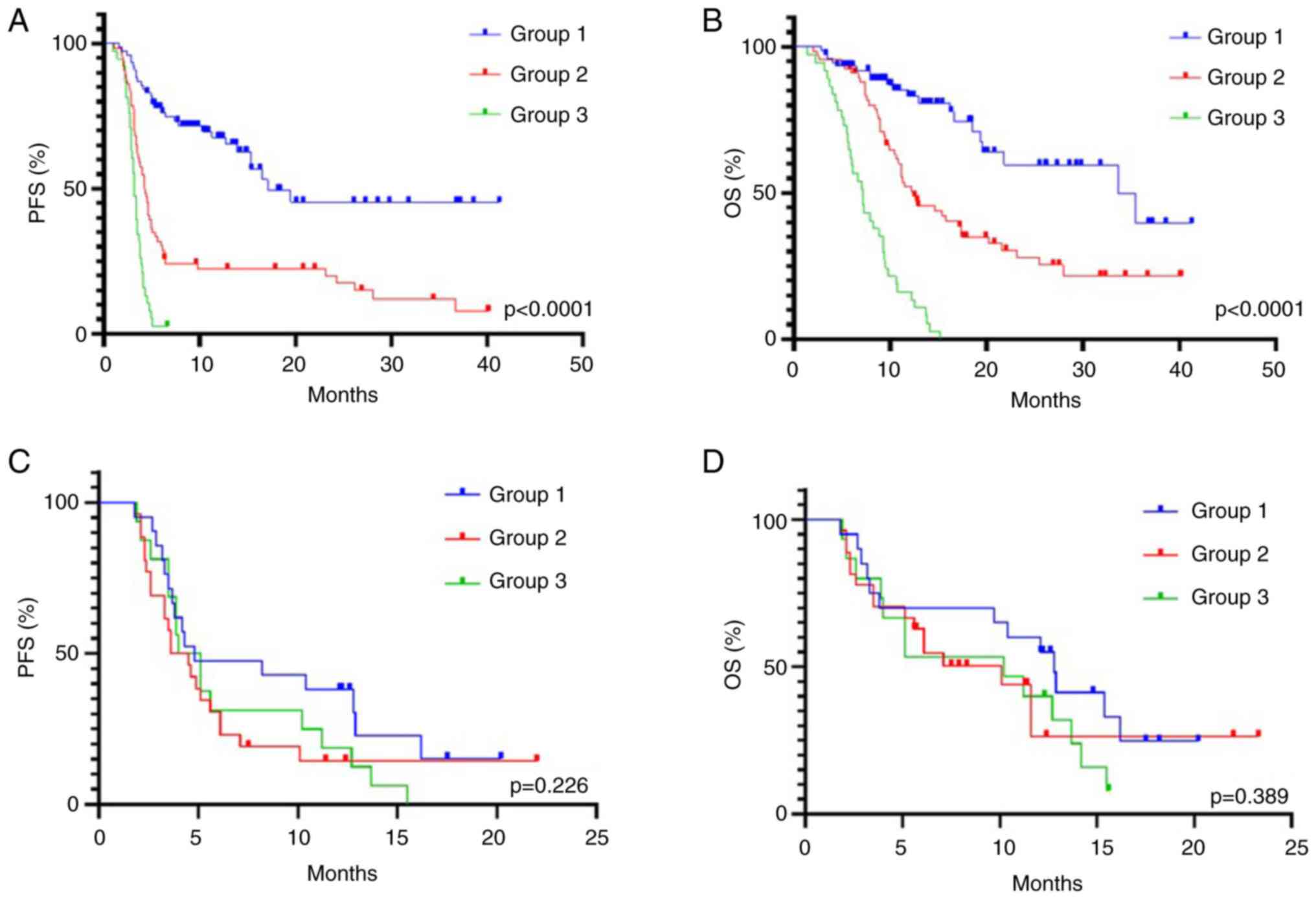
points) and poor (group 3, ≥3 points) (Table V). Among patients receiving ICIs,
77 patients (43%) belonged to the NSE group 1, 66 (36%) to group 2
and 38 (21%) to group 3. The ORR in good, intermediate and poor
prognostic groups was 35, 17 and 8%, respectively (P=0.002)
(Table II). The median PFS for
group 1 was 17.1 months, and for groups 2 and 3 it was 4.3 and 3.2
months, respectively (P<0.0001) (Fig. 5A). The median OS was 33.7, 12.2 and
7.2 months, respectively (P<0.0001) (Fig. 5B). Strong differences between these
groups retained significance upon multivariate analysis (Table VI). In addition, no significant
differences were revealed between the type of ICI (pembrolizumab,
nivolumab or atezolizumab) and the endpoints of the study in each
prognostic group (data not shown).
 | Table VNSE predictive scoring system and
risk stratification. |
Table V
NSE predictive scoring system and
risk stratification.
| Predictive
factor | Value | Points |
|---|
| NLR at
baseline | ≥4.3 | 2 |
| | <4.3 | 0 |
| Smoking status | Never | 1 |
| | Ever | 0 |
| ECOG PS | ≥2 | 1 |
| | 0-1 | 0 |
 | Table VIMultivariate analysis for PFS and OS
according to NSE score groups. |
Table VI
Multivariate analysis for PFS and OS
according to NSE score groups.
| | PFS | OS |
|---|
| NSE score | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Group 1 | 1 (Reference) | | 1 (Reference) | |
| Group 2 | 3.09
(1.94-4.99) | <0.0001 | 2.61
(1.49-4.32) | 0.0003 |
| Group 3 | 7.31
(3.95-11.68) | <0.0001 | 6.99
(3.89-10.03) | <0.0001 |
Chemotherapy cohort
Median follow-up was 15.3 months (95% CI, 11.3-20.2
months) in the chemotherapy cohort. The ORR was 27% in this group
of patients. Median PFS and OS were 4.6 months (95% CI, 4.0-5.9
months) and 10.4 months (95% CI, 8.9-14.2 months), respectively
(Table I).
No significant association was observed between
clinical parameters, peripheral blood markers (baseline NLR, NLR
after two cycles and NLR dynamic changes) and treatment efficacy
(ORR, PFS and OS) in patients receiving chemotherapy (Table VII).
 | Table VIIUnivariate analysis for PFS and OS
using Cox proportional hazards regression model in a chemotherapy
cohort. |
Table VII
Univariate analysis for PFS and OS
using Cox proportional hazards regression model in a chemotherapy
cohort.
| | PFS | OS |
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.09
(0.86-1.34) | 0.545 | 1.04
(0.86-1.32) | 0.704 |
| Sex (male vs.
female) | 1.15
(0.66-2.32) | 0.694 | 1.09
(0.46-1.74) | 0.781 |
| ECOG PS (≥2 vs.
0/1) | 1.25
(0.85-1.75) | 0.232 | 1.42
(0.90-1.81) | 0.131 |
| Smoking (never vs.
former/active) | 0.74
(0.44-1.30) | 0.292 | 0.77
(0.58-1.21) | 0.176 |
| Liver metastasis
(yes vs. no) | 1.44
(0.70-2.94) | 0.319 | 1.31
(0.68-2.98) | 0.476 |
| Bone metastasis (no
vs. yes) | 0.78
(0.63-1.42) | 0.345 | 0.64
(0.58-1.63) | 0.254 |
| Metastatic sites
(≥2 vs. <2) | 1.32
(0.81-1.86) | 0.249 | 1.22
(0.76-2.14) | 0.312 |
| Histology (squamous
vs. adenocarcinoma) | 1.16
(0.67-1.98) | 0.582 | 1.20
(0.76-2.04) | 0.483 |
| NLR baseline (≥3.1
vs. <3.1) | 1.30
(0.86-1.91) | 0.250 | 1.54
(0.88-2.82) | 0.151 |
| NLR after two
cycles (≥3.9 vs. <3.9) | 1.27
(0.77-2.11) | 0.346 | 1.37
(0.84-2.32) | 0.219 |
| NLR dynamic changes
(≥15% vs. <15%) | 1.64
(0.60-3.45) | 0.344 | 1.28
(0.68-2.38) | 0.476 |
| NSE score (group 1
vs. group 2/3) | 1.28
(0.83-1.98) | 0.276 | 1.25
(0.76-2.02) | 0.387 |
It was also addressed whether NSE score as
aforementioned is predictive for benefit from cytotoxic drugs. In
the chemotherapy cohort according to the NSE score, a total of 20
patients (32%) were assigned to group 1, 27 (42.9%) to group 2 and
16 (25%) to group 3 (data not shown). No difference was observed in
terms of response rate according to scoring groups. The PFS and OS
were similar in these groups [PFS: 4.8, 4.1 and 4.6 months,
respectively; P=0.226 (Fig. 5C);
OS: 12.8, 10.2 and 10.1 months, respectively; P=0.389 (Fig. 5D)].
Discussion
In the present study the predictive role of certain
clinical characteristics and peripheral blood markers on efficacy
to ICIs was investigated. ECOG performance status, smoking history,
presence of liver metastasis, irAEs and NLR demonstrated
associations with outcomes in metastatic patients with NSCLC
receiving single-agent ICI in second and subsequent lines of
therapy. Additionally, a combination of NLR, ECOG and smoking
history yielded an accurate prediction of ICI therapy outcome. This
combination was not predictive in the patients, who received
chemotherapy without subsequent ICI administration. The comparison
of these two groups is compromised by the fact that ICI treatment
was applied in second or subsequent lines of therapy, while
cytotoxic drugs were utilized upfront. However, it is considered
that this approach is optimal to minimize the role of confounding
factors. Notably, only a minority of patients with NSCLC receive
ICI therapy without chemotherapy in the first line and the
selection of these cases is based on strict criteria, thus, it was
not possible to collect a substantial number of subjects in this
setting. However, the responses of patients with NSCLC to
chemotherapy in the second or third lines of treatment are usually
minimal, thus this analysis was intentionally limited to the first
line platinum-based doublets.
The investigation did not demonstrate that PD-L1
expression status was significant, although some patients did not
undergo PD-L1 testing and two different IHC assays were used for
the PD-L1 analysis. These results were consistent with studies,
which utilized single-agent nivolumab or atezolizumab after failure
of cytotoxic therapy (17,18). However, a clinical trial revealed
that patients with NSCLC with PD-L1 expression in >1% of tumor
cells had improved treatment outcomes (19). A meta-analysis involving 3,688
patients from seven randomized trials, who were subjected to the
second or subsequent lines of therapy and were evaluated for OS,
revealed that ICIs outperform cytotoxic drugs across all PD-L1
expression subgroups (20). At the
same time, the most marked effect from ICIs was observed for NSCLCs
expressing PD-L1 in >50% of tumor cells (20).
In a number of studies, the immunotherapy survival
benefit was shown to be associated with the anatomic location of
metastasis (21). The liver has an
immunosuppressive microenvironment, thus the presence of metastases
in this organ may influence the efficacy of the ICI (22). A recent meta-analysis has shown
that metastatic involvement of the liver was associated with worse
PFS in patients with NSCLC, although it did not result in shorter
OS (23). The results of the
present study provided some support for the aforementioned
findings. Another clinical parameter, that may serve as a potential
predictor of ICI efficacy, is the presence of irAEs. The rationale
is that irAEs are caused by hyperactivation of the immune system
during ICI therapy and, hence, are indirectly associated with
antitumor response (24).
Similarly, to previous studies (25,26),
the presence of irAEs in the data of the present study was
associated with prolonged OS. However, this association was not
retained upon multivariate analysis.
The contribution of ECOG performance status in
determining treatment outcome is well established. Patients with
poor overall condition usually have compromised ‘defense’
mechanisms. In addition, the development of antitumor immune
response may take time, which is another factor affecting outcomes
in patients with short life expectancy (27). ECOG PS ≥2 was an independent
predictor of short PFS and OS in the present study, which is
consistent with other studies (20,28).
Smoking history is a particularly relevant
predictive marker for ICI response in patients with NSCLC.
Cigarette consumption is associated with high TMB and consequently,
increased production of tumor neoantigens (29). The data of the present study on
improved ICI treatment outcomes in smokers are consistent with the
results of meta-analysis and other individual studies (30-32).
NLR is a surrogate marker of chronic inflammation
(33). Neutrophils in the
peripheral blood represent precursors of immunosuppressive cells
(tumor-associated neutrophils and granulocytic myeloid-derived
suppressor cells) in the tumor microenvironment, which promote
tumor progression (11,34). In turn, lymphocytes are responsible
for cellular immune response (11). A high NLR ratio is a predictor of
worse prognosis in NSCLC, regardless of the treatment type.
Increased NLR may predict for poor outcomes of chemotherapy
(35), however, the data of the
present study did not confirm this association. In addition, NLR
was predictive for poor efficacy of immune therapy, which is in
agreement with previous research (36). It was also demonstrated that a high
level of NLR before and after two cycles of ICI and the dynamic
increase of this ratio during the ICI treatment, were associated
with worse survival outcomes. Similar results were produced by
several other studies (12,37,38).
The elimination of immunosuppressive derivatives of peripheral
blood neutrophils appears to be a promising strategy for overcoming
the resistance to ICIs (34). In
accordance with this hypothesis, therapeutic modulators of
immunosuppressive neutrophils increased the efficacy of ICI in
preclinical models (34). However,
only a limited number of these combinations have reached clinical
trials and are currently under investigation (39-41).
The combined assessment of different markers in a
predictive score, rather than using a single predictive marker, may
improve identification of patients who are most likely to benefit
from ICI therapy. Numerous predictive ICI-related scores have been
studied in patients with advanced NSCLC, such as EPSILoN, ALI,
LIPI, iSEND, LIPS-3 and combined NLR-TMB score (8,9,14,20,42,43).
All these indices are included in the NLR. However, some of them
included laboratory markers, such as TMB, PD-L1 and LDH, which are
not routinely studied in a number of centers for ICI therapy in
second and subsequent lines of treatment in patients with
NSCLC.
A new baseline prognostic score named NSE was
developed. It is based on markers that were independent predictors
of survival outcomes in multivariate analysis. The advantage of the
approach is the simplicity of it, as it is based on easily
accessible characteristics of the patients. In brief, the data of
the present study suggested that patients with an NLR ratio
<4.3, who have good performance status and history of smoking,
are the most likely to derive benefit from ICI therapy. By
contrast, patients with an NLR ≥4.3 coupled with poor ECOG and/or
lack of tobacco exposure, are highly unlikely to respond to ICI.
The remaining subjects consist of the group of intermediate ICI
therapy outcome. Notably, this reasoning is specific for
immunotherapy, as the same scoring was not predictive for patients
receiving cytotoxic drugs. The limitations of this investigation
were the retrospective nature of the study and the absence of other
routine blood tests, including absolute lymphocyte count and
platelet-lymphocyte ratio, which were not considered in the data
analysis.
In conclusion, the independent predictive factors
for short PFS and OS, such as a baseline NLR ≥4.3, non-smoking
status and ECOG PS ≥2 were demonstrated in the present study. In
addition, the developed new NSE score based on these markers may
assist the decision-making for NSCLC immunotherapy in second and
subsequent line settings. However, the score requires validation in
a prospective study.
Acknowledgements
The authors would like to cordially thank Professor
William R. Miller (University of Edinburgh, United Kingdom) for his
invaluable assistance in improving the language of this
manuscript.
Funding
Funding: The present study was supported by the Russian Science
Foundation (grant no. 23-45-10038).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AAM was responsible for the development of the
research design, review of publications on the topic of the
article, analysis of the data obtained, design of illustrative
material, statistical analysis and article writing. FVM was
responsible for the development of the research design, methodology
and analysis of the data obtained. TEE, APO, KAO, MAU, SVOd, IVC
and AMD were involved in curation of patients and data collection.
ALA, ENI and SVOr were responsible for conception and design
development, as well as scientific editing and research management.
AAM and SVOr confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved (approval no. 312 2022) by
the Ethics Committee of Pavlov First Saint Petersburg State Medical
University (Saint Petersburg, Russia). All participants signed
informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duchemann B, Remon J, Naigeon M, Mezquita
L, Ferrara R, Cassard L, Jouniaux JM, Boselli L, Grivel J, Auclin
E, et al: Integrating circulating biomarkers in the immune
checkpoint inhibitor treatment in lung cancer. Cancers (Basel).
12(3625)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tang S, Qin C, Hu H, Liu T, He Y, Guo H,
Yan H, Zhang J, Tang S and Zhou H: Immune checkpoint inhibitors in
non-small cell lung cancer: Progress, challenges, and prospects.
Cells. 19(320)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ding H, Xin W, Tong Y, Sun J, Xu G, Ye Z
and Rao Y: Cost effectiveness of immune checkpoint inhibitors for
treatment of non-small cell lung cancer: A systematic review. PLoS
One. 15(e0238536)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Signorelli D, Giannatempo P, Grazia G,
Aiello MM, Bertolini F, Mirabile A, Buti S, Vasile E, Scotti V,
Pisapia P, et al: Patients selection for immunotherapy in solid
tumors: Overcome the Naïve vision of a single biomarker. Biomed Res
Int. 2019(9056417)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim J, Ha H, Park J, Cho J, Lim JH and Lee
MH: Association of smoking status with efficacy of first-line
immune checkpoint inhibitors in advanced non-small cell lung
cancers: A systematic review and meta-analysis. J Cancer.
13:364–372. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Socinski M, Velcheti V, Mekhail T, Chae
YK, Leal TA, Dowell JE, Tsai ML, Dakhil CS, Stella P, Shen V, et
al: LBA83 - Final efficacy results from B-F1RST, a prospective
phase II trial evaluating blood-based tumour mutational burden
(bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L
non-small cell lung cancer (NSCLC). Ann Oncol. 30:v919–v920.
2019.
|
|
8
|
Mezquita L, Auclin E, Ferrara R, Charrier
M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L,
Audigier-Valette C, et al: Association of the lung immune
prognostic index with immune checkpoint inhibitor outcomes in
patients with advanced non-small cell lung cancer. JAMA Oncol.
4:351–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mountzios G, Samantas E, Senghas K, Zervas
E, Krisam J, Samitas K, Bozorgmehr F, Kuon J, Agelaki S, Baka S, et
al: Association of the advanced lung cancer inflammation index
(ALI) with immune checkpoint inhibitor efficacy in patients with
advanced non-small-cell lung cancer. ESMO Open.
6(100254)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Corbeau I, Jacot W and Guiu S: Neutrophil
to lymphocyte ratio as prognostic and predictive factor in breast
cancer patients: A systematic review. Cancers (Basel).
12(958)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang
G, Chen R, Jiao S and Wang J: Pretreatment neutrophil-to-lymphocyte
ratio (NLR) may predict the outcomes of advanced non-small-cell
lung cancer (NSCLC) patients treated with immune checkpoint
inhibitors (ICIs). Front Oncol. 10(654)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Diem S, Schmid S, Krapf M, Flatz L, Born
D, Jochum W, Templeton AJ and Früh M: Neutrophil-to-Lymphocyte
ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic
markers in patients with non-small cell lung cancer (NSCLC) treated
with nivolumab. Lung Cancer. 111:176–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Banna GL, Cortellini A, Cortinovis DL,
Tiseo M, Aerts JGJV, Barbieri F, Giusti R, Bria E, Grossi F,
Pizzutilo P, et al: The lung immuno-oncology prognostic score
(LIPS-3): A prognostic classification of patients receiving
first-line pembrolizumab for PD-L1 ≥50% advanced non-small-cell
lung cancer. ESMO Open. 6(100078)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J. 67:93–99.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Seymour L, Bogaerts J, Perrone A, Ford R,
Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, et
al: iRECIST: Guidelines for response criteria for use in trials
testing immunotherapeutics. Lancet Oncol. 18:e143–e152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
US Department of Health and Human
Services. Common Terminology Criteria for Adverse Events (CTCAE)
Version 5. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
|
|
17
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus Docetaxel in advanced Nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mazieres J, Rittmeyer A, Gadgeel S, Hida
T, Gandara DR, Cortinovis DL, Barlesi F, Yu W, Matheny C, Ballinger
M and Park K: Atezolizumab Versus docetaxel in pretreated patients
with NSCLC: Final results from the randomized phase 2 POPLAR and
phase 3 OAK clinical trials. J Thorac Oncol. 16:140–150.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim J, Cho J, Lee MH and Lim JH: Relative
efficacy of checkpoint inhibitors for advanced NSCLC according to
programmed death-ligand-1 expression: A systematic review and
network meta-analysis. Sci Rep. 8(11738)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Prelaj A, Rebuzzi SE, Pizzutilo P,
Bilancia M, Montrone M, Pesola F, Longo V, Del Bene G, Lapadula V,
Cassano F, et al: EPSILoN: A prognostic score using clinical and
blood biomarkers in advanced non-small-cell lung cancer treated
with immunotherapy. Clin Lung Cancer. 21:365–377.e5.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rounis K, Makrakis D, Papadaki C,
Monastirioti A, Vamvakas L, Kalbakis K, Gourlia K, Xanthopoulos I,
Tsamardinos I, Mavroudis D and Agelaki S: Prediction of outcome in
patients with non-small cell lung cancer treated with second line
PD-1/PDL-1 inhibitors based on clinical parameters: Results from a
prospective, single institution study. PLoS One.
16(e0252537)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maugeais M, Péron J, Dalle S, Boespflug A,
Duruissaux M, Corbaux P, Reverdy T, Sahin G, Rabier A, Lopez J, et
al: Impact of liver metastases and number of metastatic sites on
immune-checkpoint inhibitors efficacy in patients with different
solid tumors: A retrospective study. Biomedicines.
29(83)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xia H, Zhang W, Zhang Y, Shang X, Liu Y
and Wang X: Liver metastases and the efficacy of immune checkpoint
inhibitors in advanced lung cancer: A systematic review and
meta-analysis. Front Oncol. 12(978069)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vaddepally R, Doddamani R, Sodavarapu S,
Madam NR, Katkar R, Kutadi AP, Mathew N, Garje R and Chandra AB:
Review of immune-related adverse events (irAEs) in non-small-cell
lung cancer (NSCLC)-their incidence, management, multiorgan irAEs,
and rechallenge. Biomedicines. 10(790)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grangeon M, Tomasini P, Chaleat S, Jeanson
A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L,
Blanchon M, et al: Association between immune-related adverse
events and efficacy of immune checkpoint inhibitors in
non-small-cell lung cancer. Clin Lung Cancer. 20:201–207.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Okada N, Kawazoe H, Takechi K, Matsudate
Y, Utsunomiya R, Zamami Y, Goda M, Imanishi M, Chuma M, Hidaka N,
et al: Association between immune-related adverse events and
clinical efficacy in patients with melanoma treated with Nivolumab:
A multicenter retrospective study. Clin Ther. 41:59–67.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gärtner F, Aßmus J, Fløtten Ø, Ramnefjell
MP and Aanerud M: Characteristics and survival of patients with
non-small cell lung cancer treated with immune-checkpoint
inhibitors in the real-world: Experiences from Bergen, Norway. Acta
Oncol (Madr). 61:814–818. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takeuchi E, Kondo K, Okano Y, Kunishige M,
Kondo Y, Kadota N, Machida H, Hatakeyama N, Naruse K, Ogino H, et
al: Early mortality factors in immune checkpoint inhibitor
monotherapy for advanced or metastatic non-small cell lung cancer.
J Cancer Res Clin Oncol. 149:3139–3147. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Corke LK, Li JJN, Leighl NB and Eng L:
Tobacco use and response to immune checkpoint inhibitor therapy in
non-small cell lung cancer. Curr Oncol. 29:6260–6276.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao W, Jiang W, Wang H, He J, Su C and Yu
Q: Impact of smoking history on response to immunotherapy in
non-small-cell lung cancer: A systematic review and meta-analysis.
Front Oncol. 11(703143)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang X, Ricciuti B, Alessi JV, Nguyen T,
Awad MM, Lin X, Johnson BE and Christiani DC: Smoking history as a
potential predictor of immune checkpoint inhibitor efficacy in
metastatic non-small cell lung cancer. J Natl Cancer Inst.
113:1761–1769. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Popat S, Liu SV, Scheuer N, Gupta A, Hsu
GG, Ramagopalan SV, Griesinger F and Subbiah V: Association between
smoking history and overall survival in patients receiving
Pembrolizumab for first-line treatment of advanced non-small cell
lung cancer. JAMA Netw Open. 5(e2214046)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cedrés S, Torrejon D, Martínez A, Martinez
P, Navarro A, Zamora E, Mulet-Margalef N and Felip E: Neutrophil to
lymphocyte ratio (NLR) as an indicator of poor prognosis in stage
IV non-small cell lung cancer. Clin Transl Oncol. 14:864–869.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Raskov H, Orhan A, Gaggar S and Gögenur I:
Neutrophils and polymorphonuclear myeloid-derived suppressor cells:
An emerging battleground in cancer therapy. Oncogenesis.
11(22)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Peng B, Wang YH, Liu YM and Ma LX:
Prognostic significance of the neutrophil to lymphocyte ratio in
patients with non-small cell lung cancer: A systemic review and
meta-analysis. Int J Clin Exp Med. 8:3098–3106. 2015.PubMed/NCBI
|
|
36
|
Cao D, Xu H, Xu X, Guo T and Ge W: A
reliable and feasible way to predict the benefits of Nivolumab in
patients with non-small cell lung cancer: A pooled analysis of 14
retrospective studies. Oncoimmunology. 7(e1507262)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim
TM, Kim DW, Heo DS and Lee JS: Post-treatment
neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients
with advanced non-small cell lung cancers treated with anti-PD-1
antibody. Cancer Immunol Immunother. 67:459–470. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lim JU, Kang HS, Yeo CD, Kim JS, Park CK,
Kim JW, Kim SJ and Lee SH: Predictability of early changes in
derived neutrophil-to-lymphocyte ratio and neutrophil-to-lymphocyte
ratio in patients with advanced non-small cell lung cancer treated
with immune checkpoint inhibitors. J Thorac Dis. 13:2824–2832.
2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Simonelli M, Calvo E, Davar D, Richards D,
Gutierrez M, Garcia VM, Marron T, Rottey S, Orcurto A, Renouf DJ,
et al: 200MO Anti-IL-8 BMS-986253 + nivolumab (NIVO) ± ipilimumab
(IPI) in patients (pts) with advanced cancer: Update of initial
phase I results. Immuno-Oncology and Technology.
16(100311)2022.
|
|
40
|
Barlesi F, Isambert N, Felip E, Cho BC,
Lee DH, Peguero J, Jerusalem G, Penel N, Saada-Bouzid E, Garrido P,
et al: Bintrafusp Alfa, a Bifunctional fusion protein targeting
TGF-β and PD-L1, in patients with non-small cell lung cancer
resistant or refractory to immune checkpoint inhibitors.
Oncologist. 28:258–267. 2023.
|
|
41
|
Nadal E, Saleh M, Aix SP, Ochoa-de-Olza M,
Patel SP, Antonia S, Zhao Y, Gueorguieva I, Man M, Estrem ST, et
al: A phase Ib/II study of galunisertib in combination with
nivolumab in solid tumors and non-small cell lung cancer. BMC
Cancer. 23(708)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Park W, Mezquita L, Okabe N, Chae YK, Kwon
D, Saravia D, Auclin E, Planchard D, Caramella C, Ferrara R, et al:
Association of the prognostic model iSEND with PD-1/L1 monotherapy
outcome in non-small-cell lung cancer. Br J Cancer. 122:340–347.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Valero C, Lee M, Hoen D, Weiss K, Kelly
DW, Adusumilli PS, Paik PK, Plitas G, Ladanyi M, Postow MA, et al:
Pretreatment neutrophil-to-lymphocyte ratio and mutational burden
as biomarkers of tumor response to immune checkpoint inhibitors.
Nat Commun. 12(729)2021.PubMed/NCBI View Article : Google Scholar
|