Introduction
Although systemic chemotherapy has been the standard
therapy for advanced non-small cell lung cancer (NSCLC), its
effectiveness is limited. Recently, the identification of driver
mutations and the development of targeted therapies have improved
the prognosis of NSCLC patients with driver mutations. Among them,
in patients with NSCLC harboring the epidermal growth factor
receptor (EGFR) mutation, first-generation EGFR-TKIs showed an
improved progression-free survival (PFS) compared with chemotherapy
including carboplatin plus paclitaxel [median PFS, 10.8 months with
gefitinib versus 5.4 months with chemotherapy; hazard ratio (HR),
0.30, P<0.001] (1) and
cisplatin plus docetaxel (median PFS, 9.2 months versus 6.3 months;
HR 0.489, P<0.0001) (2).
Second-generation EGFR-TKIs, including afatinib and dacomitinib,
have also shown prolonged PFS (median PFS, 11.0 months with
afatinib versus 10.9 months with gefitinib; HR, 0.73, P=0.017)
(3) or overall survival (OS)
(median OS, 34.1 months with dacomitinib versus 26.8 months with
gefitinib; HR, 0.760, P=0.0438) (4) compared to first-generation EGFR-TKIs.
Furthermore, although exon 20 T790M mutation is one of the major
resistance mechanisms for first-/second-generation EGFR-TKIs,
osimertinib, a third-generation EGFR-TKI, can overcome the
resistance resulting from exon 20 T790M mutation. Osimertinib
showed an improved PFS (median PFS, 18.9 months versus 10.2 months;
HR, 0.46, P<0.001) and OS (median OS, 38.6 months with
osimertinib versus 31.8 months with the first-generation EGFR-TKIs;
HR, 0.80, P=0.046) compared with first-generation EGFR-TKIs
(5,6). Therefore, osimertinib has become one
of the major therapeutic options for EGFR mutant NSCLC.
However, the response of EGFR mutant NSCLC to
EGFR-TKIs is not consistent for all cases. In patients with EGFR
mutant NSCLC with positive programmed death ligand-1 (PD-L1)
expression, first-/second-generation EGFR-TKIs (7-9)
and osimertinib monotherapy (10-13)
is reported to be less effective, and combined therapy with
EGFR-TKIs plus vascular endothelial growth factor inhibitors or
cytotoxic agents may be more effective (14). On the other hand, PFS after the
treatment with osimertinib is longer in patients with EGFR mutant
NSCLC with negative or lower PD-L1 expression (10-13).
In addition, PD-L1 expression is also associated with the
acquisition rate of the T790M mutation after treatment with
first-/second-generation EGFR-TKIs (7-9).
For patients who acquired resistance through the T790M mutation,
the effectiveness of sequential therapy with osimertinib has been
demonstrated in the AURA study (15).
Thus, for EGFR mutant NSCLC with negative or lower
PD-L1 expression, both sequential therapy with
first-/second-generation EGFR-TKI plus osimertinib and first-line
osimertinib therapy are considered promising. However, there is
little information about the efficacy of these EGFR-TKI
monotherapies for this population. We conducted this observational
study to compare them.
Materials and methods
Patient selection
Data of patients who met the criteria were retrieved
from medical charts. The following inclusion criteria were used: i)
patients with NSCLC harboring the common EGFR mutation, ii)
patients with NSCLC in which the tumor proportion score (TPS) of
PD-L1 was confirmed to be less than 50% using the 22C3 antibody in
clinical practice, and iii) patients with NSCLC who were treated
with first-line EGFR-TKI monotherapy. The testing for T790M
mutations after the treatment with first-/second-generation
EGFR-TKIs was started in clinical practice in 2016. Thus, the
following exclusion criterion was established: i) patients in whom
the entire treatment for NSCLC was discontinued before 2016.
The present study was conducted following the
Declaration of Helsinki and Ethical Guidelines for Medical and
Biological Research Involving Human Subjects (Ministry of Health,
Labour and Welfare, Japan) and approved by the Ethics Committee,
University of Toyama (approval number: R2023018). Owing to the
retrospective nature of the study, the need to obtain written
informed consent was waived, and we disclosed the study information
to the subjects prior to their participation.
Driver mutation and PD-L1
expression
EGFR mutation status and PD-L1 expression were
evaluated using data retrieved from medical charts. EGFR mutation
was evaluated by polymerase chain reaction or next-generation
sequencing, and PD-L1 expression was evaluated based on TPSs
determined using the 22C3 antibody.
Statistical analysis
Patient characteristics were compared using Fisher's
exact test. To compare the efficacy of EGFR-TKI monotherapies for
EGFR mutant NSCLC with negative or lower PD-L1 expression, the PFS,
EGFR-TKI treatment duration, and OS were evaluated in the present
study. PFS was calculated from the initiation date of the treatment
with EGFR-TKIs until the date of disease progression defined by the
Response Evaluation Criteria in Solid Tumors version 1.1 or
clinically judged disease progression, whichever occurred first.
EGFR-TKI treatment duration was defined as the sum of the PFS of
first-line EGFR-TKI treatment and subsequential osimertinib therapy
after the acquisition of the T790M mutation. If the treatment was
changed because of an adverse event without disease progression,
PFS and EGFR-TKI treatment duration was censored on the day on
which the next treatment was started. OS was calculated from the
initiation date of the EGFR-TKI therapy until death and censored at
the last visit without death.
Kaplan-Meier curves were constructed, and survival
was compared by log-rank test in patients subdivided according to
treatment option. The Cox proportional hazard model was used to
assess the association between the treatment option and EGFR-TKI
treatment duration while adjusting for sex, ECOG performance status
(PS), EGFR mutation status, and brain metastases. These independent
variables were selected because they were considered to influence
the survival in patients with EGFR mutant NSCLC.
All statistical analysis was performed using the JMP
statistical software package, version 17.0.0 (SAS, Cary, NC,
USA).
Results
Patient selection
Between 2007 and 2023, 150 patients with EGFR mutant
NSCLC received first-line EGFR-TKI treatment, and PD-L1 expression
was evaluated in 74 patients. Of these 74 patients, 17 patients
were excluded for a PD-L1 TPS of ≥50%, and 2 patients were excluded
because the entire treatment for NSCLC was discontinued before
2016. Finally, 55 patients who were treated with first-line
EGFR-TKIs between 2013 and 2023 were included in the analysis.
Table I shows the
patient characteristics. Female patients with a PS of 0-1 were more
prevalent. First-/second-generation EGFR-TKIs and osimertinib were
administered in 26 and 29 patients, respectively. The
first-/second-generation EGFR-TKIs were gefitinib, erlotinib, and
afatinib. Patients aged ≥75 years were more prevalent in the
osimertinib group. First-line treatment with EGFR-TKIs were started
between 2013 and 2021 in first-/second-generation EGFR-TKI group,
while it was started between 2018 and 2023 in osimertinib group.
The median observation period was 38.3 months and 17.9 months in
the first-/second-generation EGFR-TKI group and the osimertinib
group, respectively.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics |
First-/second-generation, n (%)
(n=26) | Third-generation, n
(%) (n=29) | P-value |
|---|
| Age, years | | | |
|
<75 | 18 (69.2) | 10 (34.5) | 0.015 |
|
≥75 | 8 (30.8) | 19 (65.5) | |
| Sex | | | |
|
Male | 10 (38.5) | 9 (31.0) | 0.584 |
|
Female | 16 (61.5) | 20 (69.0) | |
| PS | | | |
|
0-1 | 20 (76.9) | 25 (86.2) | 0.490 |
|
≥2 | 6 (23.1) | 4 (13.8) | |
| Histology | | | |
|
Adenocarcinoma | 26 (100.0) | 27 (93.1) | >0.999 |
|
Squamous | 0 (0.0) | 1 (3.4) | |
|
NOS | 0 (0.0) | 1 (3.4) | |
| EGFR | | | |
|
EGFR del
19 | 13 (50.0) | 14 (48.3) | >0.999 |
|
EGFR
L858R | 13 (50.0) | 15 (51.7) | |
| PD-L1, % | | | |
|
<1 | 16 (61.5) | 14 (48.3) | 0.419 |
|
1-49 | 10 (38.5) | 15 (51.7) | |
| Brain metastases | | | |
|
Yes | 7 (26.9) | 4 (13.8) | 0.315 |
|
No | 19 (73.1) | 25 (86.2) | |
| Stage | | | |
|
IIIB | 1 (3.8) | 0 (0.0) | 0.746 |
|
IVA | 4 (15.4) | 3 (10.3) | |
|
ⅣB | 9 (34.6) | 13 (44.8) | |
|
Recurrence | 12 (46.2) | 13 (44.8) | |
Survival
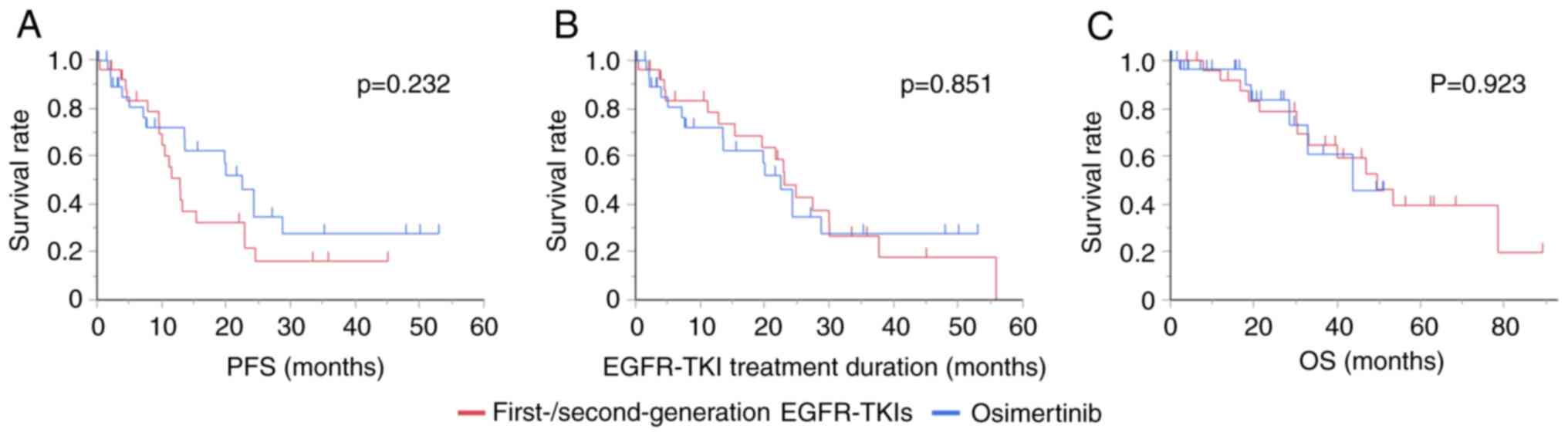
The median (95% CI) PFS was 12.9 (9.7-22.9) months
and 22.5 (7.6-28.8) months in patients who were treated with
first-/second-generation EGFR-TKIs and osimertinib, respectively
(P=0.232, log-rank test). After first-/second-generation EGFR-TKI
therapy, 18 of the 26 patients showed disease progression during
the treatment. Among them, the T790M mutation was evaluated in
16/18 (88.9%) patients, and 10/16 patients (62.5%) showed the T790M
mutation, who were subsequently treated with osimertinib. The
median (95% CI) EGFR-TKI treatment duration was 23.0 (12.9-30.0)
months and 22.5 (7.6-28.8) months in patients who were treated with
first-/second-generation EGFR-TKIs and osimertinib, respectively
(P=0.851, log-rank test). EGFR-TKI therapy was terminated in 22
patients (progression: 18, adverse event: 4) in
first-/second-generation EGFR-TKI group. Of these, 13/22 patients
(59.1%) and 8/22 patients (36.4%) were treated with platinum
doublet therapy and immune checkpoint inhibitor therapy,
respectively. On the other hand, osimertinib therapy was terminated
in 15 patients due to disease progression. Of these, 3/15 patients
(20.0%) and 2/15 patients (13.3%) were treated with platinum
doublet therapy or immune checkpoint inhibitor therapy,
respectively. The median (95% CI) OS was 49.5 (30.4-not estimated)
and 43.7 (28.5-not estimated) in patients who were treated with
first-/second-generation EGFR-TKIs and osimertinib, respectively
(P=0.923, log-rank test) (Fig.
1).
Table II shows the
results of the Cox proportional hazard model for EGFR-TKI treatment
duration. The independent variables were sex, PS, EGFR mutation
status, brain metastases, and EGFR-TKI therapy. The hazard ratio
(95% CI) of osimertinib for first-/second-generation EGFR-TKIs was
1.31 (0.55-3.13), which suggested that the EGFR-TKI treatment
duration was not statistically different between the two
monotherapies.
 | Table IIMultivariate analysis (Cox
proportional hazard model) for the EGFR-TKI treatment duration. |
Table II
Multivariate analysis (Cox
proportional hazard model) for the EGFR-TKI treatment duration.
| Characteristics | HR | 95% CI | P-value |
|---|
| Age, years | | | |
|
<75 | 1.79 | 0.75-4.25 | 0.191 |
|
≥75 | 1.00 | | |
| Sex | | | |
|
Male | 2.31 | 1.05-5.09 | 0.038 |
|
Female | 1.00 | | |
| PS | | | |
|
0-1 | 0.71 | 0.25-1.99 | 0.509 |
|
≥2 | 1.00 | | |
| EGFR | | | |
|
EGFR
L858R | 2.54 | 1.08-5.94 | 0.032 |
|
EGFR del
19 | 1.00 | | |
| Brain
metastases | | | |
|
No | 1.25 | 0.46-3.38 | 0.657 |
|
Yes | 1.00 | | |
| First-line
treatment | | | |
|
Third-generation | 1.31 | 0.55-3.13 | 0.542 |
|
First-/second-generation | 1.00 | | |
Discussion
Both sequential treatment with
first-/second-generation EGFR-TKIs plus osimertinib and first-line
treatment with osimertinib are considered promising for patients
with EGFR mutant NSCLC with negative or lower PD-L1 expression. The
present study was conducted based on this concept and confirmed
that the prognosis is equally favorable in patients with EGFR
mutant NSCLC with negative or lower PD-L1 expression who were
treated with first-/second-generation EGFR-TKIs or osimertinib.
There are several mechanisms for increasing the
PD-L1 expression (16). One is
derived by interferon-gamma produced by CD8 T lymphocytes. In other
words, increased PD-L1 expression corresponds with
tumor-infiltrating CD8 T lymphocytes, which may result in the
association between PD-L1 expression and the efficacy of immune
checkpoint inhibitors. Consistent with this, Shirasawa et al
(17) reported that NSCLC with
high PD-L1 expression and a high density of infiltrating CD8 T
lymphocytes had significantly better PFS than that with high PD-L1
expression and a low density of tumor-infiltrating CD8 T
lymphocytes after the treatment with immune checkpoint
inhibitors.
The other mechanism is based on oncogene signals. It
has been demonstrated that EGFR (18) and ALK signals (19) increase PD-L1 expression, and
depression of the ERK2 signal by siRNA decreases PD-L1 expression
(20). Furthermore, the Axl gene
mutation associated with resistance to osimertinib (21,22)
is reported to increase PD-L1 expression (23). The hypothesis was proposed that low
PD-L1 expression is associated with more homogeneous and less
immunogenic tumor cells in EGFR mutant NSCLC, resulting in a
slow-growing disease that responds well to EGFR-TKIs. In this type
of tumor, acquired resistance is more likely to occur in EGFR
pathways. Conversely, high PD-L1 expression may result from
activated oncogenes not related to the EGFR mutation, which leads
to EGFR-TKI resistance and acquired resistance outside the EGFR
pathways (7).
Therefore, in EGFR mutant NSCLC with negative or
lower PD-L1 expression, both sequential therapy with
first-/second-generation plus osimertinib and first-line
osimertinib therapy can be promising treatment strategies. In this
study, first-/second-generation EGFR-TKIs and osimertinib were
equally effective. The T790M mutation was highly detectable,
consistent with previous reports (7-9),
and the median PFS of 22.5 months in the osimertinib first-line
treatment group was longer than that shown in the FLAURA trial
(5). First-line treatment with
osimertinib may be preferable because the sequential therapy
requires re-biopsy.
The present study has several limitations. First, a
small sample size may provide insufficient statistical power for
the detection of differences in survival. In addition, it was
difficult to evaluate the survival in patients treated with
second-generation EGFR-TKI because we addressed patients treated
with first-generation EGFR-TKIs and second-generation EGFR-TKI as
one group. Finally, although we performed multivariate analysis
using the Cox proportional hazard model to adjust for patient
characteristics, some biases and confounding factors may affect the
analysis, considering the retrospective nature of the study. For
example, differences in the timing of treatment may have introduced
biases that affected the outcome. In addition, it cannot be
excluded that differences in observation duration and late-line
treatment after the EGFR-TKI therapy between the two groups might
have influenced the analysis.
In summary, the present study showed that the median
(95% CI) EGFR-TKI treatment duration was 23.0 (12.0-30.0) and 22.5
(7.6-28.8) months in patients treated with first-/second-generation
EGFR-TKIs and osimertinib, respectively. This suggests that the
sequential therapy with first-/second-generation EGFR-TKIs plus
osimertinib and first-line treatment with osimertinib are equally
effective for patients with EGFR mutant NSCLC with negative or
lower PD-L1 expression.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MI designed the study and wrote the original draft
of the manuscript. SMi, NT, KH, TH, ZS, KoT, CT, SO, KK, SI, TM, RH
and SMa contributed to the acquisition of data. KaT contributed to
the interpretation of data and supervised the study. MI and SO
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
Declaration of Helsinki and Ethical Guidelines for Medical and
Biological Research Involving Human Subjects (Ministry of Health,
Labour and Welfare, Japan) and approved by the Ethics Committee,
University of Toyama (approval no. R2023018; Toyama, Japan). The
need to obtain informed consent from the study subjects was waived
under the approval of the Ethics Committee, University of Toyama,
and information about the study was disclosed to the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Park K, Tan EH, O'Byrne K, Zhang L, Boyer
M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al: Afatinib versus
gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Lee M, Linke R, Rosell R, Corral J, et al: Improvement
in overall survival in a randomized study that compared dacomitinib
with gefitinib in patients with advanced non-small-cell lung cancer
and EGFR-activating mutations. J Clin Oncol. 36:2244–2250.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang CY, Liao WY, Ho CC, Chen KY, Tsai TH,
Hsu CL, Su KY, Chang YL, Wu CT, Hsu CC, et al: Association between
programmed death-ligand 1 expression, immune microenvironments, and
clinical outcomes in epidermal growth factor receptor mutant lung
adenocarcinoma patients treated with tyrosine kinase inhibitors.
Eur J Cancer. 124:110–122. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Inomata M, Azechi K, Takata N, Hayashi K,
Tokui K, Taka C, Okazawa S, Kambara K, Imanishi S, Miwa T, et al:
Association of tumor PD-L1 expression with the T790M mutation and
progression-free survival in patients with EGFR-mutant non-small
cell lung cancer receiving EGFR-TKI therapy. Diagnostics (Basel).
10(1006)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoon BW, Chang B and Lee SH: High PD-L1
Expression is Associated with unfavorable clinical outcome in
EGFR-mutated lung adenocarcinomas treated with targeted therapy.
Onco Targets Ther. 13:8273–8285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sakata Y, Sakata S, Oya Y, Tamiya M,
Suzuki H, Shibaki R, Okada A, Kobe H, Matsumoto H, Yokoi T, et al:
Osimertinib as first-line treatment for advanced epidermal growth
factor receptor mutation-positive non-small-cell lung cancer in a
real-world setting (OSI-FACT). Eur J Cancer. 159:144–153.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yoshimura A, Yamada T, Okuma Y, Fukuda A,
Watanabe S, Nishioka N, Takeda T, Chihara Y, Takemoto S, Harada T,
et al: Impact of tumor programmed death ligand-1 expression on
osimertinib efficacy in untreated EGFR-mutated advanced non-small
cell lung cancer: A prospective observational study. Transl Lung
Cancer Res. 10:3582–3593. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shiozawa T, Numata T, Tamura T, Endo T,
Kaburagi T, Yamamoto Y, Yamada H, Kikuchi N, Saito K, Inagaki M, et
al: Prognostic implication of PD-L1 expression on osimertinib
treatment for EGFR-mutated non-small cell lung cancer. Anticancer
Res. 42:2583–2590. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hsu KH, Tseng JS, Yang TY, Chen KC, Su KY,
Yu SL, Chen JJW, Huang YH and Chang GC: PD-L1 strong expressions
affect the clinical outcomes of osimertinib in treatment naive
advanced EGFR-mutant non-small cell lung cancer patients. Sci Rep.
12(9753)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Inomata M, Kawashima Y, Saito R, Morinaga
D, Nogawa H, Sato M, Suzuki Y, Yanagisawa S, Kikuchi T, Jingu D, et
al: A retrospective study of the efficacy of combined EGFR-TKI plus
VEGF inhibitor/cytotoxic therapy vs. EGFR-TKI monotherapy for
PD-L1-positive EGFR-mutant non-small cell lung cancer: North Japan
Lung Cancer Study Group 2202. Oncol Lett. 26(334)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Prelaj A, Tay R, Ferrara R, Chaput N,
Besse B and Califano R: Predictive biomarkers of response for
immune checkpoint inhibitors in non-small-cell lung cancer. Eur J
Cancer. 106:144–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shirasawa M, Yoshida T, Shimoda Y,
Takayanagi D, Shiraishi K, Kubo T, Mitani S, Matsumoto Y, Masuda K,
Shinno Y, et al: Differential immune-related microenvironment
determines programmed cell death protein-1/programmed death-ligand
1 blockade efficacy in patients with advanced NSCLC. J Thorac
Oncol. 16:2078–2090. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-driven NSCLC:
Implication for optional immune targeted therapy for NSCLC patients
with EGFR mutation. J Thorac Oncol. 10:910–923. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ota K, Azuma K, Kawahara A, Hattori S,
Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S,
et al: Induction of PD-L1 expression by the EML4-ALK oncoprotein
and downstream signaling pathways in non-small cell lung cancer.
Clin Cancer Res. 21:4014–4021. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sumimoto H, Takano A, Teramoto K and Daigo
Y: RAS-mitogen-activated protein kinase signal is required for
enhanced PD-L1 expression in human lung cancers. PLoS One.
11(e0166626)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Taniguchi H, Yamada T, Wang R, Tanimura K,
Adachi Y, Nishiyama A, Tanimoto A, Takeuchi S, Araujo LH, Boroni M,
et al: AXL confers intrinsic resistance to osimertinib and advances
the emergence of tolerant cells. Nat Commun. 10(259.
20190116)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yoshimura A, Yamada T, Serizawa M, Uehara
H, Tanimura K, Okuma Y, Fukuda A, Watanabe S, Nishioka N, Takeda T,
et al: High levels of AXL expression in untreated EGFR-mutated
non-small cell lung cancer negatively impacts the use of
osimertinib. Cancer Sci. 114:606–618. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsukita Y, Fujino N, Miyauchi E, Saito R,
Fujishima F, Itakura K, Kyogoku Y, Okutomo K, Yamada M, Okazaki T,
et al: Axl kinase drives immune checkpoint and chemokine signalling
pathways in lung adenocarcinomas. Mol Cancer. 18(24)2019.PubMed/NCBI View Article : Google Scholar
|