Introduction
Clear cell carcinoma (CCC) in the diaphragm is
extremely rare and, to the best of our knowledge, only four cases
have been reported in the worldwide literature (1-4).
The origin of CCC is reported to be associated with malignant
transformation of extrapelvic endometriosis (1,2).
Endometriosis is defined as the histological presence of
endometrial glands and stroma outside the uterine cavity (5). Endometriosis typically occurs in the
pelvic cavity, affecting areas such as the ovaries, uterosacral
ligaments, and pouch of Douglas. However, it can also develop in
extra-gonadal sites, including the abdominal peritoneum, diaphragm,
lungs, pleura, bowel, ureter, and brain. While most cases of
endometriosis remain benign, malignant transformation, while rare,
can occur, with an estimated incidence of up to 1%. It most
frequently involves the ovaries, which account for approximately
80% of endometriosis-associated malignancies (6). The most common histological types of
endometriosis-associated malignancies are endometrioid carcinoma
and CCC (7).
Lynch syndrome (LS), an autosomal dominant
hereditary cancer syndrome, results from germline pathogenic
variants (PVs) in the DNA mismatch repair (MMR) genes (MLH1,
MSH2, MSH6, and PMS2) or EPCAM gene.
Colorectal cancer and endometrial cancer (EC) are the most common
malignancies in LS, with lifetime risks of 53-82 and 25-60%,
respectively (8,9). Notably, approximately half of women
with LS develop EC as their first cancer (8,9).
Endometrial hyperplasia (EH) is a disordered proliferation of
epithelial cells and endometrial glands, and represents a precursor
lesion to EC. The rate of progression to EC has been reported to be
1-5% in EH patients without atypia, and increases to nearly 25% in
those with atypical EH (AEH) (10). Ovarian cancer (OC) is also
associated with LS. The incidence of OC in women with LS accounts
for 0.9-2.7% of all OC cases, with a cumulative lifetime risk of
6-17% (11). LS-related OC is
known to be associated with endometrioid carcinoma and CCC
histological types (12).
We herein present a clinical case of diaphragmatic
CCC with LS that occurred after surgery for AEH and ovarian
endometriosis.
Case report
A 48-year-old woman with abnormal genital bleeding
was referred to Fukushima Medical University Hospital (Fukushima,
Japan) in October 2017 from a gynecological outpatient clinic. She
was diagnosed as having AEH, and underwent total laparoscopic
hysterectomy and bilateral salpingo-oophorectomy. Since the
bilateral ovaries were grossly normal at the first surgery, there
was no rupture of the ovarian cyst. Pathological examination
revealed AEH of the endometrium and endometriosis in the bilateral
ovaries.
At the age of 51 years, an intra-abdominal mass was
found during a routine physical examination. The patient visited a
nearby general hospital, where an enhanced computed tomography scan
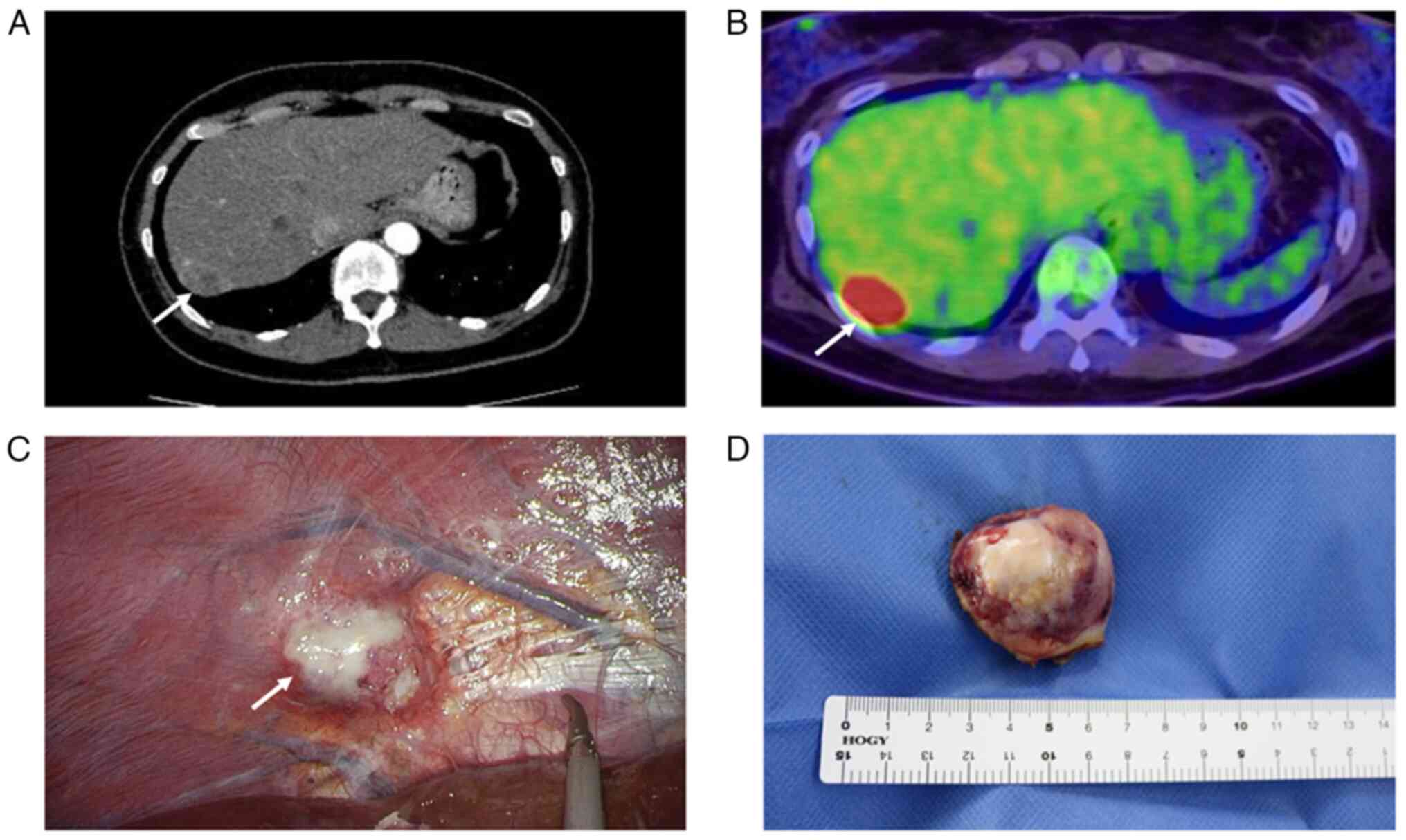
revealed a mass on the surface of the liver (Fig. 1A). She was referred to our hospital
for surgery. The laboratory examination showed CA125: 69 U/ml
(normal <35). Fluorine-18 fluorodeoxyglucose positron emission
tomography/computed tomography showed a mass with fluorine-18
fluorodeoxyglucose accumulation at the right hepatic lobe margins,
which was suspicious for malignancy (Fig. 1B). Laparoscopic examination of the
abdominal cavity revealed a tumor on the underside of the right
diaphragm, which was subsequently removed laparoscopically
(Fig. 1C).
Macroscopic examination showed a solid and flat
tumor of 4.1x3.8 cm which had a whitish cut surface (Fig. 1D). Histopathologically, the
diaphragmatic tumor was not composed of typical tubular cysts or
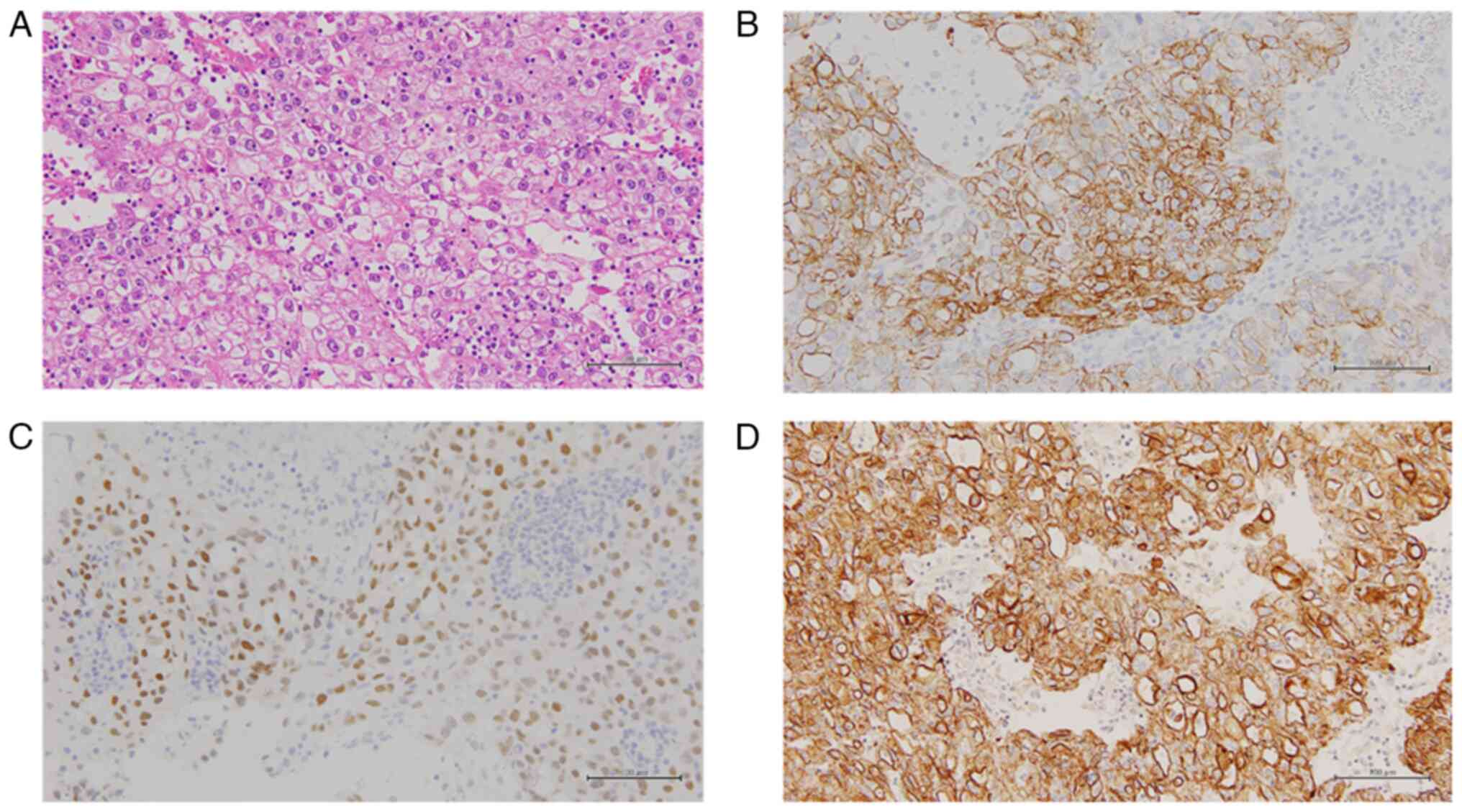
papillary structures, but mostly showed a solid pattern (Fig. 2A). Tumor cells showed proliferation
of markedly pleomorphic cells with abundant pale cytoplasm,
enlarged nuclei and prominent nucleoli (Fig. 2A). Immunohistochemistry (IHC)
revealed that the tumor cells were positive for CK7, HNF1B and
AE1/3 (Fig. 2B-D). Expression of
AFP, CD117, hCG, PLAP, S-100, ER, PgR, CK20, CK5/6, p63,
Glyppican3, Hepa-1, D2-40, GATA3, OCT3/4 and EBER-1 in the tumor
cells was negative. Hence, the morphologic findings and the
immunohistochemical profile were consistent with the diagnosis of
diaphragmatic CCC of the ovary (13). The patient received six courses of
adjuvant combination chemotherapy with paclitaxel and carboplatin.
At the time of writing, her CA125 has returned to normal level, and
she is alive without evidence of CCC recurrence 30 months after the
surgery.
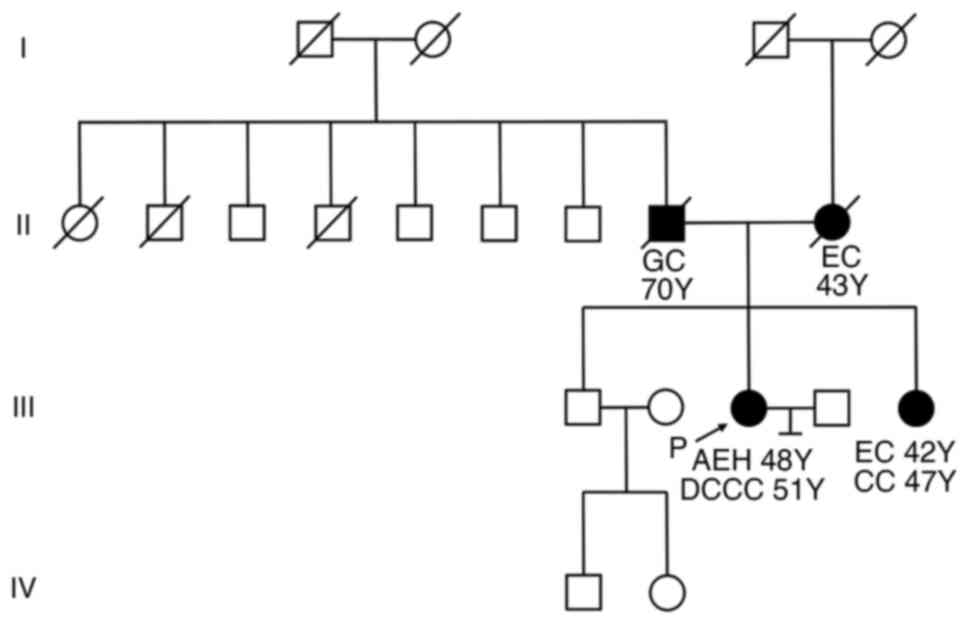
Given the family history of young-onset colorectal
cancer and EC, LS was suspected (Fig.
3). Therefore, microsatellite instability (MSI) analysis was
performed on the diaphragmatic tumor, and the results were
positive. IHC for MMR protein was performed as described
previously, with primary antibodies against MLH1 (ES05, 1:50,
Dako), MSH2 (FE11, 1:50, Dako), MSH6 (EP49, 1:200, Dako), and PMS2
(EP51, 1:50, Dako) (14). Both
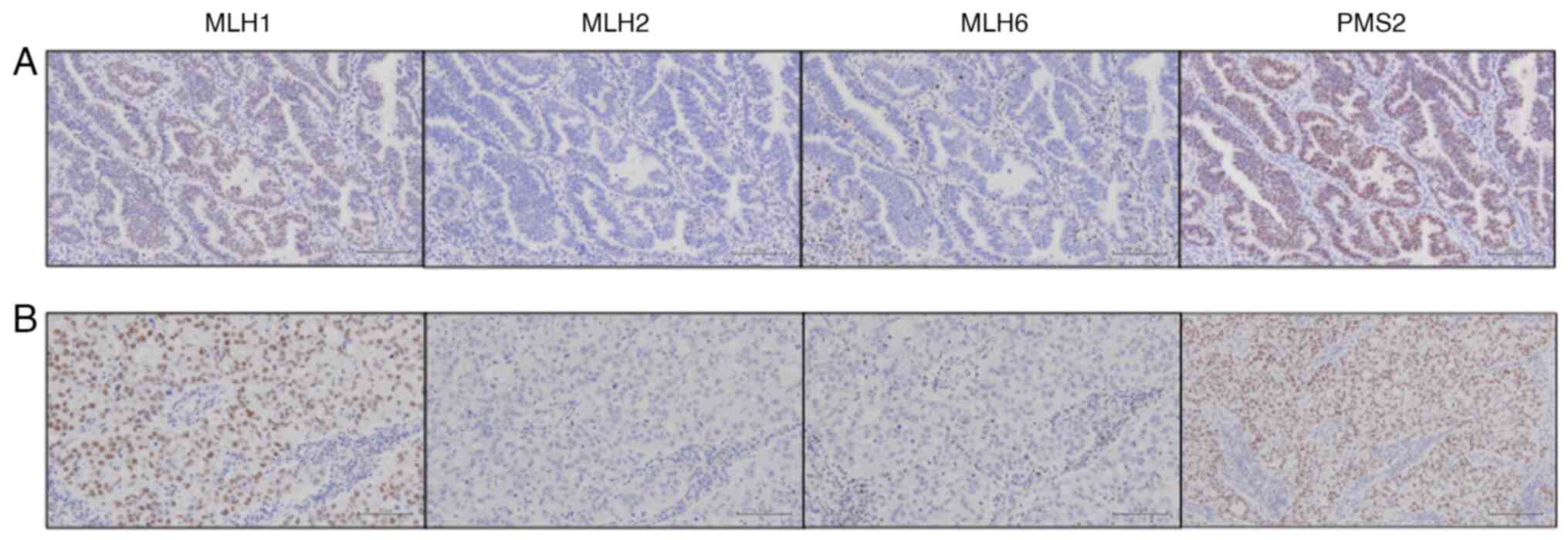
tumor cells exhibited reduced MSH2 and MSH6
expression (Fig. 4). Following
detailed genetic counseling, genetic testing of MMR genes was
performed, revealing a germline PV in MSH2 (c.1000C>T,
p.Gln344*), which confirmed the diagnosis of LS. Her sister had
early-onset endometrial and colon cancers associated with LS
(Fig. 3). Although LS is inherited
in an autosomal dominant manner, with a 50% chance of inheritance
in first-degree family members, the relatives of the patient in the
present case have not, at the time of writing, requested genetic
testing for single-site MSH2 analysis.
Discussion
Primary tumors of the diaphragm are uncommon. A
previous review found that approximately 200 primary cases of
diaphragmatic tumors have been reported (15). Moreover, metastases, including
benign lesions such as endometriosis, as well as malignant lesions
from cancers such as lung cancer, malignant mesothelioma and OC,
can occur in the diaphragm (16).
Diaphragmatic endometriosis occurs in 1.5% of surgically treated
endometriosis patients (17).
Endometriosis-related malignancy is associated with the development
of CCC and endometrioid carcinoma. To date, there have been only
Japanese case reports, including the present case (1-4).
The prevalence of ovarian CCC differed by region and is higher in
Asian populations than in Western countries (18). In particular, a recent Japanese
study has reported that ovarian CCC is increased significantly,
accounting for up to 30% of epithelial OC (19). The causes of difference are not
clear, although one reason could be that endometriosis is more
common in women of Asian origin than in Western countries (20). In addition, endometrioid carcinoma
in the diaphragm associated with endometriosis has also been
reported only in two cases (21,22).
Matsuki et al reviewed cases of CCC in the peritoneum and
diaphragm, and reported that 40% (6/15) of the patients had a
history of, or currently had, endometriosis or adenomyosis
(1). In 1925, Sampson defined
three criteria for the diagnosis of malignant transformation of
endometriosis: i) Demonstration of endometriosis within the tumor;
ii) absence of other primary tumors; and iii) histological
appearance consistent with an endometrial origin (23). Furthermore, in 1953, Scott added a
fourth criterion: iv) morphologic demonstration of benign
endometriosis contiguous with the malignant tissue (24). In the present case, previous
surgery had revealed ovarian endometriosis, but there was no
histological evidence of endometrial tissue near the diaphragmatic
tumor. One possible explanation is that any endometrial implants
originally present had undergone complete malignant transformation
into a cancerous lesion. According to a previous report,
endometriosis was present in the transition zone in only 36-42% of
patients with malignant extraovarian endometriosis (25).
Although the molecular mechanisms for the malignant
transformation of endometriosis are unknown, a review described
inflammatory responses, oxidative stress and genomic aberrations as
risk factors (26). MSI is a major
phenotype of genomic instability in cancer, and is caused by a
deficiency of the DNA MMR genes associated with LS. In recent
studies, loss of MMR proteins was identified even in normal cells,
including crypt foci and endometrial glands in women with LS
(27,28). In the present case, decreased
expression of MSH2/MSH6 and MSI-high were detected in the diaphragm
tumor, although there was no coexisting endometriosis. In Western
countries, the histological subtype of OC with MMR deficiency has
been reported in 0.3% of serous cases, 12.5% of endometrioid cases,
3.0% of CCC cases, 0.7% of mucinous cases and 7.3% of mixed cases
(29). Chui et al reported
that all LS-OCs were either pure endometrioid carcinoma (14 cases),
mixed carcinoma with an endometrioid component (four cases), or CCC
(two cases), and no high-grade or low-grade serous or mucinous
carcinomas were identified (30).
Endometrioid carcinoma and CCC are also common pathological types
of endometriosis-associated OCs. The mean interval between the last
surgery and the diagnosis of CCC of the abdominal wall was reported
to be approximately 21 years, indicating indolent tumorigenesis
(31,32). The patient in the current report
was diagnosed with CCC of the diaphragm three years after surgery,
which was a shorter period of time than those reported in previous
studies. MMR deficiency following a loss of heterozygosity of a
pathogenic MMR gene might act as an early event in the malignant
transformation of endometriosis.
To date, few papers have reported the relationship
between LS and endometrial intraepithelial neoplasia/AEH. In Lucas
et al performed IHC analysis of 118 randomly selected
endometrial intraepithelial neoplasia/AEH patients and showed loss
of MMR protein expression in four patients (3.4%), two of whom were
confirmed as having LS (33). Lu
et al reported that two (3.9%) of 51 women with LS had AEH
at the baseline endometrial biopsy (34). Loss of MMR protein expression in
endometrial lesions has been detected in LS patients with simple
EH, complicated EH, or AEH, along with MLH1 methylation (35). In a similar study, decreased MMR
protein expression was observed in 7% of LS patients with normal
endometrium, 40% of those with simple hyperplasia, 100% of those
with complex EH without atypia, 92% of those with complex AEH, and
100% of those with EC (36). It
was recently reported that elevated MSI was detected in aspirates
from premalignant and malignant lesions, as well as from normal
endometrium, and correlated with loss of MMR protein (37). Since loss of MSH2/MSH6 expression
in AEH cells was identified in our case, IHC for MMR proteins in
AEH patients may provide an opportunity to identify LS.
In conclusion, to the best of our knowledge, this is
the first reported case of diaphragmatic CCC with LS. Although
there are limitations such as the lack of MRI findings before
surgery for the diaphragm tumor and CA125 data before the removal
of the ovaries, the origin of CCC is considered to be related to
malignant transformation of extrapelvic endometriosis, given the
history of ovarian endometriosis. Since the diaphragmatic tumor
showed MSI-high and loss of MSH2/MSH6 expression by IHC, MMR
germline PVs are implicated in the malignant transformation of
endometriosis. Therefore, women with endometriosis and LS are at
risk of developing cancer not only in the ovaries, but also in
other parts of the body, such as the abdominal cavity. In addition,
our patient also had a history of AEH, which decreased the
expression of MMR proteins. For AEH patients at high risk of LS
based on family history, IHC screening for MMR proteins should be
considered to facilitate early clinical intervention, given the
possibility of EC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data presented in this manuscript are available
on request from the corresponding author.
Authors' contributions
MU and TW wrote the original draft, and contributed
to conception, design, data acquisition and data analysis. SS and
TM contributed to data analysis, supervision, and reviewed and
edited the manuscript. YK, AK, CO, TS, NK, YE and SF contributed to
management of the patient and data acquisition. KF contributed to
supervision, interpretation of the data and approved the final
manuscript version to be published. TW and TM confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient to publish this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsuki M, Numoto I, Hamakawa T, Ishii K
and Chikugo T: Primary diaphragmatic clear cell carcinoma
associated with endometriosis: A case report and literature review.
Gynecol Oncol Rep. 36(100733)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fujiu K, Miyamoto H, Hashimoto S, Suzuki
N, Takano Y, Teranishi Y, Sakuma H and Suzuki H: A case of
diaphragmatic clear cell carcinoma in a patient with a medical
history of ovarian endometriosis. Int J Clin Oncol. 15:489–492.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Harimoto N, Hagiwara K, Yamanaka T, Ishii
N, Igarashi T, Watanabe A, Kubo N, Araki K, Ikota H, Suyama M, et
al: Fairly rare clear cell adenocarcinoma mimicking liver cancer: A
case report. Surg Case Rep. 4(97)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moriyasu R, Mishima O, Sunakawa T, Otagiri
N, Ito N and Tauchi K: Case report: A surgically treated case of
diaphragmatic clear cell carcinoma without relation to
endometriosis. Int J Surg Case Rep. 105(108061)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Johnson NP, Hummelshoj L, Adamson GD,
Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R,
Sharpe-Timms KL, et al: World endometriosis society consensus on
the classification of endometriosis. Hum Reprod. 32:315–324.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Van Gorp T, Amant F, Neven P, Vergote I
and Moerman P: Endometriosis and the development of malignant
tumours of the pelvis. A review of literature. Best Pract Res Clin
Obstet Gynaecol. 18:349–371. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matias-Guiu X and Stewart CJR:
Endometriosis-associated ovarian neoplasia. Pathology. 50:190–204.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hampel H, Frankel W, Panescu J, Lockman J,
Sotamaa K, Fix D, Comeras I, La Jeunesse J, Nakagawa H, Westman JA,
et al: Screening for Lynch syndrome (hereditary nonpolyposis
colorectal cancer) among endometrial cancer patients. Cancer Res.
66:7810–7817. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Buza N, Ziai J and Hui P: Mismatch repair
deficiency testing in clinical practice. Expert Rev Mol Diagn.
16:591–604. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rakha E, Wong SC, Soomro I, Chaudry Z,
Sharma A, Deen S, Chan S, Abu J, Nunns D, Williamson K, et al:
Clinical outcome of atypical endometrial hyperplasia diagnosed on
an endometrial biopsy: institutional experience and review of
literature. Am J Surg Pathol. 36:1683–1690. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dominguez-Valentin M, Crosbie EJ, Engel C,
Aretz S, Macrae F, Winship I, Capella G, Thomas H, Nakken S, Hovig
E, et al: Risk-reducing hysterectomy and bilateral
salpingo-oophorectomy in female heterozygotes of pathogenic
mismatch repair variants: A prospective lynch syndrome database
report. Genet Med. 23:705–712. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Helder-Woolderink JM, Blok EA, Vasen HF,
Hollema H, Mourits MJ and De Bock GH: Ovarian cancer in lynch
syndrome; a systematic review. Eur J Cancer. 55:65–73.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Offman SL and Longacre TA: Clear cell
carcinoma of the female genital tract (not everything is as clear
as it seems). Adv Anat Pathol. 19:296–312. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Noda M, Okayama H, Tachibana K, Sakamoto
W, Saito K, Thar Min AK, Ashizawa M, Nakajima T, Aoto K, Momma T,
et al: Glycosyltransferase gene expression identifies a poor
prognostic colorectal cancer subtype associated with mismatch
repair deficiency and incomplete glycan synthesis. Clin Cancer Res.
24:4468–4481. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Baldes N and Schirren J: Primary and
secondary tumors of the diaphragm. Thorac Cardiovasc Surg.
64:641–646. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim MP and Hofstetter WL: Tumors of the
diaphragm. Thorac Surg Clin. 19:521–529. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ceccaroni M, Roviglione G, Giampaolino P,
Clarizia R, Bruni F, Ruffo G, Patrelli TS, De Placido G and Minelli
L: Laparoscopic surgical treatment of diaphragmatic endometriosis:
A 7-year single-institution retrospective review. Surg Endosc.
27:625–632. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iida Y, Okamoto A, Hollis RL, Gourley C
and Herrington CS: Clear cell carcinoma of the ovary: A clinical
and molecular perspective. Int J Gynecol Cancer. 31:605–616.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yen CF, Kim MR and Lee CL: Epidemiologic
factors associated with endometriosis in east Asia. Gynecol Minim
Invasive Ther. 8:4–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Agrawal A, Nation J, Ghatage P, Chu P,
Ross S and Magliocco A: Malignant chest wall endometriosis: A case
report and literature review. J Obstet Gynaecol Can. 31:538–541.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Okimura H, Tatsumi H, Ito F, Yamashita S,
Kokabu T and Kitawaki J: Endometrioid carcinoma arising from
diaphragmatic endometriosis treated with laparoscopy: A case
report. J Obstet Gynaecol Res. 44:972–977. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sampson JA: Endometrial carcinoma of the
ovary, arising in endometrial tissue in that organ. Arch Surg.
10:1–72. 1925.
|
|
24
|
Scott Rb: Malignant changes in
endometriosis. Obstet Gynecol. 2:283–289. 1953.PubMed/NCBI
|
|
25
|
Benoit L, Arnould L, Cheynel N, Diane B,
Causeret S, Machado A, Collin F, Fraisse J and Cuisenier J:
Malignant extraovarian endometriosis: A review. Eur J Surg Oncol.
32:6–11. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guidozzi F: Endometriosis-associated
cancer. Climacteric. 24:587–592. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kloor M, Huth C, Voigt AY, Benner A,
Schirmacher P, von Knebel Doeberitz M and Bläker H: Prevalence of
mismatch repair-deficient crypt foci in Lynch syndrome: A
pathological study. Lancet Oncol. 13:598–606. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wong S, Hui P and Buza N: Frequent loss of
mutation-specific mismatch repair protein expression in
nonneoplastic endometrium of Lynch syndrome patients. Mod Pathol.
33:1172–1181. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tanaka T, Takehara K, Yamashita N,
Okazawa-Sakai M, Kuraoka K, Teramoto N, Taguchi K, Yamashiro K,
Kato H, Mizunoe T, et al: Frequency and clinical features of
deficient mismatch repair in ovarian clear cell and endometrioid
carcinoma. J Gynecol Oncol. 33(e67)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chui MH, Ryan P, Radigan J, Ferguson SE,
Pollett A, Aronson M, Semotiuk K, Holter S, Sy K, Kwon JS, et al:
The histomorphology of Lynch syndrome-associated ovarian
carcinomas: Toward a subtype-specific screening strategy. Am J Surg
Pathol. 38:1173–1181. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Taburiaux L, Pluchino N, Petignat P and
Wenger JM: Endometriosis-associated abdominal wall cancer: A poor
prognosis? Int J Gynecol Cancer. 25:1633–1638. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lai YL, Hsu HC, Kuo KT, Chen YL, Chen CA
and Cheng WF: Clear cell carcinoma of the abdominal wall as a rare
complication of general obstetric and gynecologic surgeries: 15
years of experience at a large academic institution. Int J Environ
Res Public Health. 16(552)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lucas E, Chen H, Molberg K, Castrillon DH,
Rivera Colon G, Li L, Thibodeaux J, Lea J, Miller DS and Zheng W:
Mismatch repair protein expression in endometrioid intraepithelial
neoplasia/atypical hyperplasia: Should we screen for lynch syndrome
in precancerous lesions? Int J Gynecol Pathol. 38:533–542.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu KH, Loose DS, Yates MS,
Nogueras-Gonzalez GM, Munsell MF, Chen LM, Lynch H, Cornelison T,
Boyd-Rogers S, Rubin M, et al: Prospective multicenter randomized
intermediate biomarker study of oral contraceptive versus
depo-provera for prevention of endometrial cancer in women with
Lynch syndrome. Cancer Prev Res (Phila). 6:774–781. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
de Leeuw WJ, Dierssen J, Vasen HF, Wijnen
JT, Kenter GG, Meijers-Heijboer H, Brocker-Vriends A, Stormorken A,
Moller P, Menko F, et al: Prediction of a mismatch repair gene
defect by microsatellite instability and immunohistochemical
analysis in endometrial tumours from HNPCC patients. J Pathol.
192:328–335. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nieminen TT, Gylling A, Abdel-Rahman WM,
Nuorva K, Aarnio M, Renkonen-Sinisalo L, Järvinen HJ, Mecklin JP,
Bützow R and Peltomäki P: Molecular analysis of endometrial
tumorigenesis: Importance of complex hyperplasia regardless of
atypia. Clin Cancer Res. 15:5772–5783. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Canet-Hermida J, Marín F, Dorca E, Dueñas
N, Costas L, Salinas M, Velasco À, Peremiquel-Trillas P, Paytubi S,
Ponce J, et al: Highly sensitive microsatellite instability and
immunohistochemistry assessment in endometrial aspirates as a tool
for cancer risk individualization in lynch syndrome. Mod Pathol.
36(100158)2023.PubMed/NCBI View Article : Google Scholar
|