Introduction
Approximately 10% of patients with primary
malignancies develop proximal femoral metastases (1). Bone metastases, originating mainly
from breast, kidney, thyroid, prostate or myeloma cancers, are
often soluble or mixed in nature, posing a high risk of
pathological fractures to this patient population (2). A previous study published an
algorithm for treating long bone and pelvic metastases. The
patients were categorized into four classes: i) Isolated lesion
with good prognosis; ii, pathological fractures; iii, incisional
fractures; and iv), other lesions (3). Important factors influencing the
choice of treatment for long bones and the pelvis include
prognosis, disease type, visceral metastases, time from disease
onset, risk of pathologic fracture, and sensitivity to
chemotherapy, hormonal therapy and irradiation. The role of
orthopedic surgeons in evaluating patients with skeletal metastases
is expected to increase over time as improved cancer treatments
enhance survival (4).
In addition, pathological fractures are 3.5 times
more likely to occur in the proximal femur than in the proximal
humerus (5). However, studies
describing cases of pathological or impending fractures of the
lower extremities in patients with primary and metastatic
malignancies are currently lacking. Therefore, the present study
aimed to provide a detailed description of the clinical
characteristics of patients who underwent surgical treatment of
pathological or impending fractures.
Patients and methods
Patients
The study included 30 patients with impending and
pathological fractures treated at the Department of Orthopedic
Surgery at Kindai University Hospital (Osakasayama, Japan) between
January 2019 and November 2023. Inclusion and exclusion criteria:
Included were cases treated at the clinic during the period for
whom the course of treatment could be followed. Excluded were cases
for which the course of treatment could not be traced. Diagnosis:
Impending and pathological fractures were diagnosed based on the
Mirels' score (6). Number of
patients: Impending and pathological fractures were observed in 12
and 18 cases, respectively.
Parameters
The retrospective survey covered the following
parameters: Age, sex, fracture site, types of primary malignancy,
number of metastases, pre-fracture Eastern Cooperative Oncology
Group performance status score (ECOG-PS) (7), adjuvant therapy, treatment modality,
operative time, blood loss, postoperative complications,
Musculoskeletal Tumor Society (MSTS) score (8), outcome and follow-up period.
Analytical methods: Post-treatment MSTS scores between cases of
impending and pathological fractures, as well as between cases
treated with intramedullary nailing and those undergoing other
surgical procedures, were also compared. The postoperative one-year
survival rate was calculated using the Kaplan-Meier test. In
addition, the operative time, blood loss and survival rates were
compared between impending and pathological fractures.
Statistical analysis
Variables are presented as the mean ± standard
deviation (S.D.). The MSTS scores of intramedullary nailing and
other surgical procedures were compared using Student t-test. The
ECOG-PS and MSTS scores were plotted and a correlation diagram was
drawn. The coefficient of determination (R2) was
calculated by drawing an approximation line to assess the
correlation between ECOG-PS and MSTS scores. Pearson's correlation
method was used to determine these correlations. The strength of
the correlation was determined according to Pearson's correlation
coefficient R as follows: Very strong, 1.0≥|R|≥0.7; strong,
0.7≥|R|≥0.5; moderate, 0.5≥|R|≥0.4; medium, 0.4≥|R|≥0.3; weak,
0.3≥|R|≥0.2; and no correlation, 0.2≥|R|≥0.0.
The operative time between impending and
pathological fractures was compared using Student t-test. In
addition, survival rates were compared between cases of impending
and that of pathological fractures using Log-rank test. P<0.05
was considered to indicate statistical significance in all
analyses. Analyses were performed using Stat Mate 5.05 (ATMS,
Tokyo, Japan).
Results
Patient characteristics. The characteristics
of the patients [including 13 male and 17 female participants; mean
± S.D. age, 70.5±9.82 (range, 47-83) years] are summarized in
Table I and treatments were
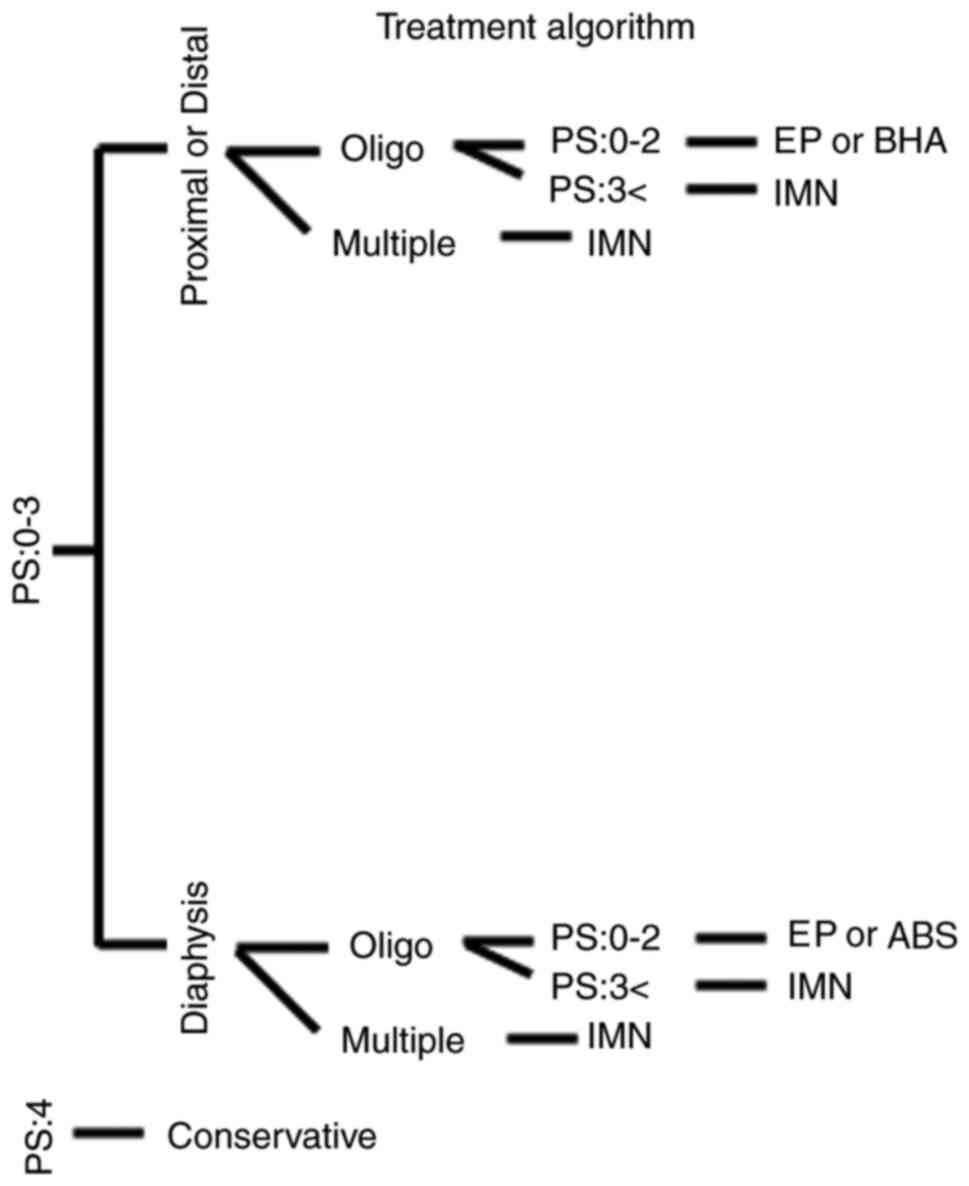
performed according to the algorithm depicted in Fig. 1.
 | Table ICharacteristics of the study
population (n=30). |
Table I
Characteristics of the study
population (n=30).
| Factor | Value |
|---|
| Age, years | |
|
Mean ±
S.D. | 70.5±9.82 |
|
≤70 | 15(50) |
|
>70 | 15(50) |
| Sex | |
|
Male | 13(43) |
|
Female | 17(57) |
| Fracture site | |
|
Femoral
neck | 5(17) |
|
Femoral
diaphysis | 5(17) |
|
Intertrochanteric | 6(20) |
|
Subtrochanteric | 10(33) |
|
Bilateral
intertrochanteric | 1(3) |
|
Proximal
tibia | 2(7) |
|
Distal
femur | 1(3) |
| Type of cancer | |
|
Lung | 9(30) |
|
Breast | 7(23) |
|
Kidney | 3(10) |
|
Multiple
myeloma | 4(13) |
|
Liver | 2(7) |
|
Gastric | 2(7) |
|
Unknown | 1(3) |
|
Esophageal | 1(3) |
|
Uterine | 1(3) |
| N. metastases | |
|
≤3 | 4(13) |
|
>3 | 26(87) |
| ECOG-PS (mean) | 2 |
|
<2 | 14(47) |
|
2-3 | 14(47) |
|
>3 | 2(7) |
| Adjuvant therapy | |
|
Radiotherapy | 2(7) |
|
Chemotherapy | 15(50) |
|
Chemotherapy
and radiotherapy | 10(33) |
|
None | 3(10) |
| Treatment
modality | |
|
Intramedullary
nail | 16(53) |
|
Endoprosthesis | 1(3) |
|
Fixation
with plate | 1(3) |
|
Bipolar head
arthroplasty | 3(10) |
|
Fixation
with CHS | 3(10) |
|
Bilateral
intermedullary nail | 2(7) |
|
Conservative | 2(7) |
|
Artificial
bone stem | 1(3) |
|
Rt. bipolar
head arthroplasty, Lt. fixation with CHS | 1(3) |
| Operating time,
min | |
|
Total | 92.0±38.7 |
|
Impending
fractures |
83.1±21.9a |
|
Pathological
fractures | 113.8±44.3 |
|
0-100 | 17 |
|
>100 | 11 |
| Blood loss, ml | |
|
Total | 50.0 (20-447) |
|
Impending
fractures | 46.4 (20-435) |
|
Pathological
fractures | 132.6 (20-447) |
|
0-60 | 15 |
|
>60 | 13 |
| MSTS score | |
|
Intramedullary
nailing | 19.9±8.8 |
|
Other
surgical procedures | 22.0±10.9 |
|
0-10 | 8(27) |
|
11-20 | 7(23) |
|
21-30 | 15(50) |
| Outcome | |
|
AWD | 19(63) |
|
DOD | 11(37) |
| Follow-up period,
months | |
|
Mean | 6.5 |
|
Range | 1-150 |
As indicated in Table
I, disease sites included the subtrochanteric region of the
femur (n=10), intertrochanteric region of the femur (n=6), femoral
diaphysis (n=5), femoral neck (n=5), bilateral intertrochanteric
femoral region (n=1), proximal tibia (n=2) and distal femur (n=1).
Pathological conditions included cases of lung cancer (n=9), breast
cancer (n=7), kidney cancer (n=3), multiple myeloma (n=4), liver
cancer (n=2), gastric cancer (n=2), unknown primary cancer (n=1),
uterine cancer (n=1) and esophageal cancer (n=1). The number of
metastases was ≤3 in 4 cases and >3 in 26 cases. The median
ECOG-PS before fracture was 2 (range, 0-4; <2, n=20; 2-3, n=15;
and >3, n=2 cases; Table I).
Adjuvant therapy comprised radiotherapy in two cases, chemotherapy
in 15 cases and a combination of radiotherapy and chemotherapy in
10 cases (Table I). Surgical
procedures included intramedullary nailing (n=16), endoprosthesis
(n=1), bipolar head arthroplasty (n=3), compression hip screw (CHS)
(n=3), conservative treatment (n=2), bilateral intramedullary
nailing (n=2), artificial bone stem (n=1), combined intramedullary
nail and plate fixation (n=1), right-sided artificial head
replacement (n=1) and left-sided CHS (n=1) (Table I).
Algorithm and planning
The algorithm was as follows: First, the ECOG-PS was
determined. In cases of PS 4, conservative treatment was indicated;
for PS 0-3, the fracture location was considered; and in addition,
for PS 0-3, the number of metastases was focused on. In cases of
distal or proximal involvement, the number of metastases was
determined. In cases of multiple metastases, intramedullary nailing
was considered. In cases of oligometastases and a PS of 3,
intramedullary nailing was performed. In cases of oligometastases
and a PS of 0-2, reconstruction with endoprosthesis or bipolar head
arthroplasty was performed.
Similarly, in cases of PS of 0-3 in the diaphysis,
the number of metastases was assessed. In cases of multiple
metastases, intramedullary nailing was considered. In cases of
oligo metastases and PS of 3, intramedullary nails were used. In
cases of oligo metastases and PS of 0-2, reconstruction was
performed using endoprosthesis or artificial bone stem.
Furthermore, the procedure plan was decided by two oncologic
surgeons (SN and KH).
Operating time, blood loss and
score
The operating time (mean ± S.D.) was 92.0±38.7 min
and the numbers of patients with operating times in different
ranges were as follows: 0-100 min, n=17; and >100 min, n=11
cases. The mean blood loss was 50.0 (range, 20-447) ml as shown in
Table I. The overall total MSTS
score was 569. The MSTS score was as follows: 0-10, 8 cases; 11-20,
7 cases; and 21-30, 15 cases. In addition, the MSTS score was
19.9±8.8 for intramedullary nailing and 22.0±10.9 for other
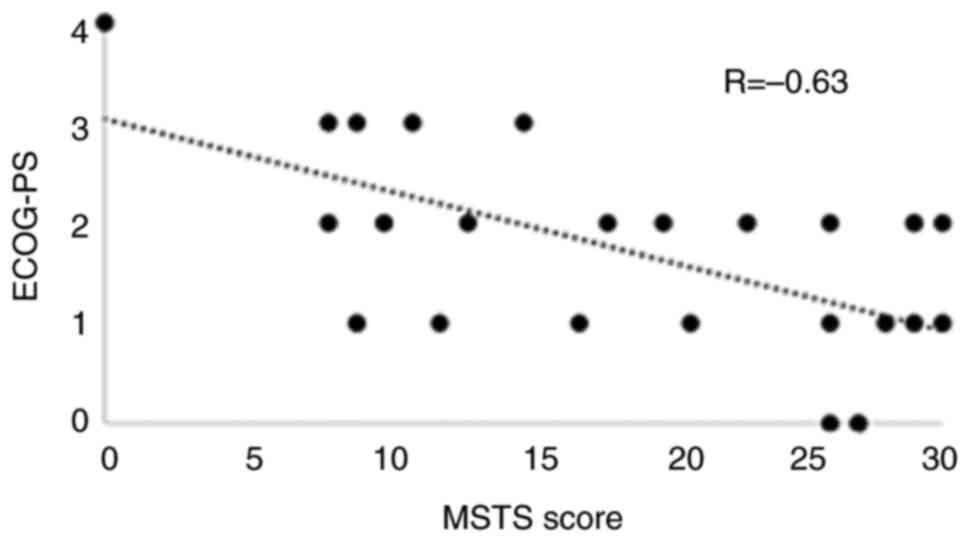
surgical procedures (P=0.23), as shown in Table I, with a negative moderate
correlation between MSTS score and pre-fracture ECOG-PS (R=-0.63;
Fig. 2).
Complications and outcomes
Postoperative complications included one case of
implant failure following the replacement of an intramedullary nail
with an endoprosthesis. The median follow-up period was 6.5 (range,
1-150) months, with outcomes categorized as alive with disease in
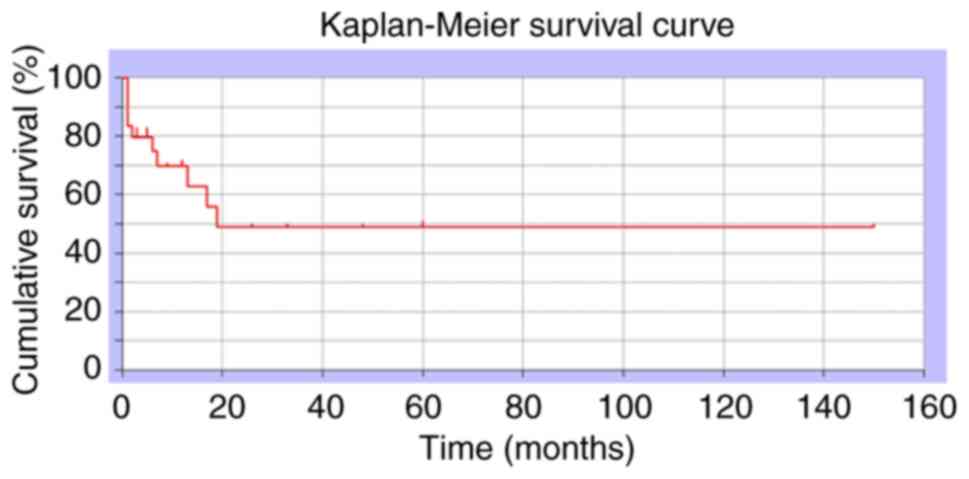
19 cases and dead of disease in 11 cases, as shown in Table I. The one-year postoperative
overall survival rate was 48.8% (Fig.
3).
Comparison of impending fractures with
pathological fractures
Furthermore, the operative time for patients with
impending fractures was significantly shorter than that for
patients with pathological fractures (83.1±21.9 vs. 113.8±44.3 min,
respectively; P=0.015), as shown in Table I. The amount of blood loss was as
follows: 0-60 ml, n=15 cases; and >60 ml, n=13 cases. The amount
of blood loss [mean (range)] of patients with impending fractures
and pathological fracture was 46.4 (20-435) and 132.6 (20-447) ml,
respectively, as shown in Table I.
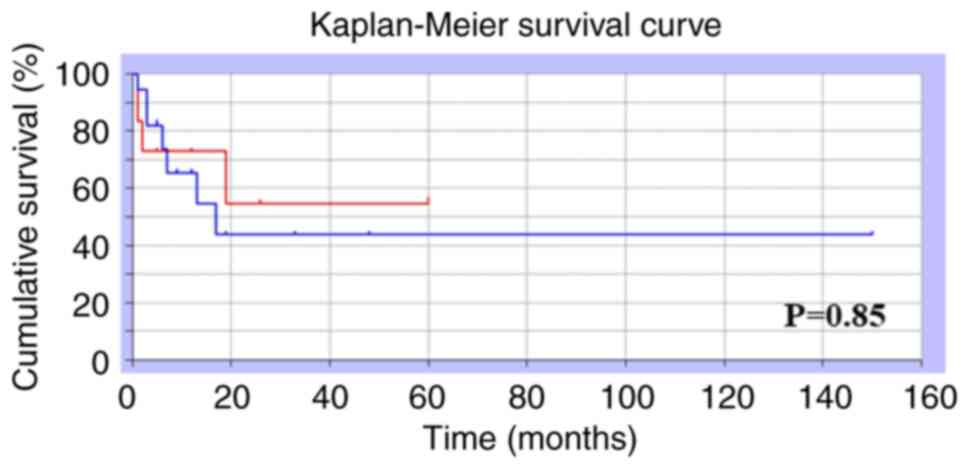
No significant difference was observed in the 1-year survival
between patients with incisional fractures and those with
pathological fractures (54.6 and 43.6%, respectively; P=0.85)
(Fig. 4).
Discussion
In the present study, the treatment outcomes of
pathological or impending fractures in metastatic bone tumors were
investigated and an algorithm was generated, with generally
favorable results. The most frequently reported sites of
pathological fractures include the femur, spine and pelvis
(9). The preferred sites of
pathological fractures in the lower extremities were the femoral
neck (50%), trochanter (30%) and subtrochanter (20%) (10). Other studies have reported 47.5% in
the femoral head and neck, 27.5% in the femoral metaphyseal area
and 25% below the femoral metaphyseal area (11). In the present study, the
subtrochanteric and trochanteric areas were more common than the
femoral neck area.
Previous studies have reported that the most common
primary sites leading to pathological femoral fractures were
multiple myeloma, breast, renal, colorectal, thyroid and lung
cancers (1). Specifically,
multiple myeloma, breast, lung and kidney cancers were the
predominant primary lesions, resulting in pathological fractures of
the proximal femur (9,11). Of note, lung cancer was relatively
common in the present study, potentially reflecting the specialized
treatments for lung cancer provided by our oncology department.
Furthermore, patients with prostate cancer were not specifically
excluded. Bone metastases from prostate cancer generally manifest
as osteosclerosis, potentially contributing to the lower incidence
of pathological fractures compared to lung and kidney cancer, which
commonly result in osteolytic metastases.
Fractures of the lower extremities are clinically
more important than those of the upper extremities because of their
weight-bearing nature (9).
Recommendations for the fixation of pathological fractures vary
depending on the anatomical site (9). Regarding femoral head and neck
fractures, treatment options include hemiarthroplasty, total hip
arthroplasty, endoprosthesis, or plate or nail fixation with void
filler. Cephalomedullary nailing is a recommended treatment for
intertrochanteric, subtrochanteric and diaphyseal fractures. In
cases of distal third femoral shaft fractures, the recommended
treatments involve locking plates or retrograde intramedullary
nails (with careful consideration by a musculoskeletal oncologist
to avoid proximal tumor spread). For supracondylar fracture, the
recommended treatment option is a distal femur periarticular plate.
A locking plate or endoprosthesis is recommended for proximal tibia
fixation, and intramedullary nails for tibial shafts.
Tumor arthroplasty offers advantages, such as rapid
stability, independence from the degree of fracture healing and
minimal risk of local progression or implant failure (12). However, it presents certain
drawbacks, including greater surgical invasiveness, bleeding,
relative difficulty in muscle reconstruction and higher costs
(12). Intramedullary nails have
the advantages of relatively low surgical invasion, the possibility
of additional radiation therapy and the ability to support load
immediately after radiation (12).
Disadvantages of intramedullary nails include the need for adequate
bone stock, instability near the joint and the risk of implant
fracture (12). Alternatively,
plate fixation provides benefits such as muscle cuff preservation,
strong fixation with locking screws, fixation of distal fractures
and a relatively large operative field allowing for visual
resection of the tumor (12). Its
disadvantages include the need for large incisions, longer surgical
procedures and lack of prophylactic fixation of the entire bone
(12). Intramedullary nails were
used in the present study. Our approach involves reconstructing
pathological fractures of the femoral neck using either artificial
head replacement or tumor arthroplasty. The choice is based on
tumor spread, prognosis, invasiveness and patient's rehabilitation
potential, including load-bearing capacity. With regard to
pathological fractures of the femoral condyle and the
subtrochanteric region, reconstruction using an intramedullary nail
was performed in anticipation of postoperative radiotherapy.
Impending fractures of the femoral neck or transverse condyle were
treated with bipolar head arthroplasty, intramedullary nails or CHS
plates. The reconstruction method was selected based on a
comprehensive evaluation of postoperative radiotherapy, fixation
stability and the amount of lesion removed. Both types of fixation
demonstrated generally good functional prognosis. However, poor
prognosis was observed when rehabilitation did not progress as
expected due to the patient's general condition.
In the present study, a protocol and treatment were
followed that resulted in the predominant use of intramedullary
nails. Previous studies have reported MSTS scores of 6.4-25.2 after
implant use for pathological fractures (11-13).
The findings of the present study align with, and corroborate the
general recommendation of our surgical indications.
Complications have been reported in 9-20% of cases
involving intramedullary nails (14,15).
The primary complications include deep infection, myocardial
infarction and stroke. In addition, 20% of patients require
revision surgery within 3 months (16). By contrast, dislocation has been
reported in 3-22% of cases as a complication of tumor arthroplasty
(11,17). The risk of periprosthetic failure
has also been reported (17-19).
In the present study, implant failure occurred in one case of
intramedullary nailing, which was subsequently replaced with an
oncological prosthesis.
Typically, patients with metastatic bone tumors are
in a terminal state (20,21). Regarding overall patient survival,
the 1-year survival range is 42-75% (15,22,23).
Fractures have been associated with an increased mortality risk in
patients with malignant bone disease (24). Although the survival rate of
patients with metastases remains low, advancements in medical
treatment have led to certain differences in tumor histology. In
this context, ‘improving the survival rate of the implant relative
to the patient's lifespan’ is essential. Furthermore, appropriate
treatment options should be selected with careful consideration of
the patient's life expectancy.
Previously, patients with pathological fractures
demonstrated similar morbidity and mortality rates to the
non-pathological fracture cohort but exhibited higher rates of
perioperative blood transfusions and unscheduled readmissions
(25). In the present study,
pathological fractures were associated with longer operative times
and greater blood loss than incisional fractures. However, no
significant difference was observed in survival rates. Therefore,
treatment should be initiated prior to the occurrence of
pathological fractures.
The present study has certain limitations. First,
the sample size was small. However, no problems were encountered
during the analyses. Second, it was a retrospective study. Finally,
the follow-up period was relatively short. Despite these
limitations, as many patients as possible were enrolled during the
study period.
Mirels' classification, which has been the most
commonly used thus far, assumes that a prognosis of at least 6
weeks is a prerequisite for surgery (26,27);
however, our algorithm is different in that surgery can be
indicated even when the prognosis is <6 weeks, which we consider
novel. In fact, the present study included six cases with a
prognosis of 1 month. Pathological fractures due to lower extremity
malignancies are load-bearing bones, thereby causing severe
activity of daily living (ADL) disability. Therefore, we think that
our algorithm will prove beneficial in maintaining ADL at an ideal
status until the patient's death.
In conclusion, oncologic surgeons must evaluate
patients' PS and other systemic conditions, including age, life
expectancy and presence of complications before considering the
optimal reconstructive approach to the anatomic site to be
treated.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization: KH, SN, TI and KG; methodology:
KH, SN, TI, RK and KG; software: KH, RK and SN; validation: SN, NS,
TI, RK and KG; formal analysis: SN, NS, TI and KG; investigation:
KH, TI, RK and SN; data curation: KH, SN, TI, RK and KG;
writing-original draft preparation: KH, SN, TI, RK and KG;
writing-review and editing: KH, SN, TI, RK and KG. Checking and
confirming the authenticity of the raw data: KH and KG. All authors
have read and agreed to the published version of the
manuscript.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
the Ethics Committee of Kindai University Hospital (Osaka, Japan;
approval no. 31-153). Written informed consent was obtained from
all participants included in the current study.
Patient consent for publication
Consent for publication was obtained from all
participants included in the current study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guzik G: Oncological and functional
results after surgical treatment of bone metastases at the proximal
femur. BMC Surg. 18(5)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fontanella C, Fanotto V, Rihawi K, Aprile
G and Puglisi F: Skeletal metastases from breast cancer:
Pathogenesis of bone tropism and treatment strategy. Clin Exp
Metastasis. 32:819–833. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Scorianz M, Gherlinzoni F and Campanacci
DA: Metastases to the long bones: Algorithm of treatment. In:
Management of Bone Metastases. Denaro V, Di Martino A and Piccioli
A (eds). Springer, Cham, pp93-102, 2019.
|
|
4
|
Hage WD, Aboulafia AJ and Aboulafia DM:
Incidence, location, and diagnostic evaluation of metastatic bone
disease. Orthop Clin North Am. 31:515–528. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Piccioli A, Spinelli MS and Maccauro G:
Impending fracture: A difficult diagnosis. Injury. 45 (Suppl
6):S138–S141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Younis M, Barnhill SW, Maguire J and
Pretell-Mazzini J: Management of humeral impending or pathological
fractures with intramedullary nailing: Reaming versus non reaming
technique-a retrospective comparative study. Musculoskelet Surg.
106:35–41. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Blagden SP, Charman SC, Sharples LD, Magee
LR and Gilligan D: Performance status score: Do patients and their
oncologists agree? Br J Cancer. 89:1022–1027. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar M and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the musculoskeletal system. Clin Orthop Relat Res. 286:241–246.
1993.PubMed/NCBI
|
|
9
|
Harrington KD: Orthopedic surgical
management of skeletal complications of malignancy. Cancer. 80
(Suppl 8):S1614–S1627. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hu YC, Lun DX and Wang H: Clinical
features of neoplastic pathological fracture in long bones. Chin
Med J (Engl). 125:3127–3132. 2012.PubMed/NCBI
|
|
11
|
Angelini A, Trovarelli G, Berizzi A, Pala
E, Breda A, Maraldi M and Ruggieri P: Treatment of pathologic
fractures of the proximal femur. Injury. 49 (Suppl 3):S77–S83.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Willeumier JJ, van der Linden YM, van de
Sande MAJ and Dijkstra PDS: Treatment of pathological fractures of
the long bones. EFORT Open Rev. 1:136–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goryń T, Pieńkowski A, Szostakowski B,
Zdzienicki M, Ługowska I and Rutkowski P: Functional outcome of
surgical treatment of adults with extremity osteosarcoma after
megaprosthetic reconstruction-single-center experience. J Orthop
Surg Res. 14(346)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wedin R, Bauer HC and Wersäll P: Failures
after operation for skeletal metastatic lesions of long bones. Clin
Orthop Relat Res. 358:128–139. 1999.PubMed/NCBI
|
|
15
|
Wedin R and Bauer HC: Surgical treatment
of skeletal metastatic lesions of the proximal femur:
Endoprosthesis or reconstruction nail? J Bone Joint Surg Br.
87:1653–1657. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jacofsky DJ, Haidukewych GJ, Zhang H and
Sim FH: Complications and results of arthroplasty for salvage of
failed treatment of malignant pathologic fractures of the hip. Clin
Orthop Relat Res. 427:52–56. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moore J, Isler M, Barry J and Mottard S:
Major wound complication risk factors following soft tissue sarcoma
resection. Eur J Surg Oncol. 40:1671–1676. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Piccioli A, Rossi B, Scaramuzzo L,
Spinelli MS, Yang Z and Maccauro G: Intramedullary nailing for
treatment of pathologic femoral fractures due to metastases.
Injury. 45:412–417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dunn J, Kusnezov N, Bader J, Waterman BR,
Orr J and Belmont PJ: Long versus short cephalomedullary nail for
trochanteric femur fractures (OTA 31-A1, A2 and A3): A systematic
review. J Orthop Traumatol. 17:361–367. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roudier MP, True LD, Higano CS, Vesselle
H, Ellis W, Lange P and Vessella RL: Phenotypic heterogeneity of
end-stage prostate carcinoma metastatic to bone. Hum Pathol.
34:646–653. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ganesh K and Massagué J: Targeting
metastatic cancer. Nat Med. 27:34–44. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mavrogenis AF, Pala E, Romagnoli C,
Romantini M, Calabro T and Ruggieri P: Survival analysis of
patients with femoral metastases. J Surg Oncol. 105:135–141.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chandrasekar CR, Grimer RJ, Carter SR,
Tillman RM, Abudu A and Buckley L: Modular endoprosthetic
replacement for tumours of the proximal femur. J Bone Joint Surg
Br. 91:108–112. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saad F, Lipton A, Cook R, Chen YM, Smith M
and Coleman R: Pathologic fractures correlate with reduced survival
in patients with malignant bone disease. Cancer. 110:1860–1867.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Boddapati V, Held MB, Levitsky M, Charette
RS, Neuwirth AL and Geller JA: Risks and complications after
arthroplasty for pathological or impending pathological fracture of
the hip. J Arthroplasty. 36:2049–2054.e5. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Errani C, Mavrogenis AF, Cevolani L,
Spinelli S, Piccioli A, Maccauro G, Baldini N and Donati D:
Treatment for long bone metastases based on a systematic literature
review. Eur J Orthop Surg Traumatol. 27:205–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mirels H: Metastatic disease in long
bones. A proposed scoring system for diagnosing impending
pathologic fractures. Clin Orthop Relat Res. 249:256–264.
1989.PubMed/NCBI
|