Introduction
Cervical cancer is the fourth most frequent cancer
among women globally. Squamous cell carcinoma (SCC) constitutes the
majority of these malignant tumors representing 80-90% of all
cancers in this location. Typically affecting women in their sixth
decade of life, patients with small tumors often do not show
symptoms, while larger tumors can manifest with abnormal vaginal
bleeding, discharge, and pain.
Most cervical SCCs are human papillomavirus (HPV)
related. Notably, HPV-related SCCs are usually less aggressive than
their HPV-independent counterparts and they arise from high-grade
squamous intraepithelial lesions (HSIL). Among the multitude of HPV
genotypes, types 16 and 18 stand out as the predominant
contributors, implicated in the majority of cervical SCC
occurrences (1).
The epidemiological landscape of cervical cancer has
witnessed significant transformations, especially in high-income
countries, where a decrease in both incidence and mortality rates
has been observed. This decline can be largely attributed to the
implementation of screening programs and the widespread HPV
vaccination initiatives. These measures have played a crucial role
in reducing the impact of cervical cancer and preventing its
harmful effects (1).
Histologically, SCCs display infiltrative nests and
cords within a desmoplastic or inflammatory stroma. Nuclear
pleomorphism and increased mitotic activity characterize these
lesions. Various subtypes, including non-keratinizing,
keratinizing, basaloid, warty, and papillary SCCs, exhibit distinct
features and a subtype of squamous cell carcinoma with giant
osteoclast-like cells has not been defined.
Immunohistochemistry plays a vital role in
diagnosing HPV-associated SCCs, with p16 immunohistochemical
testing recommended in conjunction with molecular HPV typing.
Osteoclast-like giant cells (OGCs), characterized by
their multinucleated appearance and resemblance to osteoclasts,
have been described in association with some malignant tumors at
various anatomical locations, e.g. the skin, breast and pancreas
(2,3). Despite their rarity, these unique
cells have captured attention due to their potential diagnostic
significance and implications for tumor biology. To the best of our
knowledge, only six cases of SCCs with OGCs in the uterine cervix
have been reported to date (4-8).
This limited incidence underscores the importance of meticulous
observation and thorough histopathological examination to identify
and characterize such atypical tumor features within the
cervix.
Case report
We present the case of a 38-year-old woman with a
medical background of ovarian ectopic pregnancy in 2013 and one
vaginal childbirth in 2020, who underwent periodic cervico-vaginal
cytological screening, the last of which was performed in November
2017 with no remarkable findings. In September 2021, she consulted
with the main complaint of coitorrhagia at Germans Trias i Pujol
University Hospital (Barcelona, Spain). The abdomen was depressible
and no signs of peritonism or palpable masses were detected. On
vaginal examination, a mass of approximately 4 cm was found in the
posterior lip of the uterine cervix, from which a biopsy was
taken.
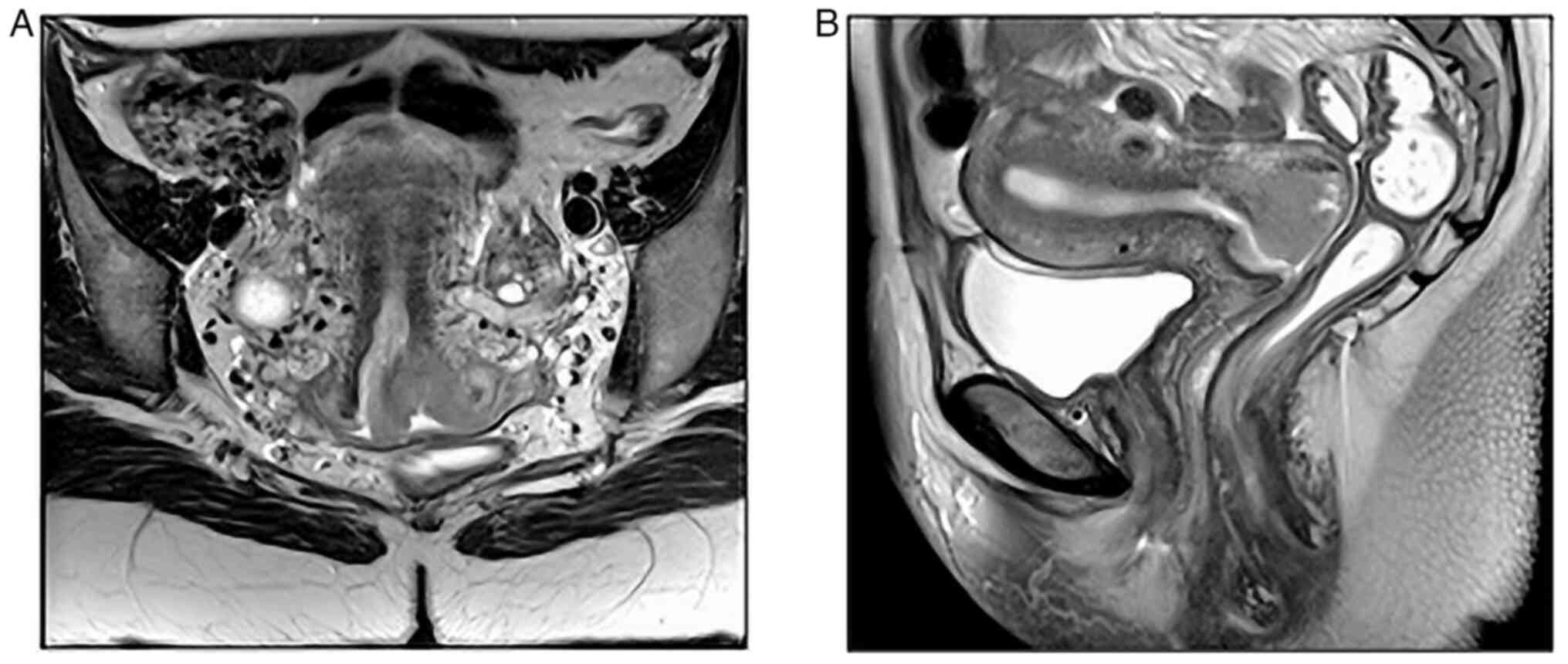
Magnetic resonance showed intimal contact of the
mass with the vaginal posterior wall and suspicion of parametrial
affection. Furthermore, an enlarged lymph node was found in the
left external iliac region, with no evidence of retroperitoneal
lymphadenopathies (Fig. 1).
The histological study on biopsy revealed a solid
proliferation composed of epithelial cells with abundant
eosinophilic cytoplasms and without evident intercellular bridges
or keratin pearls and enlarged and hyperchromatic nuclei, thus a
diagnosis of non-keratinizing SCC was made. Immunohistochemistry
showed a block positive expression of p16.
Two months later, radical hysterectomy and
iliac-obturator lymphadenectomy with ovarian preservation were
performed. The specimen exhibited a 3.5x3x2.1 cm exophytic lesion
growing at the posterior lip of the uterine cervix, with no other
structures affected, and a free closest margin of 2 mm at left
parametrium. No metastatic lymph nodes were found; thus, the
patient was a candidate for follow-up.
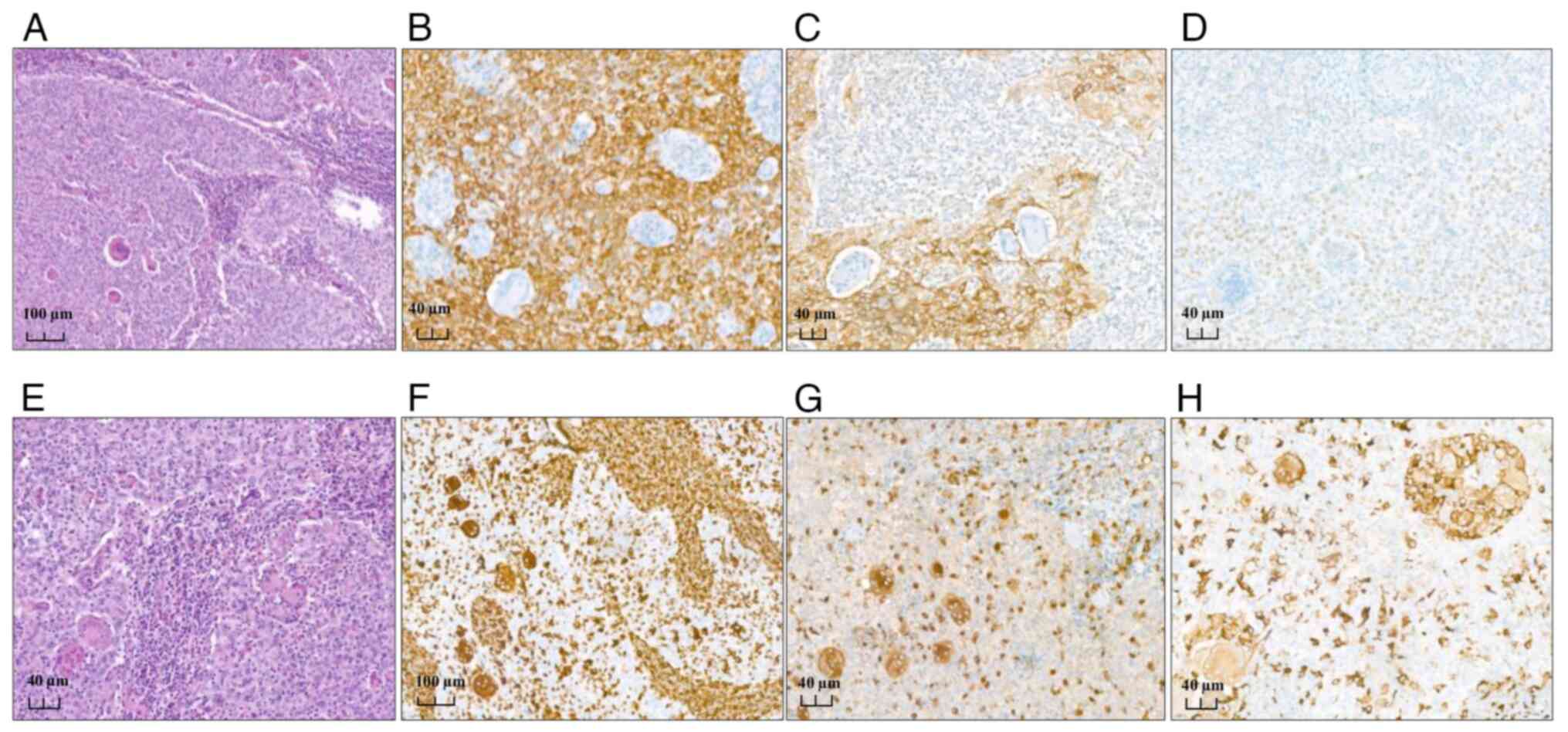
The histological morphology of the mass was similar
to the one previously described in the biopsy sample. High-grade
squamous intraepithelial lesion was found in the tumor boundaries,
and an intense intra and peritumoral inflammatory reaction was
noted, mostly lymphocytic, with a striking number of multinucleated
OGC-like cells. These cells were heterogeneously distributed
throughout the tumor with no evidence of clustering. The previous
biopsy sample was then reviewed, and some OGCs were retrospectively
observed (Fig. 2).
Immunohistochemistry was performed on whole tissue
sections using CC1 (Roche, Ventana Medical Systems) for antigen
retrieval and HRP Multimer as secondary antibody (Roche, cat. no.
253-4290, pre-diluted 55 µg/ml). Counterstaining consists in two
steps; hematoxylin (16 min) and bluing reagent (4 min) for all the
antibodies. The used antibodies for this study were: CKβE12 [34
βE12 (Roche) 95˚C, 32 min (cat. no. 790-4373) pre-diluted 1.4
µg/ml], EMA [E29 (Roche) 95˚C, 32 min (cat. no. 790-4463)
pre-diluted 0,5 µg/ml], p40 [BC28 (Roche) 36˚C, 48 min (cat. no.
790-4950) pre-diluted 0.4 µg/ml], p63 [4A4 (Roche) 95˚C, 52 min
(cat. no. 790-4509) pre-diluted 0.14 µg/ml] p16 [E6H4 Histo (Roche)
95˚C, 32 min (cat. no. 805-4713) pre-diluted 1.0 µg/ml] CD68 [kp-1
(Roche) 95˚C, 32 min (cat. no. 790-2931) pre-diluted 0.4 µg/ml],
CD163 [MRG-26 (Roche) 37˚C, 32 min (cat. no. 760-4437) pre-diluted
0.19 µg/ml], p53 [DO-7 (Roche) 95˚C, 56 min (cat. no. 800-2912)
pre-diluted 0.5 µg/ml] and Vimentin [V9 (Roche) 95˚C, 32 min (cat.
no. 790-2917) pre-diluted 2.5 µg/ml]. Positive staining was defined
according to ASCO/CAP guidelines.
Epithelial cells showed CKβE12, EMA, p40, p63 and
p16 block expression, and no reactivity for vimentin, CD68 and
CD163. p16 positivity led to HPV analysis, so DNA from the sample
was PCR amplified with the VisionArray® HPV PreCise Master Mix
(ES-0007-50, Zytovision) and genotype obtained after hybridization
with the VisionArray® HPV Chip 1.0 (VA-0001-10, Zytovision), which
allows the detection of forty-one different types, obtaining a
positive result for viral HPV 34 (classified as probable high
oncogenic risk) (Fig. 3). A
negative result was obtained for HPV 16, 18 and other high risk
types.
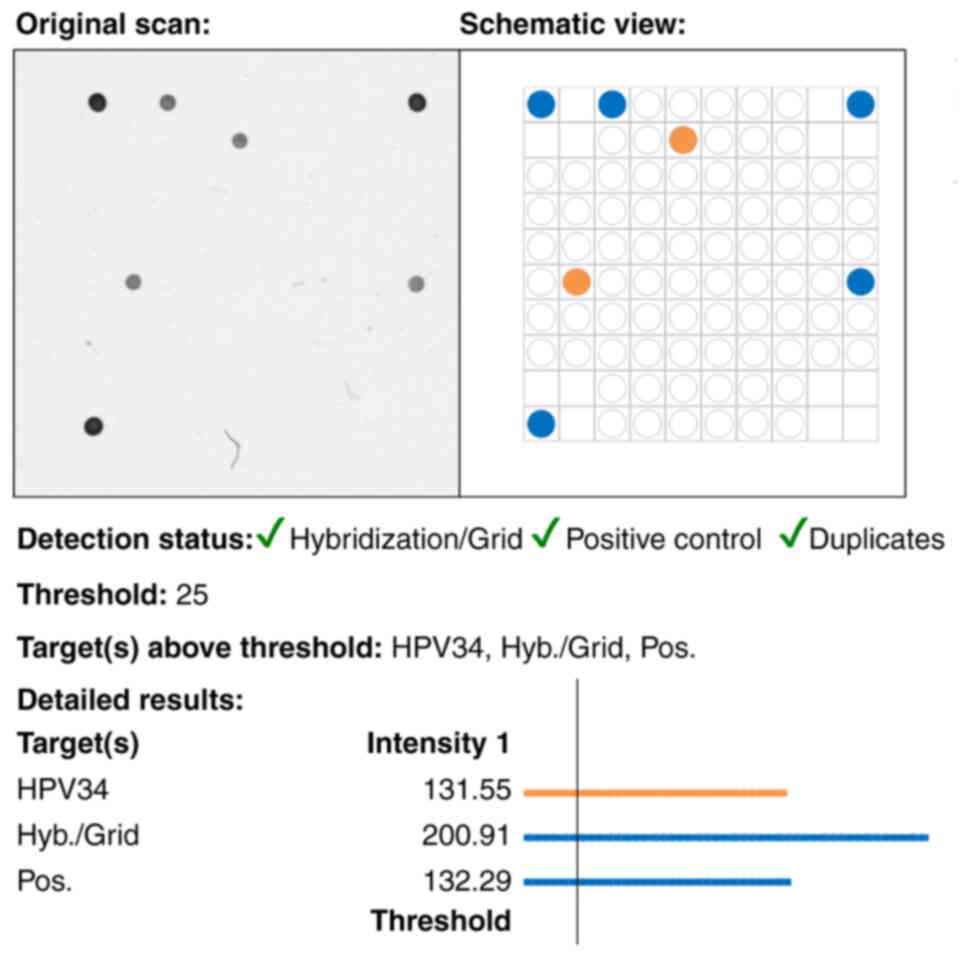 | Figure 3HPV evaluation with Vision Array
Technology. Amplified DNA is hybridized in the HPV Chip 1.0
(Zytovision) which allows the detection of high risk genotypes 16,
18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59, other probably high
risk and low risk types. The HPV Chip is scanned and processed by
the VisionArray® Analyzer Software that in the present case
highlights the 34 type detected in an orange spot. Controls are
marked as blue spots. HPV, human papillomavirus. |
OGCs were reactive to vimentin, CD68 and CD163 and
negative for CKβE12, EMA, p40, p63 and p16, elucidating an inverted
expression profile compared to the epithelial cells (Fig. 2). Staining with p53 showed a
wild-type pattern expression in both cell populations. Therefore,
the final diagnosis was a non-keratinizing poorly differentiated
(G3) SCC with HPV-associated OGCs, stage IB (FIGO 2008).
Discussion
While there are various tumors whose neoplastic
cells can show OGC morphology, neoplasms with non-neoplastic OGCs
have been also described, some of which have been accepted as new
entities.
The case we report could be one of these tumors with
immune response associated giant cells, the significance of which
is still not well understood. There are some data to support this
hypothesis. Firstly, the immunohistochemical expression of
macrophagic-histiocytic markers in the OGCs and the associated
prominent lymphocytic infiltrate suggest a reactive origin.
Moreover, some cases described in the skin (9) and the pancreas (3) have been related to a p53 mutational
pathway with an immunohistochemical aberrant expression in tumor
cells as a surrogate marker and a wild-type phenotype in associated
OGCs. However, this is not the scenario in our case, since the main
mutational pathway is probably associated with HPV, and p53 shows a
wild-type expression in both the epithelial cells and OGCs.
Finally, the non-neoplastic origin of these giant cells has been
confirmed by molecular analysis in OGCs associated with ductal
pancreatic adenocarcinoma, based on their diploid nature and the
absence of KRAS mutations (10,11).
The consistency of these results and the number of cases reported
to date in the pancreas has led to the definition of a new entity
in the latest edition of the WHO classification of tumors (12) i.e. ‘undifferentiated carcinoma with
osteoclast-like giant cells’.
Although the nature of the OGCs remains uncertain
some researchers propose a syncytial fusion of macrophages,
mirroring the process observed in osteoclastogenesis. The
osteoclast maturation is regulated by the expression of cytokines,
which are also expressed in tumor-associated macrophages and immune
cells, so it is hypothesized that OGCs share molecular features
with macrophages present within the tumor (13-15).
In fact, in the context of breast and pancreas cancer, it has been
noted that this type of tumors present a highly vascularized
microenvironment alongside inflammatory cells (lymphocytes,
histiocytes) (15,16). In other locations such as skin or
uterine cervix these characteristics are not so well defined.
Besides this, the significance of this type of
immunological response is still unknown. The biological behavior of
these tumors is not well studied; in terms of recurrence and
metastasis this tumors do not appear to have distinctive
histological characteristics. In breast cancer, prognosis seems to
be related to the intrinsic characteristics of the carcinoma and is
not associated with the presence of OGCs (17). Similar cases have been described in
lung (18) and skin squamous
carcinomas (9), where the
prognosis and clinical behavior is uncertain. In the urinary
bladder, OGC associated carcinomas seem to have an aggressive
course, but it is worth noting that most of the cases have been
diagnosed as undifferentiated carcinomas (19). In the pancreas, however, most cases
seem to have a better prognosis than the usual undifferentiated
carcinomas (20).
As is well known, the vast majority of cervical SCCs
are associated with high-risk HPV genotypes, of which 70% are
caused by types 16 and 18. In the cases of SCC with OGCs reported
to date, PCR for HPV detection has been performed in only three of
them, and just two were HPV related, with association to types 16
(case 6) (8) and 34 (present case)
(Table I). HPV type 34, in
contrast to type 16, is reported in the literature as a probable
high oncogenic risk genotype and is less frequently found.
Morphological differences have not yet been described between the
most prevalent types of HPV-associated SCC; thus, it is difficult
to find an association between the OGC variant and the subtypes of
HPV. Therefore, it would be interesting to genotype all the cases
to establish a possible relationship with morphology.
 | Table IReview of the cases of uterine SCC
associated with OGCs. |
Table I
Review of the cases of uterine SCC
associated with OGCs.
| Case | First author,
year | Age, years | Tumor diameter,
cm | Growth pattern | Stage | HPV PCR | Histological
type | Treatment | Status | Follow-up period | IHC staining of
OGCs | (Refs.) |
|---|
| 1 | Pang, 1998 | 65 | 6 | Exophytic | Ib2 | Not reported | Sarcomatoid | SP, RT and CT | DOD | 7 weeks | CD68 | (4) |
| 2 | Pang, 1998 | 61 | 5 | Exophytic | Ib2 | Not reported | Sarcomatoid | SP, RT and CT | DOD | 14 months | CD68 | (4) |
| 3 | Singh et al,
2012 | 60 | 4.5 | Infiltrating | Ib2 | Negative | Non-keratinizing | RT and CT | ACR | 6 months | CD68 | (5) |
| 4 | Yu et al,
2014 | 84 | 5 | Exophytic | Ib2 | Not reported | Non-keratinizing | RT | DOD | 8 months | CD68, vimentin | (6) |
| 5 | Alemán-Mezaet
al, 2014 | 49 | 2.7 | Exophytic | Ib1 | Not reported | Non-keratinizing | SP | ACR | 7 months | CD68, vimentin | (7) |
| 6 | Dejima et al,
2020 | 49 | 2.5 | Not reported | Ib1 | 16 | Non-keratinizing | SP, RT and CT | ACR | 22 months | CD68, CD204 | (8) |
| 7 | Present case,
2023 | 38 | 3.5 | Exophytic | Ib2 | 34 | Non-keratinizing | SP | ACR | 24 months | CD68, vimentin | - |
This case, the seventh with these characteristics in
the uterine cervix, is the first reported in Europe and notably
involves the youngest patient in the total series (Table I). Due to the small number of cases
of SCC with OGCs in this location, clinical behavior and prognosis
are still not clear.
Nevertheless, it is important to note that two out
of the three cases reported with a poor outcome had additionally a
sarcomatoid component (4), which
in itself could have explained the ominous evolution. Although our
patient achieved 24 months disease-free survival with an IB2 stage
at diagnosis, the remaining reported cases had shorter follow-up
periods, hence at present there are not enough survival data to
draw robust conclusions.
Some of these cases may have gone unnoticed,
possibly due to the associated lymphocytic inflammatory component
so OGCs can easily be overlooked among the eosinophilic background
of epithelial cells. This is further compounded by a lack of
knowledge about this variant. It is therefore advisable to report
each case presenting these histological features to contribute to
increasing the available dataset, which would help to clarify the
relationship between etiology, morphology and prognosis. Following
this approach, as happened with other locations, a new subtype of
carcinoma could potentially be defined in the not-too-distant
future if a correlation with prognosis is demonstrated.
Acknowledgements
The authors would like to thank Mr. Alan Lewis
Ritchie for their assistance with language review of the
manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
MJ and LV were involved in patient diagnosis, and in
the study conception and design. AC, CS, CPF and JGG collected the
data and performed the critical analysis. AC, JGG and CS generated
the figures and table. The draft of the manuscript was made by AC,
CPF, CS and MJ. AC and MJ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent has been obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saco A, Mills AM, Park KJ, Focchi GRA,
Carrilho C, Regauer S and Kong CS: Tumours of the uterine cervix.
In: WHO Classification of Tumours Editorial Board. Female genital
tumours [Internet]. Vol 4. 5th edition. International Agency for
Research on Cancer, Lyon, 2020. https://tumourclassification.iarc.who.int/chapters/34.
|
|
2
|
Ohashi R, Hayama A, Matsubara M, Watarai
Y, Sakatani T, Naito Z and Shimizu A: Breast carcinoma with
osteoclast-like giant cells: A cytological-pathological correlation
with a literature review. Ann Diagn Pathol. 33:1–5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Molberg KH, Heffess C, Delgado R and
Albores-Saavedra J: Undifferentiated carcinoma with osteoclast-like
giant cells of the pancreas and periampullary region. Cancer.
82:1279–1287. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pang LC: Sarcomatoid squamous cell
carcinoma of the uterine cervix with osteoclast-like giant cells:
Report of two cases. Int J Gynecol Pathol. 17:174–177.
1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Singh M, Singh S, Mahajan N and Khurana N:
Osteoclastic giant cell rich carcinoma cervix: A rare entity. J
Obstet Gynaecol. 32:499–501. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yu G, Lin C, Wang W, Han Y, Qu G and Zhang
T: Squamous cell carcinoma of the uterine cervix associated with
osteoclast-like giant cells: A case report and review of the
literature. Oncol Lett. 8:1595–1598. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alemán-Meza L, Gómez-Macías GS,
Barboza-Quintana O, Garza-Guajardo R and Loya-Solis A: Osteoclastic
giant cell rich squamous cell carcinoma of the uterine cervix: A
case report and review of the literature. Case Rep Pathol.
2014(415328)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dejima M, Hashimoto H, Sasajima Y, Nomura
N, Sugita M and Morikawa T: Uterine cervical squamous cell
carcinoma with reactive multinucleated giant cells expressing
cluster of differentiation 204: A case report and literature
review. J Obstet Gynaecol Res. 46:2174–2178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chung HJ, Wolpowitz D, Scott G, Gilmore E
and Bhawan J: Squamous cell carcinoma with osteoclast-like giant
cells: A morphologically heterologous group including
carcinosarcoma and squamous cell carcinoma with stromal changes. J
Cutan Pathol. 43:148–157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ashfaq A, Thalambedu N and Atiq MU: A rare
case of pancreatic cancer: Undifferentiated carcinoma of the
pancreas with osteoclast-like giant cells. Cureus.
14(e25118)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Campbell F and Verbeke C: Pathology of the
pancreas: A practical approach. 2nd edition. Springer, 2021.
|
|
12
|
WHO Classification of Tumours Editorial
Board: Digestive system tumours [Internet]. Vol 1. 5th edition.
International Agency for Research on Cancer, Lyon, 2019. https://tumourclassification.iarc.who.int/chapters/31.
|
|
13
|
Uehara IA, Soldi LR and Silva MJB: Current
perspectives of osteoclastogenesis through estrogen modulated
immune cell cytokines. Life Sci. 256(117921)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shishido-Hara Y, Kurata A, Fujiwara M,
Itoh H, Imoto S and Kamma H: Two cases of breast carcinoma with
osteoclastic giant cells: Are the osteoclastic giant cells
pro-tumoural differentiation of macrophages? Diagn Pathol.
5(55)2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sajjadi E, Gaudioso G, Terrasi A, Boggio
F, Venetis K, Ivanova M, Bertolasi L, Lopez G, Runza L, Premoli A,
et al: Osteoclast-like stromal giant cells in breast cancer likely
belong to the spectrum of immunosuppressive tumor-associated
macrophages. Front Mol Biosci. 9(894247)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang X, Miao J, Wang S, Shen R, Zhang S,
Tian Y, Li M, Zhu D, Yao A, Bao W, et al: Single-cell RNA-seq
reveals the genesis and heterogeneity of tumor microenvironment in
pancreatic undifferentiated carcinoma with osteoclast-like
giant-cells. Mol Cancer. 21(133)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
WHO Classification of Tumours Editorial
Board: Breast tumours [Internet]. Vol 2. 5th edition. International
Agency for Research on Cancer, Lyon 2019. https://tumourclassification.iarc.who.int/chapters/32.
|
|
18
|
Lindholm KE, Kalhor N and Moran CA:
Osteoclast-like giant cell-rich carcinomas of the lung: A
clinicopathological, immunohistochemical, and molecular study of 3
cases. Hum Pathol. 85:168–173. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Satturwar S, Parwani AV, Thomas R,
Bastacky S, Dhir R and Quiroga-Garza GM: The osteoclast-type giant
cell rich carcinoma of urinary bladder: A case series. Pathol Res
Pract. 239(154164)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Swaid MB, Vitale E, Alatassi N, Siddiqui H
and Yazdani H: Metastatic undifferentiated osteoclast-like giant
cell pancreatic carcinoma. Cureus. 14(e27586)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri: 2021 update. Int J
Gynaecol Obstet. 155 (Suppl 1):S28–S44. 2021.PubMed/NCBI View Article : Google Scholar
|