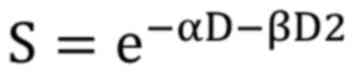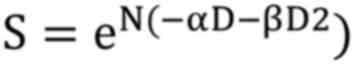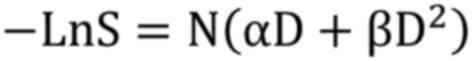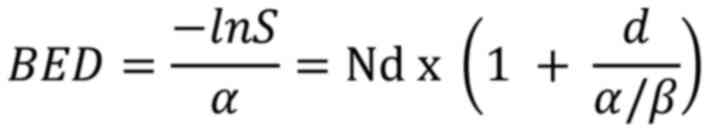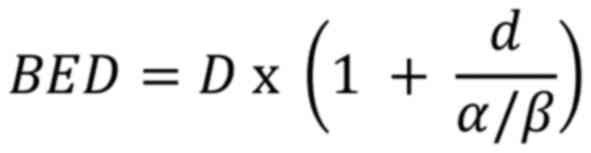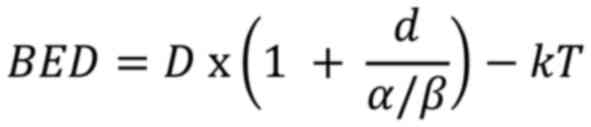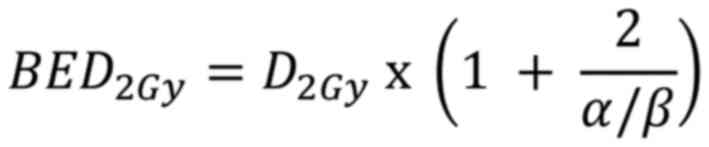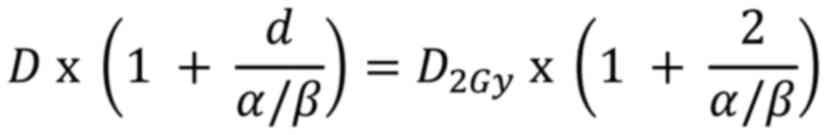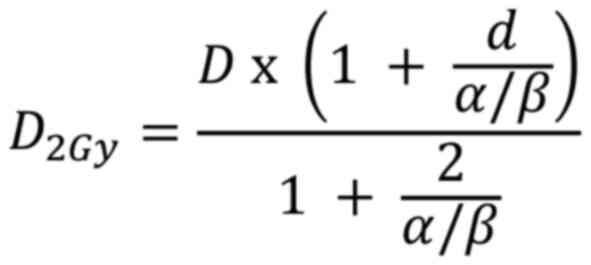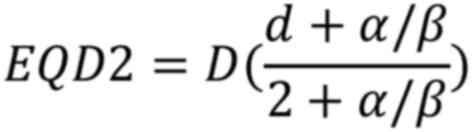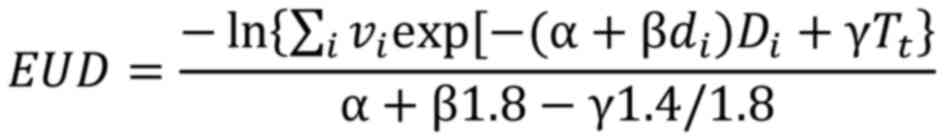1. Introduction
In radiation oncology, the tale of fractionation
schedules unfolds with the precision of a carefully crafted
narrative. In a realm where each trial and study form a piece of a
larger puzzle, the journey of breast cancer treatment has shifted
profoundly. Decades of meticulous research have unveiled pivotal
insights, reshaping how the battle against what is increasingly
becoming a chronic disease is approached.
Breast cancer is the most common malignancy in
females worldwide, with 2.3 million new cases in 2020(1). The role of adjuvant radiotherapy for
breast cancer has been extensively studied and has gone through
multiple turning points. The landmark NSABP B-06 trial showed a
notable reduction in 20-year local recurrence rates-from 39 to
14%-with addition of adjuvant radiotherapy (2). Moreover, findings from the EBCTCG
meta-analysis underscored that adjuvant radiotherapy significantly
enhances survival. It lowered the 15-year mortality risk from
breast cancer to 26% for node-negative patients and to 48% for
those with lymph node involvement (3). The analysis introduced the concept of
a ‘4:1 ratio’, illustrating that preventing 4 local recurrences by
year 5 potentially averts 1 breast cancer death by year 15(3).
Historically, breast cancer treatment relied on
delivering 50 Gy over 25 fractions spanning 5 weeks via
radiotherapy. This method sought to achieve effective tumor control
while mitigating harm to surrounding tissues using 2 Gy fractions.
However, emerging research illuminated a common responsiveness of
both healthy tissues and cancerous breast tissues to the size of
treatment fractions, as measured by the α/β ratio (4-6).
This has paved the way for clinical trials such as
the START trials, which confirmed the safety and efficacy of
hypofractionation in both early-stage breast cancer and
post-mastectomy settings (7). More
recent trials, such as FAST and FAST-Forward, have further explored
the potential of ultra-hypofractionated regimens, aiming to deliver
doses in just a few fractions (8,9).
The present scoping review seeks to synthesize
current evidence on hypofractionation in breast cancer
radiotherapy, focusing on the dose-fractionation regimens tested in
clinical trials and the radiobiological implications of varying α/β
ratios. By exploring biologically effective dose (BED) calculations
across different fractionation schedules, the present review aims
to provide insights into optimizing treatment protocols that
maximize therapeutic efficacy while minimizing toxicity.
2. The linear quadratic (LQ) model and our
understanding of hypofractionation
The LQ model is a foundational concept in
radiobiology used to describe the effects of radiation on cells
(10). It provides a mathematical
framework to predict cell survival following exposure to different
doses of radiation. The LQ model is expressed by the equation:
Where: S is the surviving fraction of cells; D is
the dose of radiation; α and β are parameters that describe the
linear and quadratic components of cell elimination, respectively.
The biologically effective dose (BED) is a measure that reflects
the biological effect of a given radiation dose. It accounts for
the total dose, dose per fraction, and the tissue response to
radiation. BED is useful for comparing different fractionation
schedules and is calculated using the formula derived from the LQ
equation as follows:
For a treatment of N fractions:
Taking the natural logarithm:
Dividing by α:
Thus:
Where: BED is the biologically effective dose; D is
the total dose in Gy (Gray); d is the dose per fraction in Gy; α/β
is the alpha/beta ratio, representing tissue sensitivity to
fraction size.
If accounting for repopulation, the formula
becomes:
Where k is a constant that depends on the
repopulation rate of the tumor, and T is the total treatment time
in days.
The equivalent dose in 2 Gy fractions (EQD2) is a
concept used to compare different radiation treatment regimens. It
normalizes doses to an equivalent dose delivered in 2 Gy fractions,
which is the ‘standard fractionation’ scheme in radiotherapy. The
EQD2 is calculated using a formula derived from the BED equation as
follows:
Normalizing the BED equation to an equivalent dose
delivered in 2 Gy fractions; the fraction size d was set to 2 Gy.
Let D2Gy be the total dose in 2 Gy fractions:
Since the BEDs for both the original and 2 Gy
fractionation regimens should be equivalent, the 2 BED expressions
were equated:
Solving for D2Gy:
Multiplying by the EQD2 equation is derived
as:
D is the total dose; d is the dose per fraction;
α/β is the tissue-specific ratio of the linear and quadratic
coefficients.
Understanding and applying these concepts are
crucial for optimizing hypofractionated regimens in breast
radiotherapy, ensuring effective and safe treatment for
patients.
Hypofractionation refers to radiation doses
exceeding 2 Gy per fraction, and ultra-hypofractionation as doses
of 5 Gy or more per fraction. Normal and malignant tissues have
different sensitivities to the size of radiotherapy fractions,
described by the α/β ratio. Lower α/β ratios (measured in Gy)
indicate greater sensitivity to changes in fraction size.
Previous studies have indicated that breast cancer
exhibits comparable sensitivity to fraction size as late-reacting
normal tissues as discussed below (5,11).
Hypofractionation involves more than just reducing the overall
treatment duration. In breast radiotherapy trials, adjustments in
the EQD2 and BED of experimental regimens aim for
iso-effectiveness, particularly concerning late tissue toxicity.
This justifies how early attempts of hypofractionation, which did
not sufficiently lower the total dose, led to high normal tissue
toxicity, increasing the inertia against moving towards
hypofractionation.
Due to the typically higher α/β ratio for acute
tissue toxicity endpoints, hypofractionated regimens often exhibit
a lower EQD2 for acute toxicity. However, this does not necessarily
translate to reduced acute toxicity because acute tissue toxicity
is sensitive to overall treatment time, often increasing as the
overall treatment time decreases, as observed in hypofractionated
schedules. Conversely, tumor cell repopulation strongly supports
the rationale for accelerating treatment through
hypofractionation.
Precisely estimating the α/β ratio is essential for
anticipating toxicity in novel hypofractionated schedules, which is
particularly highlighted in breast cancer hypofractionation trials.
The consistent results across these trials have solidified the
relevance of the LQ model, even for the most condensed
fractionation regimens employed in breast radiotherapy, as
discussed below.
3. Methods
The present study is a scoping review, aiming to
identify and describe hypofractionation regimens for whole breast
radiotherapy, and to evaluate how they differ in terms of
calculated dose using the LQ model, with and without accounting for
tumor repopulation, based on the different α/β ratios proposed in
previous studies.
Searching for hypofractionation
regimens
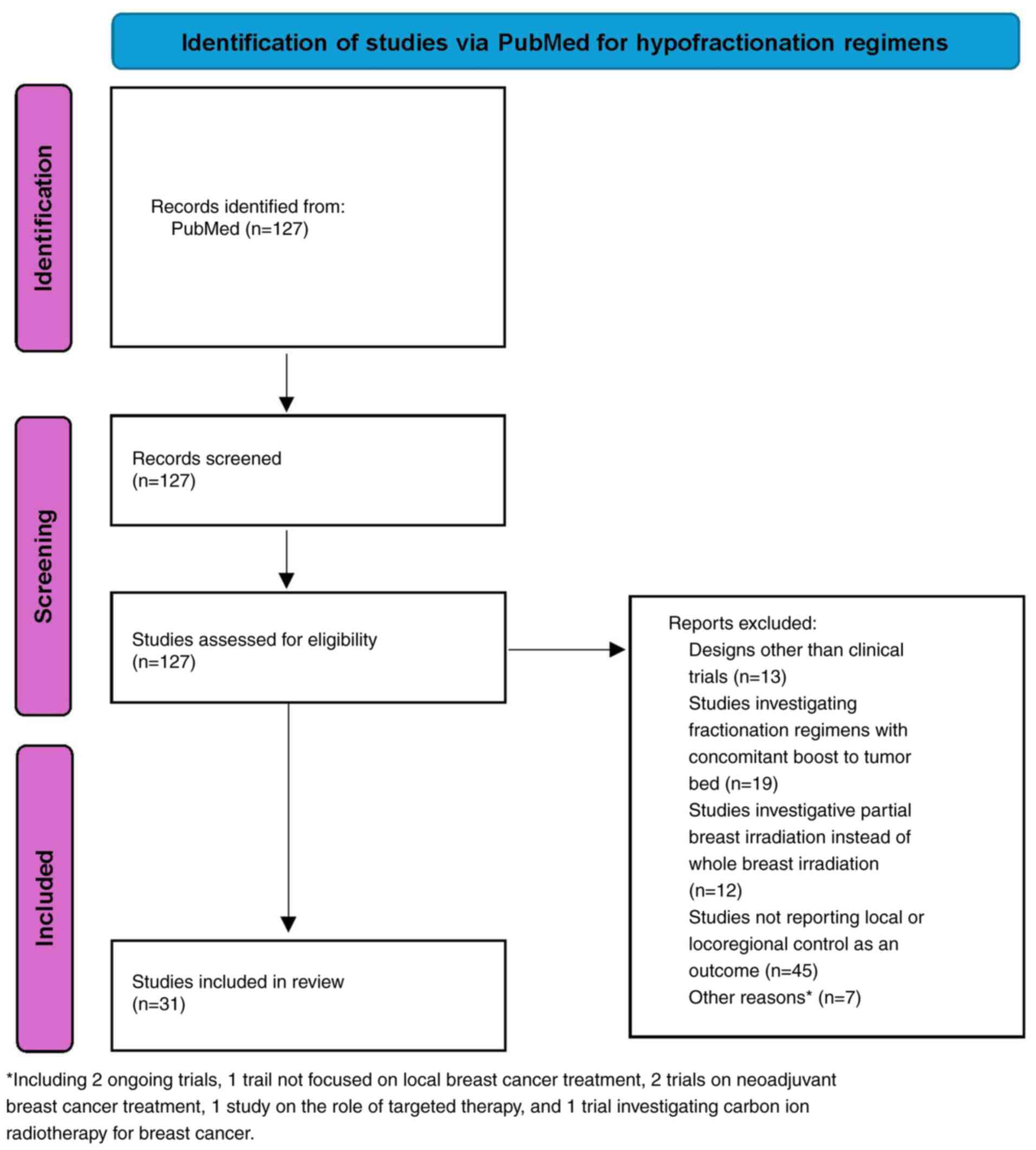
A comprehensive search of the PubMed database
(https://pubmed.ncbi.nlm.nih.gov/) was
performed to identify clinical trials on hypofractionated and
ultrahypofractionated radiotherapy for breast cancer (Fig. 1). The following search query was
used: ((‘breast neoplasms’[MeSH Terms] OR ‘breast
cancer’[Title/Abstract]) AND (‘hypofractionation’[Title/Abstract]
OR ‘hypofractionated’[Title/Abstract] OR
‘ultrahypofractionation’[Title/Abstract]) AND (‘randomized
controlled trial’[Publication Type] OR ‘clinical trial’[Publication
Type])) AND (‘2010’[Date-Publication]:
‘2024’[Date-Publication]).
Inclusion criteria. The inclusion criteria
were as follows: i) Clinical trials published after 2010 involving
patients with breast cancer receiving adjuvant radiotherapy. ii)
Studies examining hypofractionated or ultra-hypofractionated
radiotherapy. iii) Study design should be a clinical trial. iv)
Trials reporting local or locoregional control as outcomes.
Exclusion criteria. The exclusion criteria
were as follows: i) Studies with designs other than clinical
trials. ii) Studies investigating fractionation regimens with a
concomitant boost to the tumor bed. iii) Studies focusing on
partial breast irradiation instead of whole breast irradiation. iv)
Studies reporting only toxicity outcomes without local or
locoregional control. For eligible studies, data were collected on
total dose, number of fractions and fraction size.
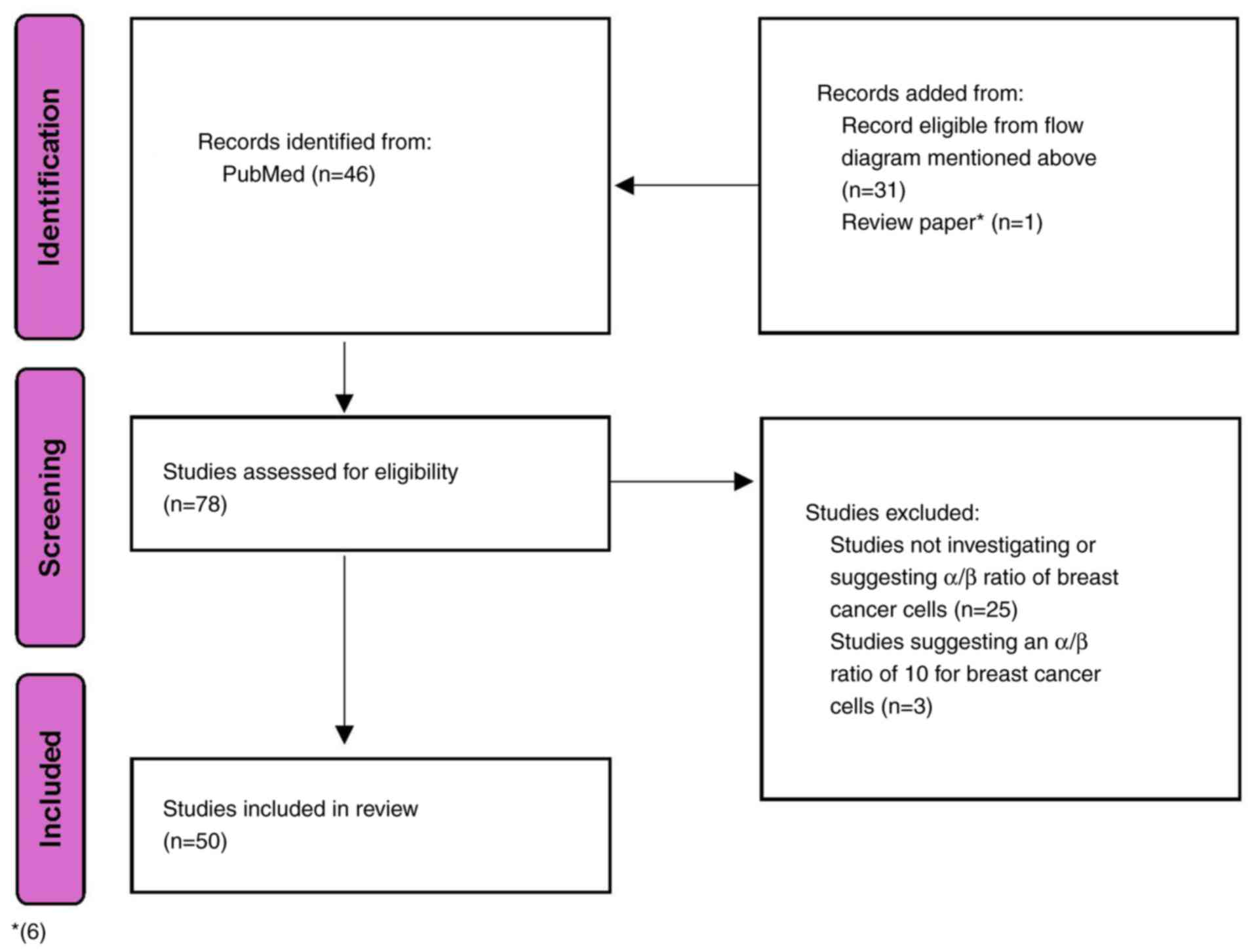
Searching for α/β ratios
An additional search was conducted in PubMed to
identify studies discussing or proposing α/β ratios for breast
cancer cells (Fig. 2). The studies
identified in the earlier search were included in this phase, as
they typically mention α/β ratios to justify the hypofractionated
regimens used. Also, a previous study that discussed possible α/β
ratios for breast cancer cells was also reviewed (12). The following search query was
applied: ((‘breast neoplasms’[MeSH Terms] OR ‘breast
cancer’[Title/Abstract] OR ‘breast carcinoma’[Title/Abstract]) AND
(‘alpha-beta ratio’[Title/Abstract] OR ‘alpha beta
ratio’[Title/Abstract] OR ‘α/β ratio’[Title/Abstract] OR
‘radiobiological parameters’[Title/Abstract]) AND (‘dose-response
relationship’[Title/Abstract] OR ‘radiotherapy’[Title/Abstract] OR
‘radiation therapy’[Title/Abstract])).
Inclusion criteria. Studies investigating,
adopting, or suggesting an α/β ratio for breast cancer cells (in
vivo or in vitro) were included.
Exclusion criteria. The exclusion criteria
were as follows: i) Studies not reporting any discussion about α/β
ratio for breast cancer cells. ii) Studies examining α/β ratio of
normal cells only. iii) Studies discussing α/β ratios for cancers
other than breast cancer. Data on the α/β ratios adopted, utilized,
or proposed by the included studies were collected.
Selection
The study selection process was conducted as
follows: First, titles and abstracts were screened to identify
studies that potentially met the inclusion criteria. Full-text
review was then carried out for selected articles to confirm their
eligibility for inclusion in the scoping review.
Subsequently, BED was analyzed for different
fractionation schedules based on the different α/β ratios.
Statistical analyses
Analysis was conducted via GraphPad Prism 10.2.2.
The BED and EQD2 were calculated using various α/β ratios extracted
from the literature. Calculated values were rounded for clarity and
ease of interpretation. To evaluate the variability of BED across
fractionation regimens, the coefficient of variation (CV) was
computed for both BED and BED adjusted for tumor repopulation
(BED-kT). The Brown-Forsythe ANOVA test was employed to assess
differences in variances across various α/β ratios. Lastly, a
discussion of different practice-changing hypofractionation trials
and elaboration of the doses used to yield equivalent tumor control
probabilities were provided.
4. Results
After identifying the relevant studies, all
hypofractionated regimens described in the eligible studies were
collected. EQD2 and BED were calculated using the different α/β
ratios obtained. To present comprehensive and clear results, some
of the calculated values were rounded. The calculated BED and EQD2
for all hypofractionated radiotherapy regimens, categorized by the
relevant α/β ratios, are included in Tables I and II.
 | Table IBiologically equivalent dose for
different radiotherapy regimens with relevant a/b ratios described
in different studies. |
Table I
Biologically equivalent dose for
different radiotherapy regimens with relevant a/b ratios described
in different studies.
| Total
dose/fractions | Total dose | Fractional
dose | Fractions | 4.8 | 4.6 | 4 | 3.7 | 3.6 | 3.5 | 3.4 | 3.1 | 3 | 2.7 | 2.5 | 2 | 1.5 | 1 |
|---|
| 50/25 | 50.00 | 2.00 | 25 | 70.8 | 71.7 | 75.0 | 77.0 | 77.8 | 78.6 | 79.4 | 82.3 | 83.3 | 87.0 | 90.0 | 100.0 | 116.7 | 150.0 |
| 43.5/15 | 43.50 | 2.90 | 15 | 69.8 | 70.9 | 75.0 | 77.6 | 78.5 | 79.5 | 80.6 | 84.2 | 85.6 | 90.2 | 94.0 | 106.6 | 127.6 | 169.7 |
| 43.2/16 | 43.20 | 2.70 | 16 | 67.5 | 68.6 | 72.4 | 74.7 | 75.6 | 76.5 | 77.5 | 80.8 | 82.1 | 86.4 | 89.9 | 101.5 | 121.0 | 159.8 |
| 42.9/13 | 42.90 | 3.30 | 13 | 72.4 | 73.7 | 78.3 | 81.2 | 82.2 | 83.3 | 84.5 | 88.6 | 90.1 | 95.3 | 99.5 | 113.7 | 137.3 | 184.5 |
| 42.5/16 | 42.50 | 2.66 | 16 | 66.0 | 67.0 | 70.7 | 73.0 | 73.9 | 74.8 | 75.7 | 78.9 | 80.1 | 84.3 | 87.7 | 98.9 | 117.8 | 155.4 |
| 42/15 | 42.00 | 2.80 | 15 | 66.5 | 67.6 | 71.4 | 73.8 | 74.7 | 75.6 | 76.6 | 79.9 | 81.2 | 85.6 | 89.0 | 100.8 | 120.4 | 159.6 |
| 41.6/13 | 41.60 | 3.20 | 13 | 69.3 | 70.5 | 74.9 | 77.6 | 78.6 | 79.6 | 80.8 | 84.5 | 86.0 | 90.9 | 94.8 | 108.2 | 130.3 | 174.7 |
| 40/15 | 40.00 | 2.67 | 15 | 62.3 | 63.2 | 66.7 | 68.9 | 69.7 | 70.5 | 71.4 | 74.5 | 75.6 | 79.6 | 82.7 | 93.4 | 111.2 | 146.8 |
| 39/13 | 39.00 | 3.00 | 13 | 63.4 | 64.4 | 68.3 | 70.6 | 71.5 | 72.4 | 73.4 | 76.7 | 78.0 | 82.3 | 85.8 | 97.5 | 117.0 | 156.0 |
| 36.63/11 | 36.63 | 3.33 | 11 | 62.0 | 63.1 | 67.1 | 69.6 | 70.5 | 71.5 | 72.5 | 76.0 | 77.3 | 81.8 | 85.4 | 97.6 | 117.9 | 158.6 |
| 36.5/10 | 36.50 | 3.65 | 10 | 64.3 | 65.5 | 69.8 | 72.5 | 73.5 | 74.6 | 75.7 | 79.5 | 80.9 | 85.8 | 89.8 | 103.1 | 125.3 | 169.7 |
| 34/10 | 34.00 | 3.40 | 10 | 58.1 | 59.1 | 62.9 | 65.2 | 66.1 | 67.0 | 68.0 | 71.3 | 72.5 | 76.8 | 80.2 | 91.8 | 111.1 | 149.6 |
| 30/5 | 30.00 | 6.00 | 5 | 67.5 | 69.1 | 75.0 | 78.6 | 80.0 | 81.4 | 82.9 | 88.1 | 90.0 | 96.7 | 102.0 | 120.0 | 150.0 | 210.0 |
| 28.5/5 | 28.50 | 5.70 | 5 | 62.3 | 63.8 | 69.1 | 72.4 | 73.6 | 74.9 | 76.3 | 80.9 | 82.7 | 88.7 | 93.5 | 109.7 | 136.8 | 191.0 |
| 27/5 | 27.00 | 5.40 | 5 | 57.4 | 58.7 | 63.5 | 66.4 | 67.5 | 68.7 | 69.9 | 74.0 | 75.6 | 81.0 | 85.3 | 99.9 | 124.2 | 172.8 |
| 26/5 | 26.00 | 5.20 | 5 | 54.2 | 55.4 | 59.8 | 62.5 | 63.6 | 64.6 | 65.8 | 69.6 | 71.1 | 76.1 | 80.1 | 93.6 | 116.1 | 161.2 |
 | Table IIEquivalent dose in 2 Gy/Fx for
different radiotherapy regimens with relevant a/b ratios described
in different studies. |
Table II
Equivalent dose in 2 Gy/Fx for
different radiotherapy regimens with relevant a/b ratios described
in different studies.
| Total
dose/fractions | Total dose | Fractional
dose | Fractions | 4.8 | 4.6 | 4 | 3.7 | 3.6 | 3.5 | 3.4 | 3.1 | 3 | 2.7 | 2.5 | 2 | 1.5 | 1 |
|---|
| 50/25 | 50.00 | 2.00 | 25 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| 43.5/15 | 43.50 | 2.90 | 15 | 49.3 | 49.4 | 50.0 | 50.4 | 50.5 | 50.6 | 50.8 | 51.2 | 51.3 | 51.8 | 52.2 | 53.3 | 54.7 | 56.6 |
| 43.2/16 | 43.20 | 2.70 | 16 | 47.6 | 47.8 | 48.2 | 48.5 | 48.6 | 48.7 | 48.8 | 49.1 | 49.2 | 49.6 | 49.9 | 50.8 | 51.8 | 53.3 |
| 42.9/13 | 42.90 | 3.30 | 13 | 51.1 | 51.4 | 52.2 | 52.7 | 52.9 | 53.0 | 53.2 | 53.8 | 54.1 | 54.8 | 55.3 | 56.8 | 58.8 | 61.5 |
| 42.5/16 | 42.50 | 2.66 | 16 | 46.6 | 46.7 | 47.1 | 47.4 | 47.5 | 47.6 | 47.7 | 48.0 | 48.1 | 48.4 | 48.7 | 49.5 | 50.5 | 51.8 |
| 42/15 | 42.00 | 2.80 | 15 | 46.9 | 47.1 | 47.6 | 47.9 | 48.0 | 48.1 | 48.2 | 48.6 | 48.7 | 49.1 | 49.5 | 50.4 | 51.6 | 53.2 |
| 41.6/13 | 41.60 | 3.20 | 13 | 48.9 | 49.2 | 49.9 | 50.4 | 50.5 | 50.7 | 50.8 | 51.4 | 51.6 | 52.2 | 52.7 | 54.1 | 55.9 | 58.2 |
| 40/15 | 40.00 | 2.67 | 15 | 43.9 | 44.1 | 44.5 | 44.7 | 44.8 | 44.9 | 45.0 | 45.3 | 45.4 | 45.7 | 46.0 | 46.7 | 47.7 | 48.9 |
| 39/13 | 39.00 | 3.00 | 13 | 44.7 | 44.9 | 45.5 | 45.8 | 46.0 | 46.1 | 46.2 | 46.6 | 46.8 | 47.3 | 47.7 | 48.8 | 50.1 | 52.0 |
| 36.63/11 | 36.63 | 3.33 | 11 | 43.8 | 44.0 | 44.7 | 45.2 | 45.3 | 45.5 | 45.7 | 46.2 | 46.4 | 47.0 | 47.5 | 48.8 | 50.5 | 52.9 |
| 36.5/10 | 36.50 | 3.65 | 10 | 45.4 | 45.6 | 46.5 | 47.1 | 47.3 | 47.5 | 47.7 | 48.3 | 48.5 | 49.3 | 49.9 | 51.6 | 53.7 | 56.6 |
| 34/10 | 34.00 | 3.40 | 10 | 41.0 | 41.2 | 41.9 | 42.4 | 42.5 | 42.7 | 42.8 | 43.3 | 43.5 | 44.1 | 44.6 | 45.9 | 47.6 | 49.9 |
| 30/5 | 30.00 | 6.00 | 5 | 47.6 | 48.2 | 50.0 | 51.1 | 51.4 | 51.8 | 52.2 | 53.5 | 54.0 | 55.5 | 56.7 | 60.0 | 64.3 | 70.0 |
| 28.5/5 | 28.50 | 5.70 | 5 | 44.0 | 44.5 | 46.1 | 47.0 | 47.3 | 47.7 | 48.0 | 49.2 | 49.6 | 50.9 | 51.9 | 54.9 | 58.6 | 63.7 |
| 27/5 | 27.00 | 5.40 | 5 | 40.5 | 40.9 | 42.3 | 43.1 | 43.4 | 43.7 | 44.0 | 45.0 | 45.4 | 46.5 | 47.4 | 50.0 | 53.2 | 57.6 |
| 26/5 | 26.00 | 5.20 | 5 | 38.2 | 38.6 | 39.9 | 40.6 | 40.9 | 41.1 | 41.4 | 42.3 | 42.6 | 43.7 | 44.5 | 46.8 | 49.8 | 53.7 |
The purpose of determining the dose for different
fractionation schedules was to ensure iso-effectiveness in terms of
late tissue endpoints. Despite differences in BED between regimens,
hypofractionated doses proved to be at least equivalent to
conventionally fractionated doses.
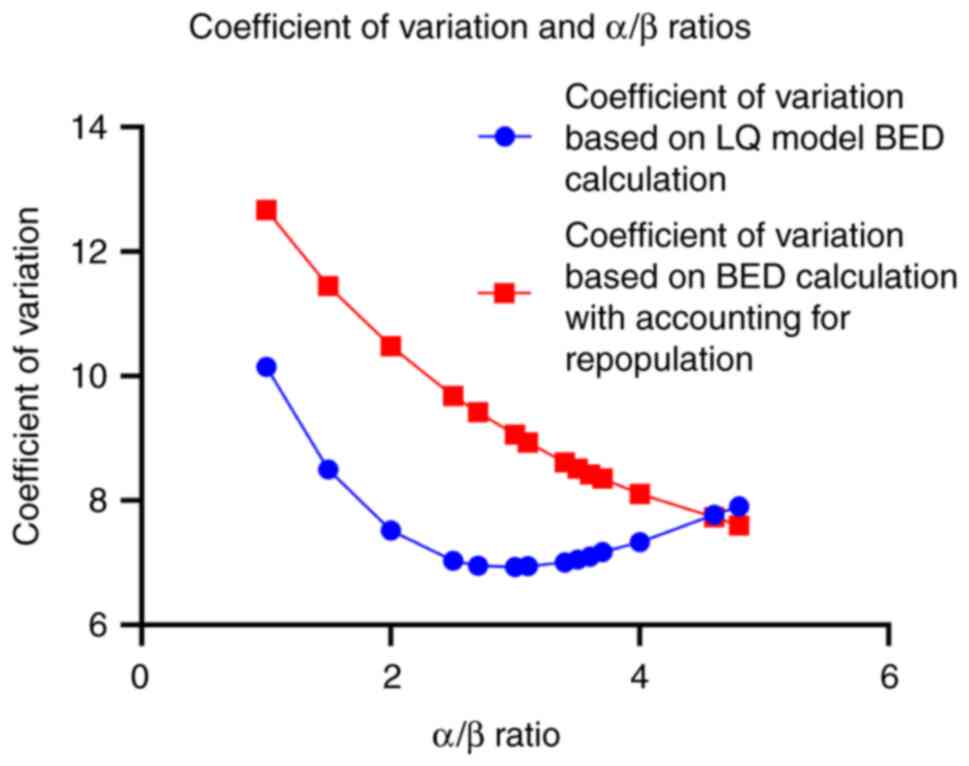
BED differs significantly between fractionation
regimens over the proposed α/β ratios. To appreciate such
variability, the coefficient of variation (CV) for the BED values
was computed with and without accounting for tumor repopulation
(BED and BED-kT, Table III). As
illustrated in Fig. 3, the CV is
lowest for an α/β ratio of ~3 when repopulation is not considered
but increases when repopulation is factored in BED calculation.
 | Table IIIBiologically equivalent dose for
different radiotherapy regimens with accounting for tumor
repopulation (BED-kT). |
Table III
Biologically equivalent dose for
different radiotherapy regimens with accounting for tumor
repopulation (BED-kT).
| Total
dose/fractions | Total dose | Fractional
dose | Fractions | 4.8 | 4.6 | 4 | 3.7 | 3.6 | 3.5 | 3.4 | 3.1 | 3 | 2.7 | 2.5 | 2 | 1.5 | 1 |
|---|
| 50/25 | 50.00 | 2.00 | 25 | 51.0 | 51.9 | 55.2 | 57.2 | 58.0 | 58.8 | 59.6 | 62.5 | 63.5 | 67.2 | 70.2 | 80.2 | 96.9 | 130.2 |
| 43.5/15 | 43.50 | 2.90 | 15 | 58.4 | 59.5 | 63.6 | 66.2 | 67.1 | 68.1 | 69.2 | 72.8 | 74.2 | 78.8 | 82.6 | 95.2 | 116.2 | 158.3 |
| 43.2/16 | 43.20 | 2.70 | 16 | 54.3 | 55.4 | 59.2 | 61.5 | 62.4 | 63.3 | 64.3 | 67.6 | 68.9 | 73.2 | 76.7 | 88.3 | 107.8 | 146.6 |
| 42.9/13 | 42.90 | 3.30 | 13 | 62.2 | 63.5 | 68.1 | 71.0 | 72.0 | 73.1 | 74.3 | 78.4 | 79.9 | 85.1 | 89.3 | 103.5 | 127.1 | 174.3 |
| 42.5/16 | 42.50 | 2.66 | 16 | 52.8 | 53.8 | 57.5 | 59.8 | 60.7 | 61.6 | 62.5 | 65.7 | 66.9 | 71.1 | 74.5 | 85.7 | 104.6 | 142.2 |
| 42/15 | 42.00 | 2.80 | 15 | 55.1 | 56.2 | 60.0 | 62.4 | 63.3 | 64.2 | 65.2 | 68.5 | 69.8 | 74.2 | 77.6 | 89.4 | 109.0 | 148.2 |
| 41.6/13 | 41.60 | 3.20 | 13 | 59.1 | 60.3 | 64.7 | 67.4 | 68.4 | 69.4 | 70.6 | 74.3 | 75.8 | 80.7 | 84.6 | 98.0 | 120.1 | 164.5 |
| 40/15 | 40.00 | 2.67 | 15 | 50.9 | 51.8 | 55.3 | 57.5 | 58.3 | 59.1 | 60.0 | 63.1 | 64.2 | 68.2 | 71.3 | 82.0 | 99.8 | 135.4 |
| 39/13 | 39.00 | 3.00 | 13 | 53.2 | 54.2 | 58.1 | 60.4 | 61.3 | 62.2 | 63.2 | 66.5 | 67.8 | 72.1 | 75.6 | 87.3 | 106.8 | 145.8 |
| 36.63/11 | 36.63 | 3.33 | 11 | 53.0 | 54.1 | 58.1 | 60.6 | 61.5 | 62.5 | 63.5 | 67.0 | 68.3 | 72.8 | 76.4 | 88.6 | 108.9 | 149.6 |
| 36.5/10 | 36.50 | 3.65 | 10 | 57.1 | 58.3 | 62.6 | 65.3 | 66.3 | 67.4 | 68.5 | 72.3 | 73.7 | 78.6 | 82.6 | 95.9 | 118.1 | 162.5 |
| 34/10 | 34.00 | 3.40 | 10 | 50.9 | 51.9 | 55.7 | 58.0 | 58.9 | 59.8 | 60.8 | 64.1 | 65.3 | 69.6 | 73.0 | 84.6 | 103.9 | 142.4 |
| 30/5 | 30.00 | 6.00 | 5 | 64.5 | 66.1 | 72.0 | 75.6 | 77.0 | 78.4 | 79.9 | 85.1 | 87.0 | 93.7 | 99.0 | 117.0 | 147.0 | 207.0 |
| 28.5/5 | 28.50 | 5.70 | 5 | 59.3 | 60.8 | 66.1 | 69.4 | 70.6 | 71.9 | 73.3 | 77.9 | 79.7 | 85.7 | 90.5 | 106.7 | 133.8 | 188.0 |
| 27/5 | 27.00 | 5.40 | 5 | 54.4 | 55.7 | 60.5 | 63.4 | 64.5 | 65.7 | 66.9 | 71.0 | 72.6 | 78.0 | 82.3 | 96.9 | 121.2 | 169.8 |
| 26/5 | 26.00 | 5.20 | 5 | 51.2 | 52.4 | 56.8 | 59.5 | 60.6 | 61.6 | 62.8 | 66.6 | 68.1 | 73.1 | 77.1 | 90.6 | 113.1 | 158.2 |
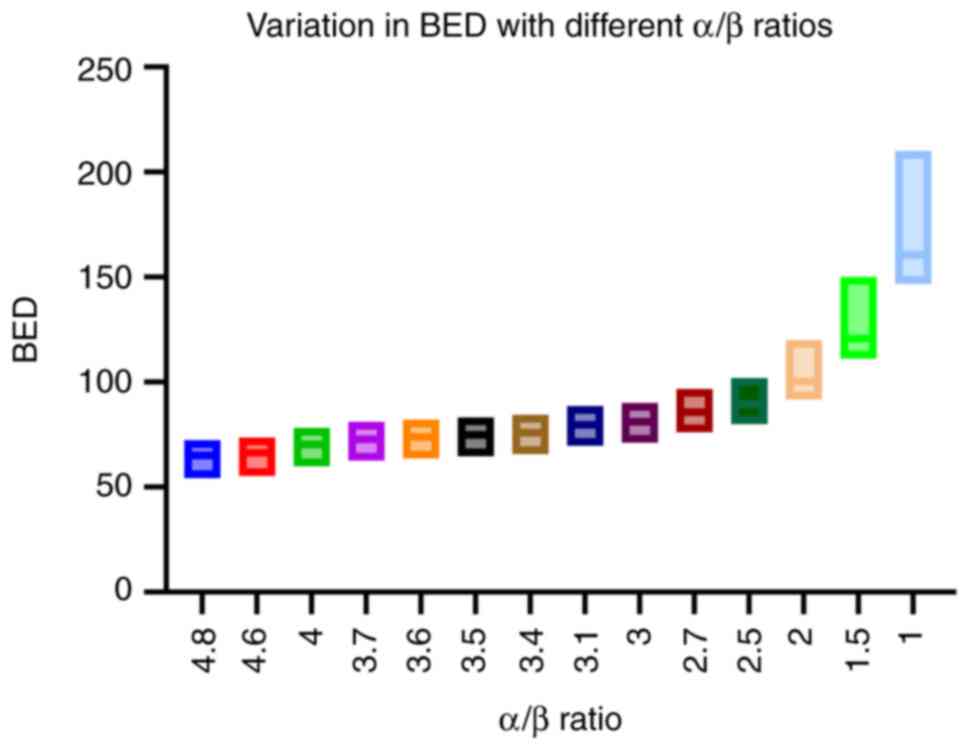
Across the various α/β ratios, there are significant
differences in the variations of BED values for different
fractionation regimens. Such variations are not consistent across
different α/β ratios. This is visually represented in Fig. 4. The Brown-Forsythe ANOVA test was
utilized to assess the differences in variances. The test yielded
an F-statistic of 219.6 with degrees of freedom (13.00, 85.11) and
a P-value of <0.0001, indicating a highly significant
result.
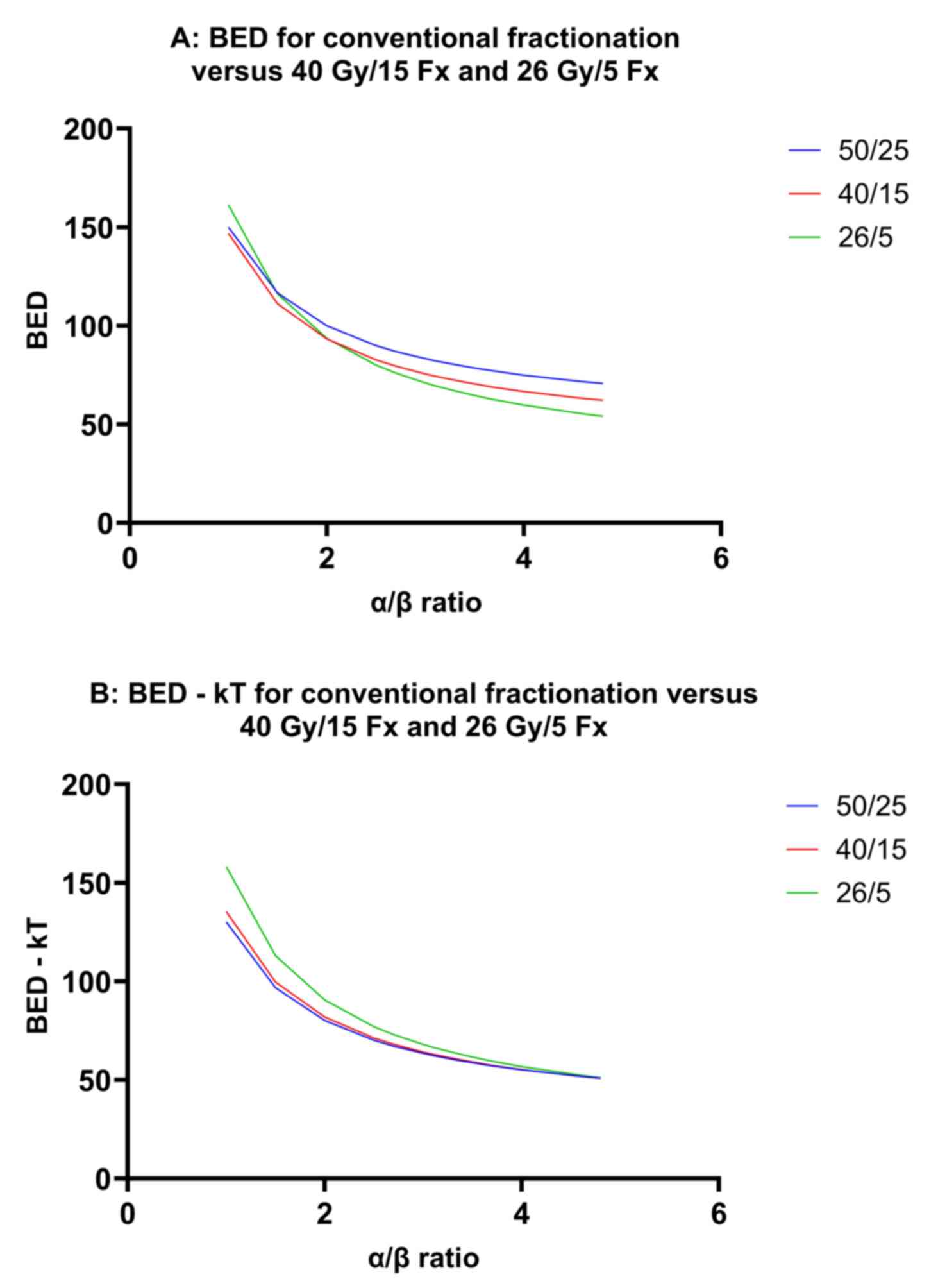
In Fig. 5, linear
graphs show how BED and BED-kT (A and B, respectively) change
across different α/β ratios for 3 common breast cancer
fractionation regimens: 50 Gy/25 Fx, 40 Gy/15 Fx, and 26 Gy/5 Fx.
Based on these figures, the α/β ratio that corresponds to an
equivalent BED across the 50 Gy/25 Fx, 40 Gy/15 Fx and 26 Gy/5 Fx
regimens appears to be <2. However, when accounting for tumor
repopulation, the α/β ratio associated with equivalent BED-kT
shifts to ~4 or slightly higher.
5. Discussion
Unraveling the α/β ratio puzzle in
breast cancer and late toxicities
Starting with the data from clinical trials, from an
analysis of 158 cases of ipsilateral local tumor recurrence, breast
cancer α/β ratio was estimated at ~4.0 Gy (11). Yet, the estimation of this value
has been evolving with the cumulative evidence of other altered
fractionation trials and studies.
The START-P and START-A trials, spanning nearly two
decades, have been pivotal in this journey. These trials were
designed to directly assess the α/β ratio for tumor control and
normal tissue effects (NTE) while standardizing overall treatment
time (5-7).
By comparing a standard 25-fraction regimen with two experimental
13-fraction hypofractionated regimens, all administered over 5
weeks, these trials provided valuable insights into the α/β ratios
for both late toxicity endpoints and tumor control without the
influence of varying overall treatment time.
START-P showed an estimated α/β value of 3.6 Gy [95%
confidence interval (CI), 1.8-5.4] for any change in breast
appearance, and an α/β value for palpable breast induration of 3.1
Gy (95% CI, 1.8-4.4) (5).
Collectively, an α/β value of ~3 Gy for late normal tissue changes
in the breast is inferred from the equivalence observed between
41.6 Gy delivered in 13 fractions and 50 Gy in 25 fractions over 5
weeks (5).
START-A trial randomized 2,236 patients to 50 Gy/25
Fx vs. 41.6 Gy or 39 Gy in 13 Fx every other day. With a median
follow up of 9.3 years, no difference in 10-year locoregional
control was found between the hypofractionated regimens and
standard fractionation (41.6 Gy vs. 50 Gy: 6.3 vs. 7.4%; P=0.65 and
39 Gy vs. 50 Gy: 8.8 vs. 7.4%; P=0.41) (6).
The α/β ratio estimated for breast cancer based on
local-regional relapse data in START Trial A is 4.8 Gy (95% CI,
0-16.3 Gy), bolstered by a meta-analysis including results from the
pilot trial, yielding an α/β ratio estimate of 4.6 Gy (95% CI,
1.1-8.1) (7). This estimate aligns
closely with the α/β ratio for NTE estimated at 3.4 Gy (95% CI,
2.3-4.5) from photographic assessments (6).
While uncertainties remain in precise fractionation
sensitivity estimation, it was evident that breast cancer appears
to differ from other cancers with higher α/β values, indicating
potential variability in response to fraction size. As the story
unfolded, START-B emerged as the next chapter, exploring how time
influences treatment outcomes.
In START B trial, 1,105 women were allocated to the
50 Gy group and 1,110 to the 40 Gy group, with a median follow-up
of 6.0 years (interquartile range, 5.0-6.2), the 5-year
local-regional tumor relapse rate was 2.2 (95% CI, 1.3-3.1) in the
40 Gy group and 3.3% (95% CI 2.2-4.5) in the 50 Gy group (13). This represents an absolute
difference of -0.7% (95% CI, -1.7 to 0.9%), suggesting that
local-regional relapse could potentially be up to 1.7% lower or at
most 0.9% higher after 40 Gy compared with 50 Gy. Both photographic
assessments and patient-reported evaluations indicated fewer late
adverse effects following treatment with 40 Gy than with 50 Gy
(side effects, including telangiectasia, breast shrinkage and edema
were significantly less frequent in the hypofractionated regimen)
(13).
Traditionally, it was anticipated that
local-regional relapse rates would be higher with 40 Gy in 15
fractions than with 50 Gy in 25 fractions, based on an α/β point
estimate of 3.5 Gy for local-regional tumor control derived from
the START-P and START-A trials. Adjusting for the EQD2 (Table II), the 40 Gy regimen in START-B
approximates closer to 45 Gy rather than 50 Gy, assuming no impact
of treatment time.
In a Canadian randomized trial involving 1,234
patients, no significant difference in ipsilateral tumor recurrence
was observed between schedules of 50 Gy in 25 fractions of 2.0 Gy
over 35 days and 42.5 Gy in 16 fractions of 2.66 Gy over 22 days to
the whole breast (11). While the
comparison based on 44 events lacks precision, if both schedules
are equally effective for tumor control, the α/β value for tumor
response could potentially be as low as 3.0 Gy, aligning with the
fractionation sensitivity observed in healthy tissues that develop
adverse effects years later.
Following these pivotal trials, the American Society
of Radiation Oncology recommends 15- or 16-fraction schedules as
preferred options for whole-breast radiotherapy (14). These trials have not only reshaped
the landscape of breast radiotherapy but also catalyzed a
renaissance in altered fractionation strategies, paving the way for
subsequent ultra-hypofractionation trials.
Ultra-hypofractionation trials for
whole breast radiotherapy
The α/β values derived from the FAST trial align
closely with those observed in the 10-year analysis of the START-A
trial, indicating estimates ranging from 3-4 Gy for late NTE in the
breast (8). This consistency
underscores the applicability of the linear-quadratic model for
fraction sizes up to 5.0-6.0 Gy.
However, there appears to be a slightly heightened
sensitivity (lower α/β value) than initially predicted (reduced
rates of moist desquamation and subsequent late skin damage) when
larger fractions are utilized. For instance, the FAST trial
randomized 915 patients with early stage invasive ductal breast
cancer (pT1-2 pN0; age ≥50) to 50 Gy/25 Fx, 30 Gy/5 Fx (once
weekly), or 28.5 Gy/5 Fx (once weekly). After a median follow up of
9.9, findings showed that patchy/confluent moist desquamation rates
were 11.7, 2.7 and 2.8% after doses of 50.0 Gy, 30.0 Gy and 28.5
Gy, respectively (8).
With an α/β value estimated at 2.7 Gy, the
15-fraction regimen equates to ~45.7 Gy in 2.0 Gy equivalents
(Table II). The FAST trial
identifies a 5-fraction schedule that appears radio-biologically
equivalent to the standard 25-fraction regimen with respect to late
NTE.
The FAST-Forward trial demonstrated the
non-inferiority, as measured by ipsilateral breast tumor relapse
rates at 5 years, of 27 Gy and 26 Gy schedules delivered in 5
fractions compared with 40 Gy in 15 fractions for patients with
early breast cancer [5-year ipsilateral breast recurrence was
similar among the three arms; 2.1 (40 Gy), 1.7 (27 Gy) and 1.4% (26
Gy)] (9). The NTE observed over 5
years with the 26 Gy regimen were comparable to those with the 40
Gy regimen.
Late NTE show a steep dose-response curve, allowing
for clinically and statistically significant differences in event
rates between the 26 Gy and 27 Gy schedules. While a 3-4 Gy
difference in EQD2 between these regimens might appear small for
detecting toxicity differences, understanding repair time can
elucidate its significance. Repair time, typically measured in
half-lives where 5 half-lives equate to ~95% repair, is crucial in
late toxicity. Previous studies proposed a half-life of ~40 days
for skin telangiectasia, suggesting a slow repair mechanism that
mitigates toxicity over time (15,16).
The 26 Gy in 5 fractions schedule, which is equally
effective with 40 Gy in 15 fractions, provides a direct estimation
of α/β for late NTE, consistent with values observed in other
trials. The α/β value of 3.7 Gy (95% CI, 0.3-7.1) for tumor control
in FAST-Forward is similar to the 3.5 Gy (1.2-5.7) estimated from
the START-P and START-A trials. Assuming no time effect, 26 Gy in 5
fractions corresponds to 46.8 Gy and 53.7 Gy in 2 Gy fractions,
assuming α/β values of 2 Gy and 1 Gy, respectively (Table II).
The 26 Gy dose level exhibits similar NTE as the 40
Gy in 15 fractions regimen, supporting its adoption as a new
standard for adjuvant breast radiotherapy. Based on the findings of
these practice-changing studies, the ultra-hypofractionated dose of
26 Gy/5 Fx was adopted in the radiotherapy clinical guidelines of
the European Society for Radiotherapy and Oncology Advisory
Committee in Radiation Oncology Practice consensus (17) and the National Institute for Health
and Care Excellence (18).
Although the LQ model has proven reliable for
predicting late tissue toxicities, the present analysis reveals
complexities when applying it to other critical endpoints, such as
tumor control. These findings underscore the necessity for a more
nuanced approach to radiobiological indices, particularly in the
context of fractionation schedules. By rigorously examining BED
variations across α/β ratios and incorporating considerations for
tumor repopulation, it becomes clearer that further research is
warranted to acquire stronger grasp on fundamental radiobiological
properties of cancer cells.
Financial impact of hypofractionation
and ultra-hypofractionation
The economic implications of hypofractionation and
ultra-hypofractionation in breast cancer radiotherapy are
substantial. Multiple studies have demonstrated the
cost-effectiveness of these approaches (19-21).
By reducing the number of treatment sessions, these fractionation
schedules not only decrease the direct costs associated with fewer
patient visits and less machine usage but also indirectly reduce
expenses related to transportation and time off work for patients.
These economic benefits make hypofractionated and
ultra-hypofractionated regimens particularly appealing, especially
in resource-limited settings. Resistance to adopting
hypofractionation has even been revealed to add extra avoidable
costs (22). Additionally, adding
radiotherapy to hormonal therapy in older patients has been found
to yield the highest clinical benefits and costs compared with
hormonal therapy alone, indicating that radiotherapy combined with
hormonal therapy is cost-effective in the US (23). Moreover, advancements in
fractionation schedules have demonstrated that a 5-fraction regimen
of radiotherapy is even more cost-effective than hormonal therapy
in older patients (24). These
findings suggested that future research should potentially shift
practice towards this regimen, as omitting hormonal therapy might
spare more side effects than omitting a 5-fraction schedule of
radiotherapy, particularly for older patients.
Advancing radiotherapy techniques and
predictive models in breast cancer treatment
Alongside advancements in fractionation schedules,
the development of radiotherapy techniques such as
three-dimensional conformal radiation therapy, intensity-modulated
radiation therapy (IMRT) and volumetric modulated arc therapy
(VMAT) has transformed the landscape of breast cancer treatment.
These technologies represent a significant leap forward in
precision medicine, offering refined control over radiation dose
distribution. IMRT and VMAT enable clinicians to achieve greater
homogeneity in dose delivery within the target area while
minimizing exposure to critical neighboring organs. Clinicians need
to be mindful of the impact that such planning techniques can have
on tumor and normal tissue radiation doses (25).
The equivalent uniform dose (EUD), pioneered by
Niemierko (26), is a method used
to address volume effects on normal tissue toxicity and tumor
control by condensing dose distributions into a single dose level
that yields equivalent biological effects. EUD is quantified as the
uniform dose delivered in daily 1.8 Gy fractions, achieving an
equivalent tumor control probability (TCP) compared with the
original dose distribution, formulated as (26,27):
Where:
vi is the fraction of the target volume
irradiated with dose Di
di is the fractional dose. In each dose
bin, di can be calculated as .
γ is ln(2)/Tpot;
Tpot is the potential doubling time;
Tt is the treatment time.
The extent of irradiated normal tissue plays a
critical role in predicting late toxicity. For example, IMRT has
demonstrated potential improvements in the homogeneity index and
EUD for targets (28). Addressing
the challenges posed by non-uniform dose distributions is crucial
for optimizing radiation plans, with methodologies including EUD
and models such as normal tissue complication probability and TCP
playing pivotal roles in plan evaluation.
Looking ahead, the incorporation of radiomics may
show potential for customizing radiation plans and techniques
according to the unique characteristics of each breast cancer
patient, with the goal of improving treatment outcomes on a
personalized basis (29-33).
Progress in biomolecular markers, radio-genomics and radiomics is
crucial in addressing individual patient vulnerability to late
toxicity (34-38).
Integrating these genomic and radiomic findings into comprehensive
clinical and dosimetric predictive models offers the possibility of
enhancing the accuracy of predictions for normal tissue toxicity.
This integrated approach also empowers radiation oncologists to
refine fractionation regimens more precisely, thereby optimizing
treatment outcomes for their patients.
As the present scoping review focused on the
radiobiology and clinical implications of whole-breast
radiotherapy, the exploration of accelerated partial breast
irradiation (APBI) is beyond its scope. APBI represents a
significant shift in radiotherapy by targeting only the tumor bed
rather than the entire breast. The rationale behind APBI is to
achieve improved geometric sparing of healthy breast tissues using
brachytherapy, external beam radiotherapy and intraoperative
radiotherapy, thereby reducing treatment-related toxicity and
improving cosmetic outcomes. The potential of APBI to further
reduce the treatment burden and enhance patient quality of life
underscores the ongoing evolution and personalization of breast
cancer radiotherapy. The extensive and intriguing radiobiology, as
well as the clinical and cost-effectiveness aspects of APBI, were
not discussed in the present study.
Ultimately, this narrative of discovery and
innovation in radiotherapy underscores a transformative era. It is
a story where science and compassion converge, promising tailored
treatments that not only combat cancer but also enhance patients'
quality of life. As these developments are put behind, the future
holds promise for personalized radiotherapy paradigms that redefine
standards of care, offering renewed hope to those battling breast
cancers.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AA designed the overall concept and outline of the
manuscript. AA, RA and FA collected the data and reviewed the
literature. AA and FA contributed to the writing and editing of the
manuscript. All authors read and approved the final version of the
manuscript. AA and FA confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–49.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241.
2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Darby S, McGale P, Correa C, Taylor C,
Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et
al: Effect of radiotherapy after breast-conserving surgery on
10-year recurrence and 15-year breast cancer death: meta-analysis
of individual patient data for 10,801 women in 17 randomised
trials. Lancet. 378 (9804):1707:–1716. 2011.PubMed/NCBI View Article : Google Scholar : https://pubmed.ncbi.nlm.nih.gov/22019144/.
|
|
4
|
Tutt A and Yarnold J: Radiobiology of
breast cancer. Clin Oncol (R Coll Radiol). 18:166–178.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yarnold J, Ashton A, Bliss J, Homewood J,
Harper C, Hanson J, Haviland J, Bentzen S and Owen R: Fractionation
sensitivity and dose response of late adverse effects in the breast
after radiotherapy for early breast cancer: Long-term results of a
randomised trial. Radiother Oncol. 75:9–17. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
START Trialists' Group. Bentzen SM,
Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J,
Dewar JA, Dobbs HJ, et al: The UK standardisation of breast
radiotherapy (START) trial A of radiotherapy hypofractionation for
treatment of early breast cancer: A randomised trial. Lancet Oncol.
9:331–341. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haviland JS, Owen JR, Dewar JA, Agrawal
RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA,
Magee BJ, et al: The UK Standardisation of breast radiotherapy
(START) trials of radiotherapy hypofractionation for treatment of
early breast cancer: 10-Year follow-up results of two randomised
controlled trials. Lancet Oncol. 14:1086–1094. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brunt AM, Haviland JS, Sydenham M, Agrawal
RK, Algurafi H, Alhasso A, Barrett-Lee P, Bliss P, Bloomfield D,
Bowen J, et al: Ten-year results of fast: A randomized controlled
trial of 5-fraction whole-breast radiotherapy for early breast
cancer. J Clin Oncol. 38:3261–3272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Murray Brunt A, Haviland JS, Wheatley DA,
Sydenham MA, Alhasso A, Bloomfield DJ, Chan C, Churn M, Cleator S,
Coles CE, et al: Hypofractionated breast radiotherapy for 1 week
versus 3 weeks (FAST-Forward): 5-year efficacy and late normal
tissue effects results from a multicentre, non-inferiority,
randomised, phase 3 trial. Lancet. 395:1613–1626. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McMahon SJ: The linear quadratic model:
Usage, interpretation and challenges. Phys Med Biol.
64(01TR01)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Owen JR, Ashton A, Bliss JM, Homewood J,
Harper C, Hanson J, Haviland J, Bentzen SM and Yarnold JR: Effect
of radiotherapy fraction size on tumour control in patients with
early-stage breast cancer after local tumour excision: Long-term
results of a randomised trial. Lancet Oncol. 7:467–471.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brunt AM, Haviland JS, Kirby AM, Somaiah
N, Wheatley DA, Bliss JM and Yarnold JR: Five-fraction radiotherapy
for breast cancer: FAST-forward to implementation. Clin Oncol (R
Coll Radiol). 33:430–439. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
START Trialists' Group. Bentzen SM,
Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss
JM, Brown J, Dewar JA, et al: The UK standardisation of breast
radiotherapy (START) trial B of radiotherapy hypofractionation for
treatment of early breast cancer: A randomised trial. Lancet.
371:1098–1107. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Smith BD, Bellon JR, Blitzblau R, Freedman
G, Haffty B, Hahn C, et al: Radiation therapy for the whole breast:
An American society for radiation oncology (ASTRO) evidence-based
guideline conflict of interest disclosure statement
acknowledgements for literature review and administrative support
and. Pract Radiat Oncol, 2018. https://pubmed.ncbi.nlm.nih.gov/29545124/.
|
|
15
|
Brand DH, Kirby AM, Yarnold JR and Somaiah
N: How low can you go? The radiobiology of hypofractionation. Clin
Oncol (R Coll Radiol). 34:280–287. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Turesson I and Thames HD: Repair capacity
and kinetics of human skin during fractionated radiotherapy:
Erythema, desquamation, and telangiectasia after 3 and 5 year's
follow-up. Radiother Oncol. 15:169–188. 1989.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meattini I, Becherini C, Boersma L,
Kaidar-Person O, Marta GN, Montero A, Offersen BV, Aznar MC, Belka
C, Brunt AM, et al: European society for radiotherapy and oncology
advisory committee in radiation oncology practice consensus
recommendations on patient selection and dose and fractionation for
external beam radiotherapy in early breast cancer. Lancet Oncol.
23:e21–e31. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Evidence review for the effectiveness of
different external beam hypofractionation radiotherapy regimens in
people with early-stage or locally advanced invasive breast cancer:
Early and locally advanced breast cancer: Diagnosis and management.
NICE Evidence Reviews Collection, 2023.
|
|
19
|
Monten C and Lievens Y: Adjuvant breast
radiotherapy: How to trade-off cost and effectiveness? Radiother
Oncol. 126:132–138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yaremko HL, Locke GE, Chow R, Lock M,
Dinniwell R and Yaremko BP: Cost minimization analysis of
hypofractionated radiotherapy. Curr Oncol. 28:716–725.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Glynn D, Bliss J, Brunt AM, Coles CE,
Wheatley D, Haviland JS, Kirby AM, Longo F, Faria R, Yarnold JR and
Griffin S: Cost-effectiveness of 5 fraction and partial breast
radiotherapy for early breast cancer in the UK: Model-based
multi-trial analysis. Breast Cancer Res Treat. 197:405–416.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Batumalai V, Delaney GP, Descallar J,
Gabriel G, Wong K, Shafiq J and Barton M: Variation in the use of
radiotherapy fractionation for breast cancer: Survival outcome and
cost implications. Radiother Oncol. 152:70–77. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ali AA, Tawk R, Xiao H, Semykina A,
Montero AJ, Moussa RK, Popoola O and Diaby V: Comparative
cost-effectiveness of radiotherapy among older women with hormone
receptor positive early-stage breast cancer. Expert Rev
Pharmacoecon Outcomes Res. 22:735–741. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ward MC, Recht A, Vicini F, Al-Hilli Z,
Asha W, Chadha M, Abraham A, Thaker N, Khan AJ, Keisch M and Shah
C: Cost-effectiveness analysis of ultra-hypofractionated whole
breast radiation therapy alone versus hormone therapy alone or
combined treatment for low-risk ER-positive early stage breast
cancer in women aged 65 years and older. Int J Radiat Oncol Biol
Phys. 116:617–626. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guerrero M and Li XA: Analysis of a large
number of clinical studies for breast cancer radiotherapy:
Estimation of radiobiological parameters for treatment planning.
Phys Med Biol. 48:3307–3326. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Niemierko A: Reporting and analyzing dose
distributions: A concept of equivalent uniform dose. Med Phys.
24:103–110. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Wang JZ and Li XA: Evaluation of external
beam radiotherapy and brachytherapy for localized prostate cancer
using equivalent uniform dose. Med Phys. 30:34–40. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Smith W, Menon G, Wolfe N, Ploquin N,
Trotter T and Pudney D: IMRT for the breast: A comparison of
tangential planning techniques. Phys Med Biol. 55:1231–1241.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Isaksson LJ, Pepa M, Zaffaroni M, Marvaso
G, Alterio D, Volpe S, Corrao G, Augugliaro M, Starzyńska A,
Leonardi MC, et al: Machine learning-based models for prediction of
toxicity outcomes in radiotherapy. Front Oncol.
10(790)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Desideri I, Loi M, Francolini G, Becherini
C, Livi L and Bonomo P: Application of radiomics for the prediction
of radiation-induced toxicity in the IMRT era: Current
state-of-the-art. Front Oncol. 10(1708)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Feng H, Wang H, Xu L, Ren Y, Ni Q, Yang Z,
Ma S, Deng Q, Chen X, Xia B, et al: Prediction of radiation-induced
acute skin toxicity in breast cancer patients using data
encapsulation screening and dose-gradient-based multi-region
radiomics technique: A multicenter study. Front Oncol.
12(1017435)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi BS, Yoo SK, Moon J, Chung SY, Oh J,
Baek S, Kim Y, Chang JS, Kim H and Kim JS: Acute coronary event
(ACE) prediction following breast radiotherapy by features
extracted from 3D CT, dose, and cardiac structures. Med Phys.
50:6409–6420. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Avanzo M, Pirrone G, Vinante L, Caroli A,
Stancanello J, Drigo A, Massarut S, Mileto M, Urbani M, Trovo M, et
al: Electron density and biologically effective dose (BED)
radiomics-based machine learning models to predict late
radiation-induced subcutaneous fibrosis. Front Oncol.
10(490)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Durdik M, Markova E, Kosik P, Vigasova K,
Gulati S, Jakl L, Vrobelova K, Fekete M, Zavacka I, Pobijakova M,
et al: Assessment of individual radiosensitivity in breast cancer
patients using a combination of biomolecular markers. Biomedicines.
11(1122)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Somaiah N, Rothkamm K and Yarnold J: Where
do we look for markers of radiotherapy fraction size sensitivity?
Clin Oncol (R Coll Radiol). 27:570–578. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ahmed KA, Liveringhouse CL, Mills MN,
Figura NB, Grass GD, Washington IR, Harris EE, Czerniecki BJ,
Blumencranz PW, Eschrich SA, et al: Utilizing the genomically
adjusted radiation dose (GARD) to personalize adjuvant radiotherapy
in triple negative breast cancer management. EBioMedicine.
47:163–169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Anbalagan S, Ström C, Downs JA, Jeggo PA,
McBay D, Wilkins A, Rothkamm K, Harrington KJ, Yarnold JR and
Somaiah N: TP53 modulates radiotherapy fraction size sensitivity in
normal and malignant cells. Sci Rep. 11(7119)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Seibold P, Webb A, Aguado-Barrera ME,
Azria D, Bourgier C, Brengues M, Briers E, Bultijnck R,
Calvo-Crespo P, Carballo A, et al: REQUITE: A prospective
multicentre cohort study of patients undergoing radiotherapy for
breast, lung or prostate cancer. Radiother Oncol. 138:59–67.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. PLOS Med. 18(e1003583)2021.PubMed/NCBI View Article : Google Scholar
|