Introduction
Approximately 90,000 people develop colorectal
cancer and 40,000 succumb to the disease annually in Japan,
demonstrating a markedly increased prevalence among Japanese people
(1).
Surgical treatment is known to be effective for
colorectal cancer and laparoscopic surgery (Lap) became widely used
in the first half of the 1990s. Its advantages are the cosmetic
appearance of the incision, reduced postoperative pain, early
improvement of intestinal movement following surgery and early
return to social activities (2).
In addition, low-level invasiveness and favorable short-term
outcomes have been reported (3–5). Its
indications have also been gradually expanded in the Japanese
Guidelines for the Treatment of Colorectal Cancer (6). However, reports on the long-term
outcomes of advanced colorectal cancer, including transverse colon
and rectal cancer, are considered insufficient (7–9).
Free cancer cells have recently attracted attention
as a marker indicating micro-metastasis, while the carcinoembryonic
antigen (CEA) mRNA has been associated with outcome (10,15–19).
It was suggested that the short-term equivalence of Lap and
conventional open colectomy (OC) with regard to the oncological
viewpoint can be investigated by measuring peripheral blood CEA
mRNA expression, which represents free cancer cells, in the
perioperative period and comparing it between Lap and OC. For this
purpose, we measured peripheral blood CEA mRNA expression in the
perioperative period and investigated whether there were
differences in the CEA mRNA-positive rate due to the different
surgical approaches (OC and Lap), surgical content and
clinicopathological characteristics.
Patients and methods
Patients
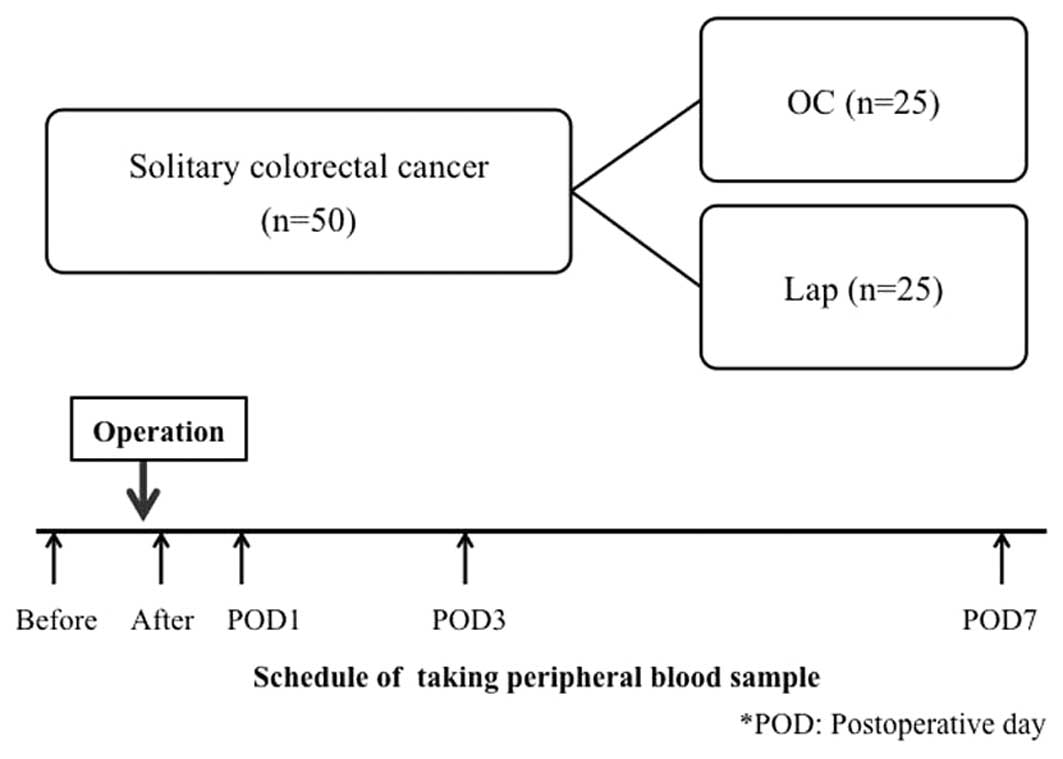
A total of 50 patients who underwent curative
surgery for solitary colorectal cancer at our department, between
June, 2008 and February, 2011 were included in the present study.
The patients were divided into OC and Lap groups (25 patients each)
(Fig. 1). The exclusion criteria
for Lap at our department were as follows: i) large tumor size,
exceeding the laparotomy incision (≥8); ii) apparent invasion of
other organ(s); iii) stage IV disease, with metastasis to other
organ(s); and iv) ileus or intestinal perforation. Accordingly,
patients with large tumors (≥8 cm), invasion and/or metastasis to
other organ(s) and ileus, for which pressure reduction was
impossible, were excluded. Lap was not converted to OC in any of
the patients and patients receiving preoperative chemotherapy or
radiotherapy were also excluded. Written informed consent was
obtained from the patients. The study protocol was approved by the
Ethics Committee of Juntendo University (approval no.: 556).
Patients who offered their consent selected OC or Lap and were
prospectively investigated.
Methods
Blood sampling
Peripheral blood was collected from the colorectal
cancer patients prior to surgery, immediately following surgery and
1, 3 and 7 days after surgery (Fig.
1). To prevent contamination with skin tissue during blood
sampling, the first 5 ml of blood was discarded. Blood was
collected in an RNA stabilizing agent-containing PAXgene Blood RNA
tube (Qiagen, Valencia, CA, USA) and stored at −80°C until use.
RNA extraction
Total RNA was extracted from blood samples using the
PAXgene Blood RNA kit (Qiagen). To remove contaminating genomic
DNA, the blood sample was treated with the RNase-Free DNase I set
(Qiagen) and dissolved with 80 μl of RNase-free water. The
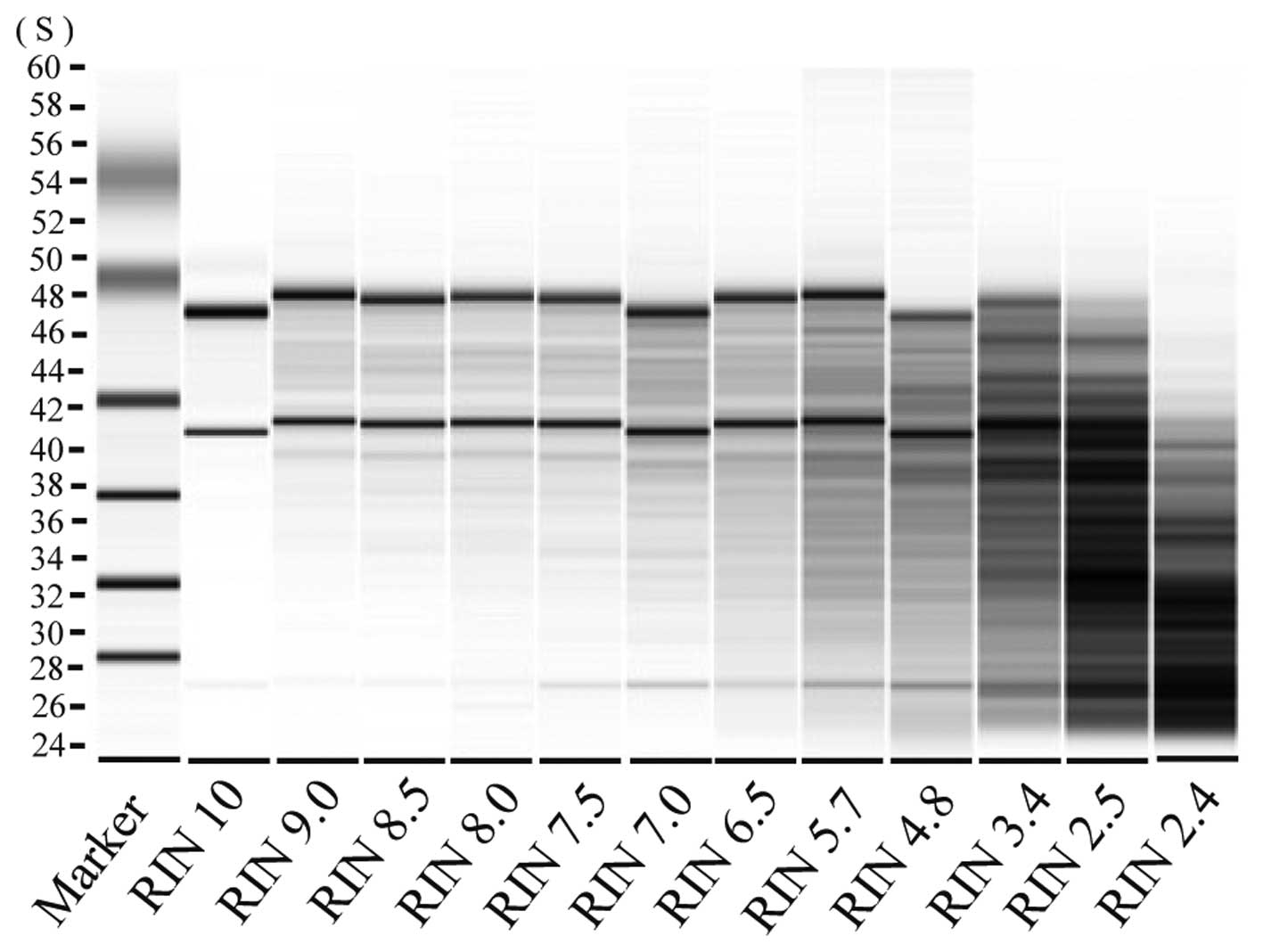
RNA decomposition level was evaluated using the Bio Analyzer
(Agilent, Santa Clara, CA, USA) and samples with an RNA integrity
number (RIN) of ≥6.5 were analyzed (Fig. 2).
Reverse transcription
Using 100 ng of total RNA as a template, reverse
transcription was performed using the SuperScript III First-Strand
synthesis system for qRT-PCR (Invitrogen, Carlsbad, CA, USA).
Synthesized cDNA was stored at −80°C until use.
Real-time PCR
Using 5 μl of cDNA (corresponding to 25 ng of
total RNA) as a template, CEA-specific primers and the Power
SYBR-Green PCR Master Mix (Applied Biosystems, Foster City, CA,
USA) PCR reactions were conducted using the Applied Biosystems
7,500 real-time PCR system (Applied Biosystems) under the reaction
condition of 95°C for 10 min, followed by 40 cycles of heat
denaturation at 95°C for 15 sec and annealing/elongation at 60°C
for 1 min. In order to eliminate false positivity due to
non-specific amplification, the experiment was repeated twice and
samples confirmed to be amplified in both experiments were accepted
as positive (Fig. 3). The
CEA-specific primers used are shown below: CEA sense:
5′-GCCTGTTTTGTCTCTAACTTGGC-3′ and antisense:
5′-CAACCAGCACTCCAATCATGAT-3′.
Investigation items
Changes in the positive rate over the perioperative
period were investigated in the patients. The patients were divided
into the OC and Lap groups and patient background factors,
including age, gender, tumor site, intraoperative blood loss,
operative time, maximum tumor diameter, depth of invasion,
lymphatic or vascular invasion, histological type and stage, were
investigated. The CEA mRNA-positive rate and its changes over the
perioperative period were evaluated.
Patients who were positive and negative for CEA mRNA
after surgery were designated as postoperative positive and
negative groups, respectively, and the associations with
clinicopathological characteristics (age, gender, preoperative CEA
level, tumor site, maximum tumor diameter, depth of invasion,
histological type, lymphatic or vascular invasion and stage) and
surgical factors (surgical approach, intraoperative blood loss,
operative time and surgical procedure) were investigated. Staging
was performed according to the TNM classification established by
the UICC.
Statistical analysis
The significance of differences was analyzed by
employing the Fisher’s exact, χ2, Mann-Whitney U and
t-tests, using statistical analysis software SPSS v.17.0 (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
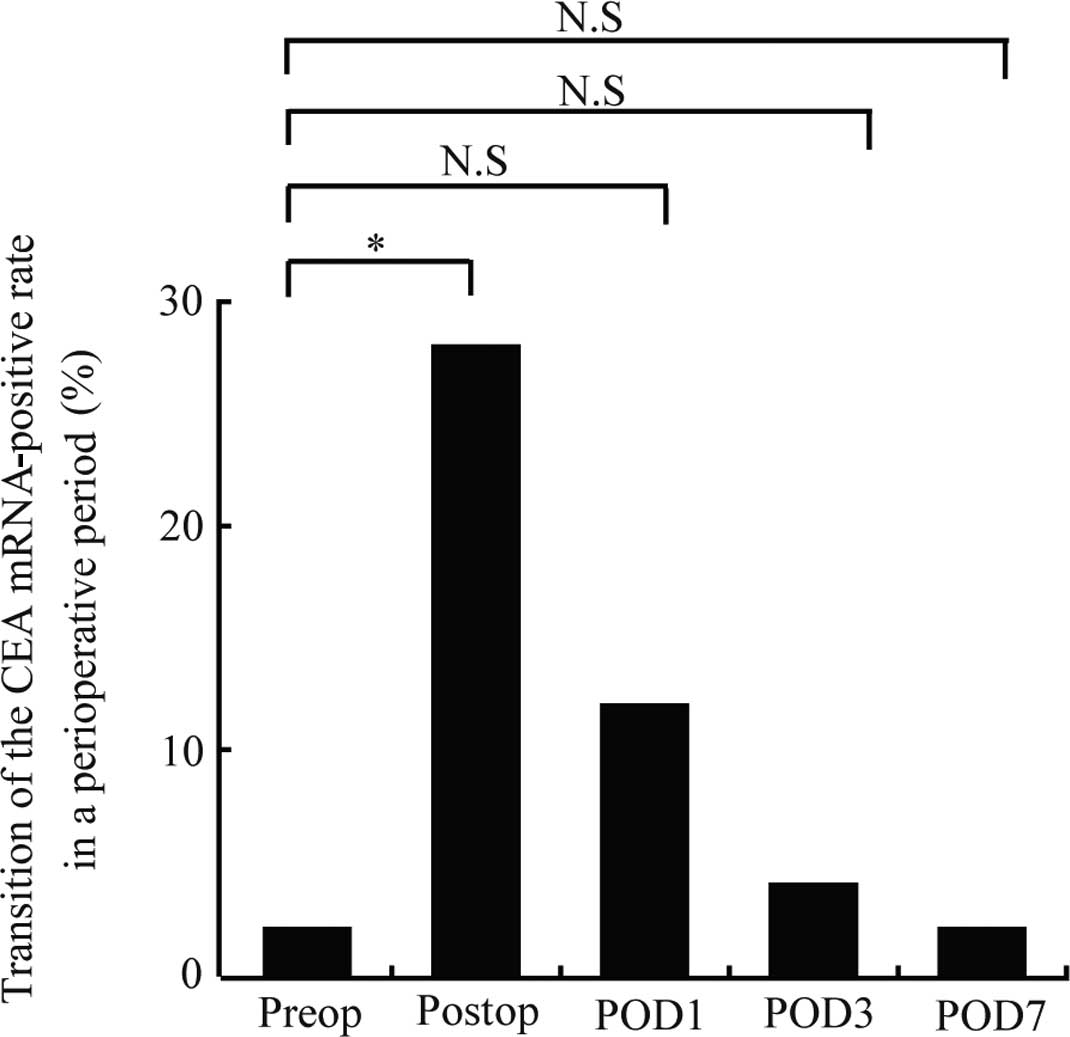
The results from the patients are presented in
Table I. Only one patient (2%) was
positive for CEA mRNA prior to surgery. The positive rate was
significantly increased immediately after surgery to 28% (14
patients) (P=0.001), but decreased over time to 12, 4 and 2% (6, 2
and 1 patient, respectively) at postoperative days 1, 3 and 7,
respectively. No significant differences from the preoperative
positive rate were noted after postoperative day 1 (Fig. 4).
 | Table IComparative results from open
colectomy and laparoscopic surgery groups on different
postoperative days (POD). |
Table I
Comparative results from open
colectomy and laparoscopic surgery groups on different
postoperative days (POD).
| No. | Before | After | POD1 | POD3 | POD7 |
|---|
| Open colectomy
group |
| 1 | − | − | − | − | − |
| 2 | − | + | + | − | − |
| 3 | − | + | + | − | − |
| 4 | − | − | − | − | − |
| 5 | − | − | − | − | − |
| 6 | − | − | − | − | − |
| 7 | − | − | − | − | − |
| 8 | − | − | − | − | − |
| 9 | − | − | − | − | − |
| 10 | − | + | − | − | − |
| 11 | − | + | − | − | − |
| 12 | − | − | − | − | − |
| 13 | − | − | − | − | − |
| 14 | − | + | + | − | − |
| 15 | − | + | − | − | − |
| 16 | − | − | − | − | − |
| 17 | − | − | − | − | − |
| 18 | − | + | − | − | − |
| 19 | − | + | − | − | − |
| 20 | − | − | − | − | − |
| 21 | − | + | − | − | − |
| 22 | − | − | − | − | − |
| 23 | − | − | − | − | − |
| 24 | − | − | − | − | − |
| 25 | − | − | − | − | − |
| Total | 0/25 (0%) | 9/25 (36%) | 3/25 (12%) | 0/25 (0%) | 0/25 (0%) |
| Laparoscopic
surgery group |
| 1 | − | − | − | − | − |
| 2 | − | − | − | − | − |
| 3 | − | − | − | − | − |
| 4 | − | − | − | − | − |
| 5 | − | − | − | − | − |
| 6 | − | + | − | − | − |
| 7 | − | + | + | − | − |
| 8 | − | − | − | − | − |
| 9 | − | − | − | − | − |
| 10 | − | − | − | − | − |
| 11 | − | + | + | − | − |
| 12 | − | + | − | − | − |
| 13 | − | − | − | − | − |
| 14 | + | − | − | + | − |
| 15 | − | − | − | − | − |
| 16 | − | − | − | − | − |
| 17 | − | − | − | − | − |
| 18 | − | − | − | − | − |
| 19 | − | − | − | − | − |
| 20 | − | − | + | + | + |
| 21 | − | − | − | − | − |
| 22 | − | − | − | − | − |
| 23 | − | − | − | − | − |
| 24 | − | − | − | − | − |
| 25 | − | + | − | − | − |
| Total | 1/25 (4%) | 5/25 (25%) | 3/25 (12%) | 2/25 (8%) | 1/25 (4%) |
Differences in the background factors were
investigated between the OC and Lap groups. In the OC group, the
tumor size was significantly larger compared to that in the Lap
group (OC group, 40 mm; Lap group, 28 mm; P=0.04), the
intraoperative blood loss was greater (OC group, 200 ml; Lap group,
50 ml; P<0.001) and fewer cases were lymphatic or vascular
invasion-positive (P=0.04). However, there were no significant
differences between the other factors (Table II).
 | Table IIOC vs. Lap patient
characteristics. |
Table II
OC vs. Lap patient
characteristics.
| Variables | OC (n=25) | Lap (n=25) | P-value |
|---|
| Age (years) | 66.7±11.0 | 64.0±11.3 | 0.41 |
| Gender | | | |
| Male/female | 11/14 | 17/8 | 0.15 |
| Tumor site | | | |
| Right
colon/other | 6/19 | 9/16 | 0.27 |
| Left
colon/other | 9/16 | 7/18 | 0.38 |
| Upper
rectum/other | 5/20 | 7/18 | 0.37 |
| Lower
rectum/other | 5/20 | 2/23 | 0.21 |
| Blood loss in ml
(range) | 200 (10–880) | 50 (15–255) | <0.00 |
| Time of operation
in min (average) | 140–584 (235) | 210–510 (263) | 0.07 |
| Tumor size in mm
(average) | 11–87 (40) | 10–85 (28) | 0.04 |
| Depth of
invasion | | | |
| T1-2/T3-4 | 9/16 | 15/10 | 0.16 |
| Lymphatic or venous
invasion | | | |
|
Absent/present | 12/13 | 5/20 | 0.04 |
| Histological
type | | | |
|
TUB1/TUB2-POR | 8/17 | 11/14 | 0.28 |
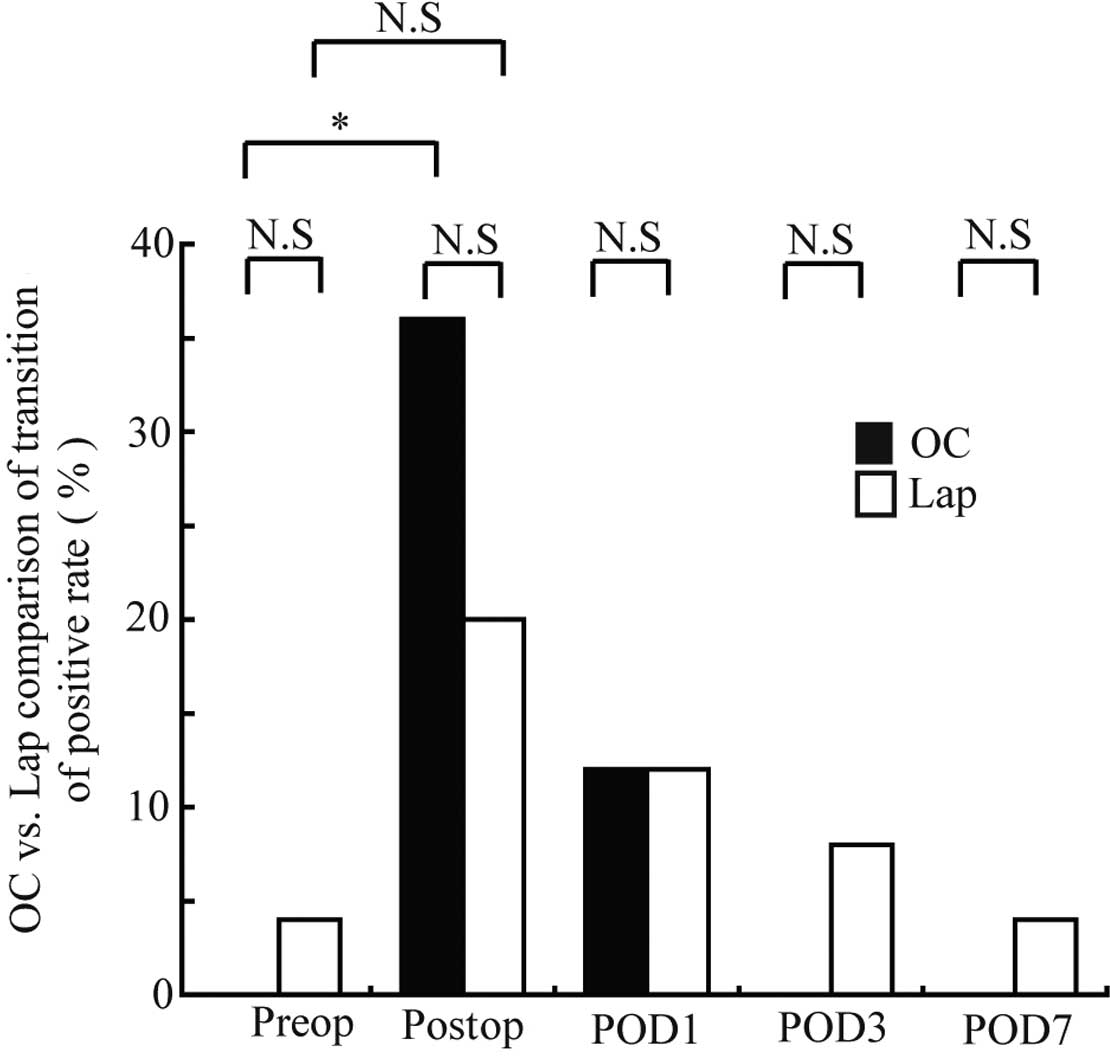
Changes in the peripheral blood CEA mRNA-positive
rate over the perioperative period in the OC and Lap groups are
shown in Fig. 5. Only one patient
(4%) in the Lap group was positive prior to surgery. In the OC
group, 9 patients (36%) were positive immediately following
surgery, exhibiting a significantly higher positive rate compared
to the preoperative rate (P=0.004). In the Lap group, 5 patients
(20%) were positive immediately following surgery, although the
increase in the rate was not significant. The positive rate
decreased over time thereafter in the two groups: 3 patients (12%)
in each group were positive at postoperative day 1; 0 (0%) and 2
(8%) at day 3, respectively; and 0 (0%) and 1 (4%) at day 7,
respectively, exhibiting no significant differences in the positive
rates compared to those prior to surgery. In addition, no
significant differences were noted between the 2 groups at any
blood-sampling time point.
The patients were divided into those positive and
negative for CEA mRNA following surgery, designated as
postoperative positive (14 patients) and negative (36 patients)
groups, respectively, and the clinicopathological and surgical
factors were compared between the groups (Tables III and IV). Regarding tumor site (belonging to
the clinicopathological factors), the positive rate was
significantly higher in patients with lower rectal cancer compared
to those with cancer located elsewhere (P=0.001). Regarding the
surgical factors, no significant difference was noted between the
surgical approaches (OC vs. Lap), although the intraoperative blood
loss was significantly greater in the positive group (P= 0.01) and
a significant difference in relation to the surgical procedure was
noted (P=0.02). A comparison of the surgical procedures, revealed
the positive rate to be significantly higher in the patients
treated with inter-sphincteric resection (ISR) and abdominoperineal
resection (APR) of the rectum, compared to those treated with other
procedures (P=0.01).
 | Table IIIPositive vs. negative group
clinicopathological characteristics. |
Table III
Positive vs. negative group
clinicopathological characteristics.
| Variables | Positive group
1a (n=14) | Negative group
2b (n=36) | P-value |
|---|
| Age (years) | 62.9±10.5 | 66.3±11.3 | 0.34 |
| Gender | | | |
| Male/female | 7/7 | 15/21 | 0.75 |
| Preoperative serum
CEA in ng/ml (average) | 0.3–68.5 (4.4) | 0.7–14.3 (2.9) | 0.20 |
| Tumor site | | | |
| Right
colon/other | 4/10 | 11/25 | 0.59 |
| Left
colon/other | 3/11 | 13/23 | 0.26 |
| Upper
rectum/other | 1/13 | 11/25 | 0.08 |
| Lower
rectum/other | 6/8 | 1/35 | 0.001 |
| Tumor size in mm
(average) | 20–45 (32) | 10–87 (34) | 0.93 |
| Depth of
invasion | | | |
| T0-2/T3-4 | 7/7 | 17/19 | 0.55 |
| Histological
type | | | |
|
TUB1/TUB2-POR | 5/9 | 14/22 | 0.55 |
| Lymphatic or venous
invasion | | | |
|
Absent/present | 5/9 | 12/24 | 0.56 |
 | Table IVPositive vs. negative group surgical
factors. |
Table IV
Positive vs. negative group surgical
factors.
| Variables | Positive group
(n=14) | Negative group
(n=36) | P-value |
|---|
| Approach | | | |
| OC/Lap | 9/5 | 15/21 | 0.13 |
| Blood loss in ml
(average) | 30–880 (182) | 10–500 (80) | 0.01 |
| Time of operation
in min (average) | 190–430 (261) | 140–584 (250) | 0.15 |
| Procedure | | | |
| Partial
resection | 6 | 19 | |
|
Hemicolectomy | 1 | 3 | |
| Anterior
resectiona | 2 | 13 | 0.02 |
| ISR or
APRb | 5 | 2 | |
| ISR or
APR/other | 5/9 | 2/34 | 0.01 |
Discussion
Lap for colorectal cancer has become popular, due to
its low invasiveness compared to conventional open surgery.
However, despite the rapid rise in the popularity of this
technique, data on its oncological safety are limited (12,13).
Following the initial success of Smith et al
(14) in detecting circulating
melanoma cells in peripheral blood using RT-PCR, their method has
been applied for the detection of cancer cells and cytokeratin 20
and CEA mRNA have been reported to reflect the presence of free
cancer cells in peripheral blood. There have been numerous reports
on the detection of CEA mRNA using RT-PCR and its association with
the outcome (10,15–20).
Serum CEA is a tumor-related glycoprotein, most commonly used for
the management of colorectal cancer patients in clinical practice.
Thus, we used peripheral blood CEA mRNA expression to measure free
cancer cells with RT-PCR, as described above. We considered that
the short-term equivalence of Lap and OC with regard to the
oncological viewpoint may be investigated by measuring peripheral
blood CEA mRNA expression, representing free cancer cells, in the
perioperative period, during which time the severest surgical
stress is experienced.
The association between the timing of detection of
free cancer cells in peripheral blood and the outcome varies among
different studies. Taniguchi et al (16) and Ito et al (17) reported that the outcome was
significantly poorer in patients who were positive during and
immediately after surgery. By contrast, Allen-Mersh et al
(18) reported a significantly
poorer outcome in patients who were positive one day after surgery,
and Sadahiro et al (19)
and Chen et al (15)
reported that the outcome was significantly poorer in patients who
were positive at 7–14 days after surgery. Regarding the time course
of positivity, the positive rate was significantly higher
immediately following surgery in the OC group (P=0.004), whereas
the rate increased, albeit not significantly in the Lap group. The
increase in the positive rate may have been due to the surgical
operation (tumor dissection) in the two groups, but the rate was
lower in the Lap compared to the OC group, suggesting that the
non-touch isolation technique was complied with in Lap. The
positive rate was decreased at postoperative days 1, 3 and 7
compared to that immediately after surgery in the two groups, while
no significant differences were noted in the rate or its time
course changes between the groups. It was suggested that there is
no difference between the surgical approaches regarding
perioperative appearance of free cancer cells.
According to Peach et al (10), two processes are responsible for
the appearance of free cancer cells. In the first process, cells
are released from the primary lesion by a surgical operation, such
as dissection and mobilization; in the other process, free cells
appear due to disseminating micro-metastasis. Based on these
processes, it was hypothesized that the appearance of free cancer
cells during and immediately after surgery was significantly
affected by the former process, whereas the latter process was
responsible for the appearance observed at 3 and 7 days after
surgery. However, it must not be ruled out that free cancer cells
released by a surgical operation influence micro-metastasis. It has
been hypothesized that a surgical stress-induced reduction of
immunity contributes to metastasis by free cancer cells entering
the circulation during surgery (20) and it has recently been reported
that the outcome was significantly poorer in the group in which
free cancer cells were detected during or immediately after surgery
compared to the group with no detection of free cancer cells
(10).
We considered that postoperative positivity is
important, rather than the timing of blood sampling. We compared
patients who were positive at least once in the period immediately
following surgery and thereafter (postoperative positive group)
with those who showed no positivity (postoperative negative group).
As demonstrated by the results, significant differences were noted
in patients in the postoperative positive group in whom the cancer
was located in the lower rectum (P=0.0001), with a large volume of
intraoperative blood loss (P=0.001) and in whom the surgical
procedure was ISR or APR (P=0.01), although no significant
difference was observed between the surgical approaches (P=0.13).
Regarding the tumor site, a significant difference was noted in
patients with lower rectal cancer, but not in those with cancer
located elsewhere. Similarly, a significant difference was noted in
patients treated by ISR or APR, but not in those treated by other
surgical procedures. It was assumed that cancer cells are readily
released from the lower rectum due to the absence of serosa, which
is a patient factor. In addition, this procedure is more complex
and the dissection distance is longer in surgery for lower rectal
cancer compared to other colorectal cancers, which are surgical
factors. ISR or APR is frequently employed for lower rectal cancer.
The blood loss is generally greater with this procedure compared to
other procedures. Although blood loss is dependent on the location
of the tumor (lower rectal cancer), it may serve as an index from
the oncological viewpoint. No significant differences were noted in
the background clinicopathological characteristics of the patients
between the Lap and OC groups and the blood loss was significantly
lower in the Lap compared to the OC group. It was suggested that
Lap for colorectal cancer is better or at least equivalent to OC
with regard to peripheral blood free cancer cells, which is
considered as micro-metastasis.
In conclusion, in colorectal cancer patients, there
were no significant differences in the perioperative peripheral
blood CEA mRNA-positive rate, or its short-term changes, between
the patients receiving open and laparoscopic surgeries. It was
suggested that Lap is equivalent to OC with regard to free cancer
cells. Additional studies are necessary in order to assess more
cases, verify the findings and investigate their association with
long-term outcome.
Abbreviations:
|
Lap
|
laparoscopic surgery;
|
|
OC
|
open colectomy;
|
|
RIN
|
RNA integrity number;
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction;
|
|
ISR
|
intersphincteric resection;
|
|
APR
|
abdominoperitoneal resection
|
References
|
1.
|
Center for Cancer Control and Information
Services: National Cancer Center; Japan: http://ganjoho.jp/professional/statistics/statistics.html.
Accessed August 31, 2012.
|
|
2.
|
Abraham NS, Young JM and Solomon MJ:
Meta-analysis of short-term outcomes after laparoscopic resection
for colorectal cancer. Br J Surg. 91:1111–1124. 2004. View Article : Google Scholar
|
|
3.
|
Lancy AM, Delgado S, Castells A, Prins HA,
Arroyo V, Ibarzabal A and Pique JM: The long-term results of a
randomized clinical trial of laparoscopy-assisted versus open
surgery for colon cancer. Ann Surg. 248:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Jayne DG, Guillou PJ, Thorpe H, et al:
Randomized trial of laparoscopic-assisted resection of colorectal
carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin
Oncol. 25:3061–3068. 2007.PubMed/NCBI
|
|
5.
|
Clinical Outcomes of Surgical Therapy
Study Group: A comparison of laparoscopically assisted and open
colectomy for colon cancer. N Engl J Med. 350:2050–2059. 2004.
View Article : Google Scholar
|
|
6.
|
Watanabe T, Itabashi M, Shimada Y, et al:
Japanese Society for Cancer of the Colon and Rectum (JSCCR)
Guidelines 2010 for the treatment of colorectal cancer. Int J Clin
Oncol. 17:1–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Guillow PJ, Quirke P, Thorpe H, et al:
Short-term endpoints of conventional versus laparoscopic-assisted
surgery in patients with colorectal cancer (MRC CLASSIC trial):
multicentre, randomized controlled trial. Lancet. 365:1718–1726.
2005. View Article : Google Scholar
|
|
8.
|
Aly EH: Laparoscopic colorectal surgery:
summary of the current evidence. Ann R Coll Surg Engl. 91:541–544.
2009. View Article : Google Scholar
|
|
9.
|
Martel G, Crawford A, Barkun JS, Boushey
RP, Ramsay CR and Fergusson DA: Expert opinion on laparoscopic
surgery for colorectal cancer parallels evidence from a cumulative
meta-analysis of randomized controlled trials. PLoS One.
7:e352922012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Peach G, Kim C, Zacharakis E, Perkayastha
S and Ziprin P: Prognostic significance of circulating tumor cells
following surgical resection of colorectal cancers: a systematic
review. Br J Cancer. 102:1327–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
International Union Against Cancer (UICC):
TNM Classification of Malignant Tumours. 7th edition. Sobin LH,
Gospodarwicz MK and Wittekind C: Wiley; New York: 2010
|
|
12.
|
Vukasin P, Ortega AE, Greene FL, et al:
Wound recurrence following laparoscopic colon cancer resection:
results of The American Society of Colon and Rectal Surgeons
Laparoscopic Registry. Dis Colon Rectum. 39:S20–S23. 1996.
View Article : Google Scholar
|
|
13.
|
Bruch HP and Schwandner O: Current status
of laparoscopic surgery in colorectal cancer. Onkologie. 24:29–32.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Smith B, Selby P, Southgate J, Pittman K,
Bradley C and Blair GE: Detection of melanoma cells in peripheral
blood by means of reverse transcriptase polymerase chain reaction.
Lancet. 338:1227–1229. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chen WS, Chung MY, Liu JH, Liu JM and Lin
JK: Impact of circulating free tumor cells in the peripheral blood
of colorectal cancer patients during laparoscopic surgery. World J
Surg. 28:552–557. 2004.PubMed/NCBI
|
|
16.
|
Taniguchi T, Makino M, Suzuki K and
Kaibara N: Prognostic significance of reverse
transcriptase-polymerase chain reaction measurement of
carcinoembryonic antigen mRNA levels in tumor drainage blood and
peripheral blood of patients with colorectal carcinoma. Cancer.
89:970–976. 2000. View Article : Google Scholar
|
|
17.
|
Ito S, Nakanishi H, Hirai T, et al:
Quantitative detection of CEA expressing free tumor cells in the
peripheral blood of colorectal cancer patients during surgery with
real-time RT-PCR on a LightCycler. Cancer Lett. 183:195–203. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Allen-Mersh TG, McCullough TK, Patel H,
Wharton RQ, Glover C and Jonas SK: Role of circulating tumor cells
in predicting reccurence after excision of primary colorectal
carcinoma. Br J Surg. 94:96–105. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sadahiro S, Suzuki T, Maeda Y, et al:
Detection of carcinoembryonic antigen messenger RNA-expressing
cells in peripheral blood 7 days after curative surgery is a novel
prognostic factor in colorectal cancer. Ann Surg Oncol.
14:1092–1098. 2007. View Article : Google Scholar
|
|
20.
|
Lundy J: Anesthesia and surgery: a
double-edged sword for the cancer patient. J Surg Oncol. 14:61–65.
1980. View Article : Google Scholar : PubMed/NCBI
|