Introduction
Cholangiocarcinoma (CCA) is a fatal neoplasm with a
poor prognosis. Despite developments in its detection and
treatment, only approximately 5–15% of CCA patients survive for 5
years after the diagnosis (1–4). Surgery
is the only curative treatment for CCA; however, the overall
survival (OS) is not satisfactory, with a 5-year OS of 40% in
patients treated with surgery (5–7). To
improve the OS, several adjuvant chemotherapies after surgery for
CCA have been performed. However, despite a number of studies
demonstrating the potential efficacy of adjuvant chemotherapies
(8–10), there is no secure evidence on which
to highly recommend adjuvant chemotherapy be administered for CCA.
One reason for this is that patients are unlikely to tolerate
chemotherapy after surgery, especially after hepatectomy. We
therefore need to identify those patients who will receive a
substantial clinical benefit from adjuvant chemotherapy.
Among preoperative systemic immunological and
inflammatory clinical variables, the neutrophil-to-lymphocyte ratio
(NLR), platelet-lymphocyte ratio (PLR) and prognostic nutritional
index (PNI) have been reported as predictors of the therapeutic
outcome in cancer, including CCA (11–13). The
postoperative immune response was also reported as a risk factor of
the prognosis among patients with other cancers (14–16). For
hepatocellular carcinoma after hepatectomy, it was reported that
the perioperative change in the leukocyte number and levels of
postoperative C-reactive protein (CRP) were associated with the
survival (17,18). However, the relationship between the
long-term prognosis and the perioperative immune responses in
patients with CCA has remained unclear.
In this study, we investigated the clinical impact
of the perioperative immune response on the long-term prognosis in
patients receiving hepatectomy for CCA.
Materials and methods
Rules for CCA used in the present
study
In this study, we used the 6th edition of the
general rules for clinical and pathological studies on cancer of
the biliary tract for extra-hepatic CCA (eCCA) and the 6th edition
of the general rules for the clinical and pathological study of
primary liver cancer for intra-hepatic CCA (iCCA) (19,20).
Because R0 resection in the 6th edition of the general rules for
clinical and pathological studies on cancer of the biliary tract
are equivalent to Cur A and B in the 6th edition of the general
rules for the clinical and pathological study of primary liver
cancer, we defined Cur A and B as R0 in this article.
Patients
Between February 2000 and October 2012, 109 patients
diagnosed with iCCA or eCCA underwent R0 resection in the
Department of Gastroenterological Surgery at Osaka University
Hospital. After a routine examination, including a blood test,
computed tomography, endoscopic retrograde cholangiography and/or
percutaneous transhepatic cholangiography, 81 patients (57 iCCA
patients and 24 eCCA patients) underwent hepatectomy.
In this study, written informed consent to receive
perioperative management was obtained from all of the patients.
The criteria used for surgery were based on three
factors; the preoperative liver function, the cut margin and the
size of the remnant liver. In brief, the cutting line of the
biliary duct should be 5 mm away from the CCA, and the estimated
remnant liver volume should be >30% of the total liver volume
with a normal liver function. If needed, percutaneous transhepatic
portal embolization was performed before surgery.
Adjuvant chemotherapy and
follow-up
Several kinds of adjuvant chemotherapies following
hepatectomy were performed for some patients in this cohort as
clinical trials in 2011–2012 (3,9).
Patients with major hepatectomy were assigned to KHBO 1003, ‘Phase
I study of adjuvant gemcitabine or S-1 in patients with biliary
tract cancers undergoing major hepatectomy’ (Clinical-Trials.gov ID NCT01291615; UMIN ID
000004682), and were treated with GEM or S-1 chemotherapy. Patients
with small hepatectomy were assigned to KHBO 1004, ‘A Phase I study
of adjuvant chemotherapy with gemcitabine plus cisplatin in
patients with biliary tract cancer undergoing curative resection
without major hepatectomy’ (Clinical-Trials.gov ID NCT01297998; UMIN ID
000004622), and were treated with GEM plus cisplatin therapy. The
median follow-up period of all patients was 50.6±38.1 months.
Patients received regular follow-up with abdominal computed
tomography and measurement of the serum CEA and CA19-9 levels every
three months for the first two years and every six months
thereafter. The last follow-up date of this study was November
2017.
Treatment for recurrence
Almost all of patients with recurrence were first
treated by gemcitabine (GEM). The chemotherapy comprised oral
5-fulorouracil (5FU) and/or tegafur or the combination of 5FU,
adriamycin, and cisplatin until June 2006, when GEM for CCA was
approved in Japan.
Evaluations of the clinicopathological
features
We collected all information related to three types
of factors: patient factors, tumor factors and treatment factors.
Regarding patient factors, we evaluated each patient's condition
from multiple perspectives. Because several reports have suggested
that the nutritional state or inflammation condition affects the
prognosis of CCA, we evaluated whether or not these factors were
associated with the long-term prognosis. The nutritional state of
the patients before surgery was evaluated with Onodera's PNI. The
inflammation-associated status at the same time point was also
evaluated with two scores: the NLR and the PLR. These statuses were
evaluated based on blood tests within one week before surgery. We
checked the postoperative first peak of the level of CRP and the
numbers of each kind of leukocyte (neutrophils, lymphocytes,
eosinophils, monocytes and basophils). Each kind of leukocytes
reached the peak at the various date after surgery; the median date
of each kind of leukocytes
(leukocytes/neutrophils/lymphocytes/eosinophils/monocytes/basophils)
was 2nd/1st/13th/14th/6th/11th date, respectively. Regarding to
neutrophils, they usually reached the peak around the same time as
leukocytes, but the peak of eosinophils was usually delayed several
days from the peak of leukocytes.
Regarding tumor factors, we collected all tumor
information relevant to the pathological diagnosis. The TNM and
stage of tumors were classified by different rules between eCCA and
iCCA. The 6th edition of the general rules for clinical and
pathological studies on cancer of the biliary tract was used for
eCCA, and the 6th edition of the general rules for the clinical and
pathological study of primary liver cancer was used for iCCA. The
levels of the tumor markers CEA and CA19-9 were evaluated the day
before surgery. Treatment factors consisted of information
concerning the surgery type, the complications following surgery
and the presence of adjuvant therapy. All morbidities were judged
as clinically relevant postoperative complications when a surgical
or interventional radiology approach was required (Table I).
 | Table I.Clinicopathological features in 81
CCA patients by each factor type. |
Table I.
Clinicopathological features in 81
CCA patients by each factor type.
| Variable | n=81 |
|---|
| Patient
factors |
|
| Age
(years) | 63.7±10.9 |
| Sex
(male:female) | 52:29 |
|
Jaundice (present:absent) | 6:75 |
|
PNI | 44.1±4.8 |
|
NLR | 2.8±1.5 |
|
PLR | 174.6±89.5 |
|
Postoperative maximum number
of leukocytes (/µl) | 12182±3748 |
|
Postoperative
maximum number of neutrophils (/µl) | 9986±3730 |
|
Postoperative
maximum number of lymphocytes (/µl) | 1542±529 |
|
Postoperative
maximum number of eosinophils (/µl) | 466±419 |
|
Postoperative
maximum number of monocytes (/µl) | 879±316 |
|
Postoperative
maximum number of basophils (/µl) | 106±364 |
|
Postoperative peak value of
CRP (mg/dl) | 10.2±4.2 |
| Tumor factors |
|
| Tumor
type (intrahepatic CCA:perihilar CCA) | 57:24 |
|
Differentiation
(tub1:tub2:por:muc:small cell:unknown) |
8:51:12:6:1:1:2 |
|
pTa(1:2:3:4) | 7:38:27:8 |
|
Historical
vascular invasion (present absent) | 35:44 |
|
Tumor size
(cm) | 4.1±2.7 |
|
pNa(1:0) | 24:56 |
|
pMNa(1:0) | 1:80 |
|
pStageNa(I:II:III:IV) | 7:33:19:21 |
| CEA
(>5:<5 ng/ml) | 10:71 |
| CA19-9
(>37:<37 U/ml) | 36:45 |
| Treatment
factors |
|
|
Adjuvant therapy (yes:no) | 29:52 |
|
Treatment after recurrence
with gemcitabine (yes:no:unknown:no recurrence) | 24:36:2:19 |
|
Operative method (HPD:
trisectionectomy:hemihepatectomy:
segmentectomy:subseqmentectomy:partial hepatectomy) | 1:8:49:5:2:16 |
|
Operation time (min) | 536.8±202.2 |
| Blood
loss (ml) | 1451.1±1148.8 |
|
Morbidity
(present:absent) | 14:67 |
|
Resected liver weight (g) | 420.4±316.1 |
Statistical analyses
All data are expressed as the mean ± standard
deviation. The chi-squared test and Fisher's exact test were used
to compare categorical variables, when appropriate. The
Kaplan-Meier analysis and the log-rank test were used to construct
the survival curve and to evaluate differences for the univariate
analysis. A multivariate analysis of the detected factors was
performed with a Cox regression analysis. All analyses were
conducted with the JMP 13 software program (SAS Institute, Cary,
NC, USA). Statistical significance was defined as a P-value of
0.05.
Ethical guidelines followed in this
study
This study was conducted in accordance with the
Declaration of Helsinki. This study was performed at Osaka
University Hospital, Japan, and approved by the local ethics
committee (no. 18261).
Results
The peak number of leukocytes after
hepatectomy was an individual significant risk factor for the OS in
CCA patients
Eighty-one patients of CCA received liver resection;
57 of them had intrahepatic CCA, and 24 had perihilar CCA (Table I). The univariate analysis detected
several risk factors for the OS in CCA patients receiving
hepatectomy, including 2 among the patient factors (the PLR
(P=0.0251) and the postoperative maximum number of leukocytes (PNL,
P=0.0111)); 6 among the tumor features [TNM classification (T/N/M,
P=0.0091/0.0003/0.0002), historical vascular invasion
(P<0.0001), staging (P=0.0030) and level of CA19-9 (P=0.0383)];
and 4 among the treatment features (operation time (P=0.0113),
blood loss (P=0.0372), morbidity (P=0.0040) and resected liver
weight (P=0.0103)]. A multivariate analysis of those risk factors
revealed 2 significant risk factors for the OS: The PNL among
patient factors (P=0.0406) and the TNM-stage among tumor factors
(P=0.0059). There were no significant risk factors for the OS among
treatment factors (Table II).
 | Table II.Results of univariate and
multivariate analyses for the OS. |
Table II.
Results of univariate and
multivariate analyses for the OS.
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variable | MST (months) | Univariate analysis
P-value | HR | (95% CI) | P-value |
|---|
| Patient
factors |
|
|
|
|
|
| Age
(<65:≥65 years) | 39.4:69.2 | 0.3968 |
|
|
|
| Sex
(male:female) | 69.9:43.1 | 0.2617 |
|
|
|
|
Jaundice (present:absent) | 56.5:51.8 | 0.9873 |
|
|
|
| PNI
(≤45:>45) | 52.2:51.8 | 0.8540 |
|
|
|
| NLR
(≤2.5:>2.5) | 69.2:39.4 | 0.1215 |
|
|
|
| PLR
(≤150:>150) | 74.8:43.1 | 0.0251 | 0.59 | (0.32–1.08) | 0.0906 |
|
Postoperative maximum number
of leukocytes (≤11580:>11580/µl) | 74.8:32.7 | 0.0111 | 0.54 | (0.29–0.97) | 0.0406 |
|
Postoperative peak value of
CRP (≤9.7:>9.7 mg/dl) | 59.2:43.1 | 0.5203 |
|
|
|
| Tumor factors |
|
|
|
|
|
| Tumor
type (intrahepatic CCA:perihilar CCA) | 53.8:43.7 | 0.1210 |
|
|
|
|
Differentiation (tub1, tub2:
others) | 47.7:- | 0.1818 |
|
|
|
|
pTa(1,2:3,4) | 70.5:30.3 | 0.0091 |
|
| N.A. |
|
Historical
vascular invasion (present: absent) | 29.9:- | <0.0001 |
|
| N.A. |
|
Tumor size
(≤2:>2 cm) | 69.9:51.5 | 0.5559 |
|
|
|
|
pNa(0:1) | 69.9:18.8 | 0.0003 |
|
| N.A. |
|
pMa(1:0) | 6.4:51.8 | 0.0002 |
|
| N.A. |
|
pStagea(I, II:III, IV) | 78.3:29.9 | 0.0030 | 0.44 | (0.24–0.79) | 0.0059 |
| CEA
(≤5:>5 ng/ml) | 52.2:27.2 | 0.0688 |
|
|
|
| CA19-9
(≤37:>37 U/ml) | 69.2:30.8 | 0.0383 | 0.60 | (0.33–1.08) | 0.0888 |
| Treatment
factors |
|
|
|
|
|
|
Adjuvant therapy (yes:no) | 39.4:59.2 | 0.9716 |
|
|
|
|
Treatment after recurrence
with gemcitabine (yes:no) | 43.7:27.9 | 0.3503 |
|
|
|
|
Operative method of
hepatectomy (major:minor) | 47.7:- | 0.1265 |
|
|
|
|
Operation time (≤545:>545
min) | 74.8:27.4 | 0.0113 | 0.66 | (0.32–1.29) | 0.2238 |
| Blood
loss (<1,000:≥1,000 ml) | 70.5:30.3 | 0.0372 | 0.92 | (0.44–1.89) | 0.8249 |
|
Morbidity
(present:absent) | 20.4:59.2 | 0.0040 | 1.86 | (0.82–3.97) | 0.1346 |
|
Resected liver weight
(≤340:>340 g) | -: 39.4 | 0.0103 | 0.64 | (0.30–1.34) | 0.2365 |
To clarify which kinds of leukocytes were
significantly associated with the OS, we evaluated the distribution
of each kind among neutrophils, lymphocytes, eosinophils, monocytes
and basophils. A multivariate analysis was performed, and both the
postoperative maximum number of neutrophils (PNN) and postoperative
maximum number of eosinophils (PNE) were detected as significant
factors among leukocytes (PNN/PNE, P=0.0367/0.0083, Table III).
 | Table III.Results of a multivariate analysis
for the OS in leukocytes. |
Table III.
Results of a multivariate analysis
for the OS in leukocytes.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | HR | (95% CI) | P-value |
|---|
| Postoperative
maximum number of leukocytes (≤11580:>11580/µl) |
|
| N.A. |
|
Postoperative maximum number
of neutrophils (≤9807:>9807/µl) | 0.53 | (0.28–0.96) | 0.0367 |
|
Postoperative maximum number
of lymphocytes (≤1513:>1513/µl) | 1.06 | (0.56–2.01) | 0.8549 |
|
Postoperative maximum number
of eosinophils (≤356:>356/µl) | 2.20 | (1.22–4.04) | 0.0083 |
|
Postoperative maximum number
of monocytes (≤815:>815/µl) | 0.95 | (0.52–1.74) | 0.8752 |
|
Postoperative maximum number
of basophils (≤58:>58/µl) | 0.94 | (0.50–1.77) | 0.8527 |
PNN
We divided patients into a high-neutrophil group
(high-PNN group, n=40) and a Lowl-neutrophil group (low-PNN group,
n=41) based on the median PNN (9807/µl). The high-PNN group showed
a poorer prognosis than the low-PNN group with regard to the OS
(P=0.0406). To assess the influence of the increase in neutrophils
after hepatectomy, a sub-analysis for the OS was performed. The
high-PNN group showed a poorer prognosis than the low-PNN group
(P=0.0318) in the survival time after recurrence (SAR). However, no
significant difference in the disease-free survival (DFS) was noted
between the groups (P=0.8809) (Fig.
1A).
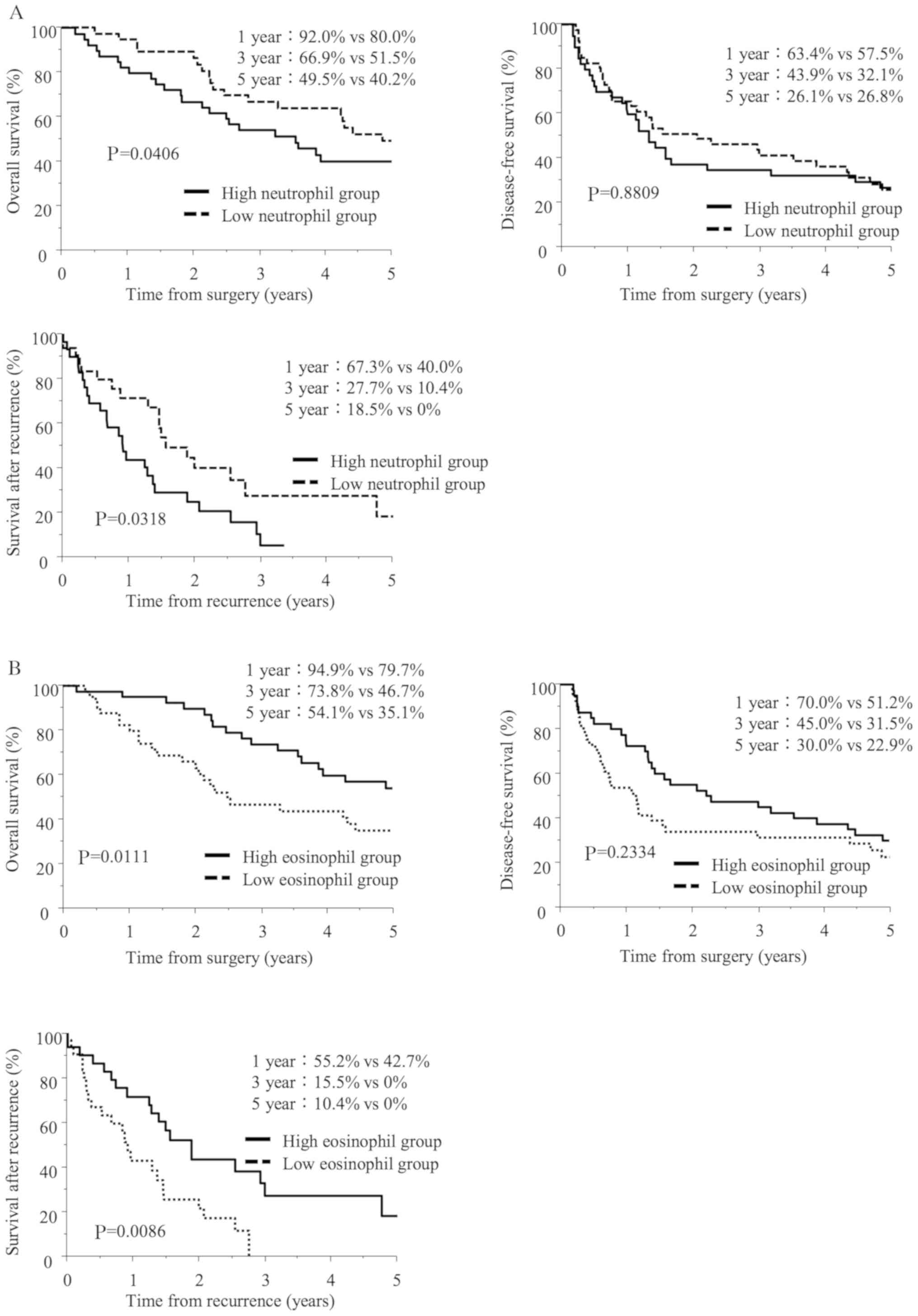 | Figure 1.The long-term prognosis of patients
after hepatectomy for cholangiocarcinoma (CCA). (A) The overall
survival (OS, upper left), disease-free survival (DFS, upper right)
and survival after recurrence (SAR, lower left) curves after
surgery for 81 patients with CCA. Patients were divided into two
groups according to the median postoperative peak number of
neutrophils. The median OS in the high-neutrophil group (n=40) and
low-neutrophil group (n=41) was 43.1 and 59.2 months, respectively;
P=0.0406. The median DFS in the high-neutrophil group and
low-neutrophil group was 16.1 and 25. months, respectively;
P=0.8809. The median SAR in the high-neutrophil group and
low-neutrophil group was 11.0 and 18.8 months, respectively;
0.0318. (B) The OS (upper left), DFS (upper right) and SAR (lower
left) curves after surgery for 81 patients with CCA. Patients were
divided into two groups according to the median postoperative peak
number of eosinophils. The median OS in the high-eosinophil group
(n=40) and low-eosinophil group (n=41) was 74.8 and 30.3 months,
respectively; P=0.0111. The median DFS in the high-eosinophil group
and low-eosinophil group was 27.3 and 13.8 months, respectively;
P=0.2334. The median SAR in the high-eosinophil group and
low-eosinophil group was 22.8 and 11.0 months, respectively;
P=0.0086. |
To investigate the factors associated with the PNN,
we compared the clinicopathological factors between the two groups.
The comparison revealed significant differences in three factors:
the level of CA19-9, CCA tumor type and the PLR. The high-PNN group
contained more patients showing a higher preoperative CA19-9 level
(P=0.0259) and high PLR (P=0.0018). In addition, the number of
neutrophils in the serum was higher in iCCA patients than in eCCA
patients after hepatectomy (P=0.0035) (Table IV).
 | Table IV.Results of a univariate analysis of
patient characteristics and postoperative peak number of
neutrophils. |
Table IV.
Results of a univariate analysis of
patient characteristics and postoperative peak number of
neutrophils.
| Variable | n | High neutrophils
(n=40) | Low neutrophils
(n=41) | P-value |
|---|
| Age (<65:≥65
years) | 37:44 | 19:21 | 18:23 | 0.8249 |
| Sex
(male:female) | 52:29 | 24:16 | 28:13 | 0.4919 |
| CEA (≤5:>5
ng/ml) | 71:10 | 34:6 | 37:4 | 0.5187 |
| CA19-9 (≤37:>37
U/ml) | 45:36 | 17:23 | 28:13 | 0.0259 |
| Jaundice
(present:absent) |
6:75 | 5:35 | 1:40 | 0.1088 |
| Adjuvant therapy
(yes:no) | 29:52 | 14:26 | 15:26 | 0.1394 |
| Tumor type
(intrahepatic CCA:perihilar CCA) | 57:24 | 22:18 | 35:6 | 0.0035 |
| Differentiation
(tub1, tub2:others) | 59:20 | 27:11 | 32:9 | 0.6059 |
| Operation time
(≤545:>545 min) | 41:39 | 16:23 | 25:16 | 0.1168 |
| Blood loss
(<1,000:≥1,000 ml) | 36:45 | 18:22 | 18:23 | 1.0000 |
| Morbidity
(present:absent) | 14:67 | 10:30 | 4:37 | 0.0843 |
| Resected liver
weight (≤340:>340 g) | 37:39 | 15:23 | 22:16 | 0.1681 |
| Operative method of
hepatectomy (major:minor) | 58:23 | 32:8 | 26:15 | 0.1394 |
| pTa(1, 2:3, 4) | 45:35 | 22 :17 | 23:18 | 1.0000 |
|
Historical vascular invasion
(present:absent) | 35:44 | 21:17 | 32:9 | 0.6059 |
| Tumor
size (≤2:>2 cm) | 18:62 | 11:28 | 7:34 | 0.2890 |
| pNa(0:1) | 56:24 | 26:13 | 30:11 | 0.6276 |
| pMa(1:0) |
1:80 | 1:39 | 0:41 | 0.4938 |
| pStagea(I, II:III, IV) | 40:40 | 18:21 | 22:19 | 0.6549 |
| PNI
(≤45:>45) | 49:32 | 27:13 | 22:19 | 0.2576 |
| NLR
(≤2.5:>2.5) | 40:41 | 18:22 | 22:19 | 0.5077 |
| PLR
(≤150:>150) | 39:42 | 12:28 | 27:14 | 0.0018 |
| Postoperative
maximum number of lymphocytes (≤1513:>1513/µl) | 41 :40 | 18:22 | 23:18 | 0.3771 |
| Postoperative
maximum number of eosinophils (≤356:>356/µl) | 41:40 | 18:22 | 23:18 | 0.3771 |
| Postoperative
maximum number of monocytes (≤815:>815/µl) | 41:40 | 17:23 | 24:17 | 0.1848 |
| Postoperative
maximum number of basophils (≤58:>58/µl) | 41:40 | 16:24 | 25:16 | 0.0766 |
| Postoperative peak
value of CRP (≤9.7:>9.7 mg/dl) | 41:40 | 19:21 | 22:19 | 0.6590 |
PNE
When patients were divided into a high-eosinophil
group (high-PNE group, n=40) and a low-eosinophil group (low-PNE
group, n=41) based on the median PNE (356/µl), the low-PNE group
showed a poorer prognosis with regard to the OS (P=0.0111) and the
SAR (P=0.0086) (Fig. 1B). However,
there was no significant difference in the DFS between the groups
(P=0.2334) (Fig. 1B).
We investigated the factors associated with the PNE
and determined that only the preoperative serum level of CA19-9 was
associated with the PNE (P=0.0445) (Table V).
 | Table V.Results of a univariate analysis of
patient characteristics and postoperative peak number of
eosinophils. |
Table V.
Results of a univariate analysis of
patient characteristics and postoperative peak number of
eosinophils.
| Variable | n | High eosinophils
(n=40) | Low eosinophils
(n=41) | P-value |
|---|
| Age (<65:≥65
years) | 37:44 | 17:23 | 20:21 | 0.6575 |
| Sex
(male:female) | 52:29 | 27:13 | 25:16 | 0.6445 |
| CEA (≤5:>5
ng/ml) | 71:10 | 34:6 | 37:4 | 0.5187 |
| CA19-9 (≤37:>37
U/ml) | 45:36 | 27:13 | 18:23 | 0.0445 |
| Jaundice
(present:absent) |
6:75 | 4:36 | 2:39 | 0.4321 |
| Adjuvant therapy
(yes:no) | 29:52 | 16:24 | 13:28 | 0.4919 |
| Tumor type
(intrahepatic CCA:perihilar CCA) | 57:24 | 28:12 | 29:12 | 1.0000 |
| Differentiation
(tub1, tub2:others) | 59:20 | 32:8 | 27:12 | 0.3095 |
| Operation time
(≤545:>545 min) | 41:39 | 23:17 | 18:22 | 0.3711 |
| Blood loss
(<1,000:≥1,000 ml) | 36:45 | 20:20 | 16:25 | 0.3749 |
| Morbidity
(present:absent) | 14:67 | 7:33 | 7:34 | 1.0000 |
| Resected liver
weight (≤340:>340 g) | 37:39 | 22:17 | 15:22 | 0.1786 |
| Operative method of
hepatectomy (major:minor) | 58:23 | 27:13 | 31:10 | 0.4670 |
| pTa(1, 2:3, 4) | 45:35 | 26:14 | 19:21 | 0.1759 |
|
Historical vascular invasion
(present:absent) | 35:44 | 16:24 | 19:20 | 0.5005 |
| Tumor
size (≤2:>2 cm) | 18:62 | 9:31 | 9:31 | 1.0000 |
| pNa(0:1) | 56:24 | 32:8 | 24:16 | 0.0866 |
| pMa(1:0) |
1:80 | 0:40 | 1:40 | 1.0000 |
| pStagea(I, II:III, IV) | 40:40 | 24:16 | 16:24 | 0.1170 |
| PNI
(≤45:>45) | 49:32 | 21:19 | 28:13 | 0.1763 |
| NLR
(≤2.5:>2.5) | 40:41 | 22:18 | 18:23 | 0.3771 |
| PLR
(≤150:>150) | 39:42 | 22:18 | 17:24 | 0.2692 |
| Postoperative
maximum number of neutrophils (≤9807:>9807/µl) | 41:40 | 18:22 | 23:18 | 0.3771 |
| Postoperative
maximum number of lymphocytes (≤1513:>1513/µl) | 41:40 | 19:21 | 22:19 | 0.6590 |
| Postoperative
maximum number of monocytes (≤815:>815/µl) | 41:40 | 18:22 | 23:18 | 0.3771 |
| Postoperative
maximum number of basophils (≤58:>58/µl) | 41:40 | 18:22 | 23:18 | 0.3771 |
| Postoperative peak
value of CRP (≤9.7:>9.7 mg/dl) | 41:40 | 19:21 | 22:19 | 0.6590 |
The rule of peak number of leukocytes
after hepatectomy for the SAR in CCA patients
Because both PNN and PNE were associated with the
SAR, we investigated the univariate analysis for the SAR including
other factors (Table SI). The
univariate analysis detected several risk factors for the SAR in
CCA patients receiving hepatectomy, including the postoperative
maximum number of leukocytes (PNL, P=0.0076). Among leukocytes, PNN
and PNE were risk factors for the SAR (PNN/PNE, P=0.0039/0.0008,
Table SII).
Regarding to the treatment after recurrence, there
were 16 patients without treatments after recurrence and 44
patients treated by mainly chemotherapy. Evaluating with PNN and
PNE, there was no significant difference among high/low-PNN group
in the presence of treatment after recurrence (respectively,
64.3%/81.3%, P=0.1569), and among high/low-PNE group (respectively,
83.9%/62.1%, P=0.0807). Whereas, we investigated clinical impact of
the site of recurrence on SAR. There were 62 recurrence cases; 29
cases in the liver, 8 cases at the peritoneal dissemination, 7
cases in the lymph node, and 7 cases in other sites. There was no
significant association with the site of recurrence among between
high and low-PNN/PNE groups (respectively, P=0.7418/P=0.7311).
Discussion
We revealed that the postoperative peak number of
leukocytes for CCA patients after hepatectomy was associated with
the long-term prognosis, especially with regard to the OS and SAR,
and the PNN and PNE were dominant components in the increase in
leukocyte numbers. We also investigated the prognosis for patients
of eCCA who underwent surgery without hepatectomy. There was no
significant difference in OS, DFS and SAR with PNN (OS/DFS/SAR,
P=0.6514/0.6630/0.6817) and PNE (OS/DFS/SAR, P=0.5980/05254/0.6372)
(Fig. S1). Because the clinical
impact of postoperative leukocytes was observed in only CCA
patients with hepatectomy, we considered that the immune response
might be different between patients with hepatectomy and patients
without hepatectomy. Thus, we focused on the immune response for
CCA patients after hepatectomy in this study.
The immune response following liver resection has
been shown to influence various immune functions represented by
several cytokines and growth factors (21). As we previously reported, these
reactions and changes in levels of cytokines/growth factors
facilitate CCA progression (22–26).
Thus, we assumed that the postoperative peak number of
leukocytes/CRP would be good surrogate markers and useful
prediction tools for the OS.
The postoperative level of CRP, which has been
described as a postoperative risk marker in other tumors (18,27) was
not associated with the prognosis in CCA. In contrast, an increased
peak number of leukocytes was associated with a poorer OS, showing
some degree of contradiction between these two findings. We
therefore considered that the liver may not fully react and produce
CRP sufficiently after hepatectomy in some cases, as CRP is
produced in mainly by the liver.
The PNN was a significant risk factor of the OS.
Historically, neutrophils were considered to have no effect on
chronic or progressive diseases, such as cancer, due to the
relatively short survival of such patients. Recently, however,
several studies have revealed that the presence of neutrophils in
tumors is associated with a poor prognosis in some tumors, such as
bronchoalveolar carcinoma, melanoma, renal carcinoma and head and
neck squamous cell carcinoma (28–32).
Regarding a tumor-promoting role, neutrophils in inflammatory
response product cytokines, proteases, and reactive oxygen species
(ROS) (33). Interleukin-6 (IL-6) in
particular is a marker of inflammation and the neuroendocrine
stress response after surgery for primary biliary cancer and is a
dominant inflammatory cytokine in the postoperative period
(34,35). We previously revealed that IL-6 was
associated with malignant features in CCA (23,25). The
IL-6 expression was found to induce chemoresistance in CCA cells
through epithelial-mesenchymal transition (EMT) (23). Thus, neutrophils might be induced in
the acute inflammation period after hepatectomy by cytokines,
including IL-6, and CCA exacerbated by IL-6 exposure carries a poor
prognosis for patients.
In contrast to findings concerning neutrophils,
eosinophils have rarely shown any particular association with
cancer. Albeit in only a few reports, eosinophils have been found
to be associated with both favorable and unfavorable prognoses.
Most reports on eosinophils in cancer have demonstrated the
presence of eosinophil infiltration in tissues surrounding tumors,
such as nasopharyngeal carcinoma, colorectal tumor, oral squamous
cell carcinoma, pulmonary adenocarcinoma and laryngeal carcinoma
(36–38). Increased eosinophil counts in
patients with prostate cancer and metastatic colon cancers have
been shown to be associated with a prolonged survival (39,40).
Recently, there have been reports that the short-term
administration of IL-33 facilitates the development of a murine
genetic model of CCA (22). However,
the clinical role of IL-33 in CCA has not been investigated. IL-33,
a member of the IL-1 family, has been shown to be a crucial
costimulator of the adaptive immune response, exerting effects on
antiviral CD8+ cytotoxic T lymphocytes, CD4+
T helper 1 cell reactions and immune regulation by regulatory T
cells. We therefore initially assumed that an increase in
eosinophils might be a surrogate marker of an increase in the
expression of IL-33 and thus a risk factor of the prognosis of CCA
patients. However, we observed the opposite results. Although these
findings seemed to conflict with our hypothesis, several reports
have shown that IL-33 does not increase the number of eosinophils
in all cases. Tjota and Stolarski suggested that IL-33 was involved
in both increases and decreases in the number of eosinophils. In
contrast, Dyer et al determined that IL-33 antagonized
IL-5-dependent eosinophilopoiesis (41–43).
Those previous results may partially explain the findings in the
present study.
After getting the result, we reanalyzed the
prognosis dividing CCA patients into iCCA patients and eCCA
patients. Particularly, OS and SAR for the high-PNN group in iCCA
patients seemed to be inferior comparing to the low-PNN group in
iCCA patients, although there was no significant different, maybe
because of the small number (OS/SAR, P=0.1124/0.1629) (Fig. S2). We need to accumulate more cases
for further investigation.
In conclusion, the postoperative peak number of
leukocytes after hepatectomy was significantly associated with the
long-term prognosis in patients with CCA. Although studies in a
larger group are still needed, changes in the numbers of leukocytes
after hepatectomy may be a marker on treatment for CCA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GS made substantial contributions to acquisition of
data and drafting the manuscript. DY and HE made substantial
contributions to conception, design, revising the manuscript. HE,
YI, HA, TA, TN and KG made contribution to acquisition of data and
revising manuscript. SK, YT and MT made substantial contributions
to analysis and interpretation of data. YD and MM revising it
critically for important intellectual content, given final approval
of the version. And All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants. This retrospective study protocol was approved by the
institutional reviewer board of the Osaka University Graduate
School of Medicine (Suita, Japan) (no. 18261).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson CD, Pinson CW, Berlin J and Chari
RS: Diagnosis and treatment of cholangiocarcinoma. Oncologist.
9:43–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howlader N, Noone AM and Krapcho M: SEER
Cancer Statistics Review 1975–2008. National Cancer Institute;
Bethesda, MD: 2018
|
|
3
|
Toyoda M, Ajiki T, Fujiwara Y, Nagano H,
Kobayashi S, Sakai D, Hatano E, Kanai M, Nakamori S, Miyamoto A, et
al: Phase I study of adjuvant chemotherapy with gemcitabine plus
cisplatin in patients with biliary tract cancer undergoing curative
resection without major hepatectomy (KHBO1004). Cancer Chemother
Pharmacol. 73:1295–1301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colvin H, Mizushima T, Eguchi H, Takiguchi
S, Doki Y and Mori M: Gastroenterological surgery in Japan: The
past, the present and the future. Ann Gastroenterol Surg. 1:5–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyakawa S, Ishihara S, Horiguchi A,
Takada T, Miyazaki M and Nagakawa T: Biliary tract cancer
treatment: 5,584 results from the Biliary Tract Cancer Statistics
Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg.
16:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wellner UF, Shen Y, Keck T, Jin W and Xu
Z: The survival outcome and prognostic factors for distal
cholangiocarcinoma following surgical resection: A meta-analysis
for the 5-year survival. Surg Today. 47:271–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higuchi R, Ota T, Yazawa T, Kajiyama H,
Araida T, Furukawa T, Yoshikawa T, Takasaki K and Yamamoto M:
Improved surgical outcomes for hilar cholangiocarcinoma: Changes in
surgical procedures and related outcomes based on 40 years of
experience at a single institution. Surg Today. 46:74–83. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi S, Miyamoto A, Shimizu J,
Kashiwazaki M, Takeda Y, Ueshima S, Kim Y, Kitagawa T, Dono K, Mori
M, et al: Comparison of 4-weekly vs 3-weekly gemcitabine as
adjuvant chemotherapy following curative resection for biliary
tract cancer: A prospective randomized controlled trial. J Cancer
Ther. 02:703–709. 2011. View Article : Google Scholar
|
|
9
|
Kobayashi S, Nagano H, Sakai D, Eguchi H,
Hatano E, Kanai M, Seo S, Taura K, Fujiwara Y, Ajiki T, et al:
Phase I study of adjuvant gemcitabine or S-1 in patients with
biliary tract cancers undergoing major hepatectomy: KHBO1003 study.
Cancer Chemother Pharmacol. 74:699–709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuno T, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Yamaguchi J and Nagino M: Adjuvant gemcitabine
monotherapy for resectable perihilar cholangiocarcinoma with lymph
node involvement: A propensity score matching analysis. Surg Today.
47:182–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Q, Dai Z, Yin D, Yang LX, Wang Z,
Xiao YS, Fan J and Zhou J: Negative impact of preoperative
platelet-lymphocyte ratio on outcome after hepatic resection for
intrahepatic cholangiocarcinoma. Medicine (Baltimore). 94:e5742015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gomez D, Morris-Stiff G, Toogood GJ, Lodge
JP and Prasad KR: Impact of systemic inflammation on outcome
following resection for intrahepatic cholangiocarcinoma. J Surg
Oncol. 97:513–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Wang H, Ning Z, Xu L, Zhuang L,
Wang P and Meng Z: Prognostic nutritional index serves as a
predictive marker of survival and associates with systemic
inflammatory response in metastatic intrahepatic
cholangiocarcinoma. OncoTargets Ther. 9:6417–6423. 2016. View Article : Google Scholar
|
|
14
|
Tibau A, Ennis M and Goodwin PJ:
Post-surgical highly sensitive C-reactive protein and prognosis in
early-stage breast cancer. Breast Cancer Res Treat. 141:485–493.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito T, Kurokawa Y, Miyazaki Y, Makino T,
Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M and Doki
Y: Which is a more reliable indicator of survival after gastric
cancer surgery: Postoperative complication occurrence or C-reactive
protein elevation? J Surg Oncol. 112:894–899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Y, Huang S, Li P, Chen B, Liu W, Chen Z
and Yin F: Prognostic evaluation of preoperative serum C-reactive
protein concentration in patients with epithelial ovarian cancer.
Exp Ther Med. 9:2003–2007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujiwara Y, Shiba H, Furukawa K, Iida T,
Sakamoto T, Gocho T, Wakiyama S, Hirohara S, Ishida Y, Misawa T, et
al: Perioperative change in white blood cell count predicts outcome
of hepatic resection for hepatocellular carcinoma. J Hepatobiliary
Pancreat Sci. 17:892–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiba H, Furukawa K, Fujiwara Y, Futagawa
Y, Haruki K, Wakiyama S, Ishida Y, Misawa T and Yanaga K:
Postoperative peak serum C-reactive protein predicts outcome of
hepatic resection for hepatocellular carcinoma. Anticancer Res.
33:705–709. 2013.PubMed/NCBI
|
|
19
|
Japanese Society of
Hepato-Biliary-Pancreatic Surgery, . General Rules for Clinical and
Pathological Studies on Cancer of the Biliary Tract. 6th. Kanehara
and Co., Ltd.; Tokyo: 2013
|
|
20
|
Liver Cancer Study Group of Japan, .
General Rules for the Clinical and Pathological Study of Primary
Liver Cancer. 6th. Kanehara and Co., Ltd.; Tokyo: 2015
|
|
21
|
Ando T, Ito H, Kanbe A, Hara A and
Seishima M: Deficiency of NALP3 Signaling Impairs Liver
Regeneration After Partial Hepatectomy. Inflammation. 40:1717–1725.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada D, Rizvi S, Razumilava N, Bronk SF,
Davila JI, Champion MD, Borad MJ, Bezerra JA, Chen X and Gores GJ:
IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an
interleukin-6-sensitive mechanism. Hepatology. 61:1627–1642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamada D, Kobayashi S, Wada H, Kawamoto K,
Marubashi S, Eguchi H, Ishii H, Nagano H, Doki Y and Mori M: Role
of crosstalk between interleukin-6 and transforming growth
factor-beta 1 in epithelial-mesenchymal transition and
chemoresistance in biliary tract cancer. Eur J Cancer.
49:1725–1740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakamoto T, Kobayashi S, Yamada D, Nagano
H, Tomokuni A, Tomimaru Y, Noda T, Gotoh K, Asaoka T, Wada H, et
al: A histone deacetylase inhibitor suppresses
epithelial-mesenchymal transition and attenuates chemoresistance in
biliary tract cancer. PLoS One. 11:e01459852016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi S, Werneburg NW, Bronk SF,
Kaufmann SH and Gores GJ: Interleukin-6 contributes to Mcl-1
up-regulation and TRAIL resistance via an Akt-signaling pathway in
cholangiocarcinoma cells. Gastroenterology. 128:2054–2065. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada D, Kobayashi S, Yamamoto H,
Tomimaru Y, Noda T, Uemura M, Wada H, Marubashi S, Eguchi H,
Tanemura M, et al: Role of the hypoxia-related gene, JMJD1A, in
hepatocellular carcinoma: Clinical impact on recurrence after
hepatic resection. Ann Surg Oncol. 19 (Suppl 3):S355–S364. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ibuki Y, Hamai Y, Hihara J, Emi M, Taomoto
J, Furukawa T, Yamakita I, Kurokawa T and Okada M: Role of
postoperative C-reactive protein levels in predicting prognosis
After surgical treatment of esophageal cancer. World J Surg.
41:1558–1565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trellakis S, Bruderek K, Dumitru CA,
Gholaman H, Gu X, Bankfalvi A, Scherag A, Hütte J, Dominas N,
Lehnerdt GF, et al: Polymorphonuclear granulocytes in human head
and neck cancer: Enhanced inflammatory activity, modulation by
cancer cells and expansion in advanced disease. Int J Cancer.
129:2183–2193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmidt H, Bastholt L, Geertsen P,
Christensen IJ, Larsen S, Gehl J and von der Maase H: Elevated
neutrophil and monocyte counts in peripheral blood are associated
with poor survival in patients with metastatic melanoma: A
prognostic model. Br J Cancer. 93:273–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uribe-Querol E and Rosales C: Neutrophils
in cancer: Two sides of the same coin. J Immunol Res.
2015:9836982015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wislez M, Rabbe N, Marchal J, Milleron B,
Crestani B, Mayaud C, Antoine M, Soler P and Cadranel J: Hepatocyte
growth factor production by neutrophils infiltrating
bronchioloalveolar subtype pulmonary adenocarcinoma: Role in tumor
progression and death. Cancer Res. 63:1405–1412. 2003.PubMed/NCBI
|
|
32
|
Jensen HK, Donskov F, Marcussen N,
Nordsmark M, Lundbeck F and von der Maase H: Presence of
intratumoral neutrophils is an independent prognostic factor in
localized renal cell carcinoma. J Clin Oncol. 27:4709–4717. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cata JP, Velasquez JF, Ramirez MF, Vauthey
JN, Gottumukkala V, Conrad C, Kim BJ and Aloia T: Inflammation and
pro-resolution inflammation after hepatobiliary surgery. World J
Surg Oncol. 15:1522017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alazawi W, Pirmadjid N, Lahiri R and
Bhattacharya S: Inflammatory and immune responses to surgery and
their clinical impact. Ann Surg. 264:73–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fujii M, Yamashita T, Ishiguro R, Tashiro
M and Kameyama K: Significance of epidermal growth factor receptor
and tumor associated tissue eosinophilia in the prognosis of
patients with nasopharyngeal carcinoma. Auris Nasus Larynx.
29:175–181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nielsen HJ, Hansen U, Christensen IJ,
Reimert CM, Bru N and Moesgaard F: Independent prognostic value of
eosinophil and mast cell infiltration in colorectal cancer tissue.
J Pathol. 189:487–495. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jain M, Kasetty S, Sudheendra US, Tijare
M, Khan S and Desai A: Assessment of tissue eosinophilia as a
prognosticator in oral epithelial dysplasia and oral squamous cell
carcinoma-an image analysis study. Pathol Res Int. 2014:5075122014.
View Article : Google Scholar
|
|
39
|
McNeel DG, Gardner TA, Higano CS, Kantoff
PW, Small EJ, Wener MH, Sims RB, DeVries T, Sheikh NA and Dreicer
R: A transient increase in eosinophils is associated with prolonged
survival in men with metastatic castration-resistant prostate
cancer who receive sipuleucel-T. Cancer Immunol Res. 2:988–999.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yalcin AD, Kargi A and Gumuslu S: Blood
eosinophil and platelet levels, proteomics patterns of trail and
CXCL8 correlated with survival in bevacizumab treated metastatic
colon cancers. Clin Lab. 60:339–340. 2014.PubMed/NCBI
|
|
41
|
Tjota MY, Williams JW, Lu T, Clay BS, Byrd
T, Hrusch CL, Decker DC, de Araujo CA, Bryce PJ and Sperling AI:
IL-33-dependent induction of allergic lung inflammation by FcγRIII
signaling. J Clin Invest. 123:2287–2297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stolarski B, Kurowska-Stolarska M, Kewin
P, Xu D and Liew FY: IL-33 exacerbates eosinophil-mediated airway
inflammation. J Immunol. 185:3472–3480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dyer KD, Percopo CM and Rosenberg HF:
IL-33 promotes eosinophilia in vivo and antagonizes IL-5-dependent
eosinophil hematopoiesis ex vivo. Immunol Lett. 150:41–47. 2013.
View Article : Google Scholar : PubMed/NCBI
|















