Introduction
Colorectal cancer is one of the leading causes of
cancer mortality worldwide (1,2). Since
the introduction of 5-fluorouracil (5-FU) in the 1960s, 5-FU has
been widely used in colorectal cancer treatment, as well as for
other malignancies, including gastric cancer, breast cancer, and
head and neck cancer (3).
Subsequently, 5-FU-based combination therapies, such as 5-FU plus
folinic acid-oxaliplatin (FOLFOX) and 5-FU plus folinic
acid-irinotecan (FOLFIRI) regimens, have been developed (4–6).
Previously, the addition of anti-epidermal growth factor receptor
monoclonal antibodies, such as cetuximab and panitumumab, and
humanized anti-vascular growth factor receptor monoclonal
antibodies, such as bevacizumab, have also proven to enhance the
anti-tumor effect (7–12). Despite the development of combined
therapy, 5-FU has continued to be the main drug for the treatment
of colorectal cancer. However, the calculation of the dosing amount
of 5-FU is still simply based only on the body surface area (BSA)
(13).
Dosing based on BSA is considered convenient and
easy to use, yet previous studies have indicated that many patients
treated with 5-FU are not given the appropriate dose to achieve
optimal plasma concentration (13,14).
According to a previous study, only 20–30% of patients are treated
with the appropriate therapeutic range, while 40–60% are under
dosed and 10–20% are overdosed (15). Previous results have suggested the
existence of interpatient and intrapatient pharmacokinetic (PK)
variation in 5-FU clearance (13,16–19).
Collectively, these previous results indicated that dosing based on
BSA is of limited use (13,19,20).
Therefore, the concept of direct monitoring of 5-FU plasma
concentrations with appropriate dose adjustments has been
introduced. A number of previous studies, including a large
multicenter randomized trial, have shown that PK-guided dose
adjustment of 5-FU in metastatic colorectal cancer has resulted in
an improvement in therapeutic outcomes and reduced the severity of
adverse events (14,20). The aim of the present study was to
determine an optimal method of determining the 5-FU dosage for
patients with colorectal cancer undergoing chemotherapy. In the
present prospective study, the transition of 5-FU plasma levels in
patients with colorectal cancer during treatment with 5-FU combined
chemotherapy was monitored. Furthermore, the association between
the tumor response and adverse events [classified by the Common
Terminology Criteria for Adverse Events (CTCAE) version 5.0]
(21) based on the 5-FU plasma
levels was evaluated.
Patients and methods
Patient treatment and eligibility
The present prospective study was performed using 15
patients with colorectal cancer treated with 5-FU combined
chemotherapy at The Tobu Chiiki Hospital. All patients were
subjected to standardized clinical practice, according to the
Japanese Society for Cancer of the Colon and Rectum Guidelines 2016
for the Treatment of Colorectal Cancer (22). 5-FU combined chemotherapy included
modified FOLFOX6 (mFOLFOX6) (n=6): Oxaliplatin 85 mg/m2
for 2 h, leucovorin (folinic acid) 200 mg/m2 for 2 h,
5-FU 400 mg/m2 bolus and 5-FU 2,400 mg/m2
continuous intravenous infusion for 46 h; or FOLFIRI (n=9):
Irinotecan 150 mg/m2 for 2 h, leucovorin 150
mg/m2 for 2 h, 5-FU 400 mg/m2 bolus and 5-FU
2,400 mg/m2 continuous intravenous infusion for 46 h.
All patients received the dose of 5-FU based on their BSA (5-FU 400
mg/m2 bolus and 5-FU 2,400 mg/m2 continuous
intravenous infusion for both mFOLFOX6 and FOLFIRI regimens). The
Eastern Cooperative Oncology Group Performance Status (ECOG-PS)
classification (23) was used to
evaluate the performance status (PS) of each patient. Patients were
required to have a PS ≤2. The criteria for the classification of PS
were as follows: i) PS 0, fully active, able to carry on
pre-disease performances without restriction; ii) PS 1, restricted
in physically strenuous activity but ambulatory and is able to
carry out work of a light or sedentary nature; and iii) PS 2,
ambulatory and capable of all selfcare but unable to carry out any
work activities; up and about more than 50% of waking hours. All
the patients included in the present study were treated under the
guidance of professional medical staff members. Patients were
required for 4 days in admission for treatment and were readmitted
every 2 weeks for 3 months (a total of six admissions). The present
study was approved by the Ethics Committee of Tobu Chiiki Hospital
(approved December 21, 2015; IRB nos. 15 and 4), and performed
between January 1, 2017 and March 31, 2017. The present study was
conducted in accordance with the principles of the amended
‘Declaration of Helsinki and Ethical Guidelines for Epidemiological
Research’ (established by the Japanese Government in 2008)
(24). Written informed consent was
obtained from all patients before they were enrolled in the present
study. Patients with PS >2 or requiring radiation or dialysis,
or those unable to provide informed consent were excluded.
Measurement of 5-FU plasma levels
Venous blood samples (10 ml) were collected three
times during admission (prior to the start of infusion, and 22 and
40 h after the start of continuous 5-FU infusion). 5-FU plasma
levels were measured using the My-5FU® assay, a
competitive homogeneous nanoparticle agglutination immunoassay
(FALCO Biosystems Ltd.). This assay is used for patients receiving
5-FU by continuous infusion to facilitate PK dose adjustment at the
next cycle and drug monitoring to achieve an optimal plasma level
of the drug. The assay uses two reagents and when they are mixed,
the nanoparticles aggregate together. Therefore, the amount of
aggregation of nanoparticles determines the 5-FU concentration in
plasma samples. 5-FU plasma levels at 0 (prior to the start of
infusion), 22 and 40 h after the start of continuous 5-FU infusion
in each patient were calculated as median values of six admissions
and statistically analyzed.
Analysis of tumor response and adverse
events
Tumor response to treatment was classified according
to Response Evaluation Criteria in Solid Tumors Group (RECIST 1.1)
criteria (25) and was evaluated
after the 3 month monitoring period. A complete response required
the disappearance of every lesion. A partial response required a
≥30% reduction in the cross-sectional area of all lesions. Stable
disease required a lesion size decrease <30%. Progressive
disease categorization encompassed any situation in which any one
lesion increased in cross-sectional size by >25% or a new lesion
appeared.
Adverse events during chemotherapy were evaluated
just after the infusion of 5-FU (for 46 h) on the 4th day of
admission, according to the Common Terminology Criteria for Adverse
Events (CTCAE) v5.0 (21), based on
physical examination and laboratory tests. The severity of adverse
events was graded between 1 and 5. Grade 1 consists of the
following criteria: i) Mild; ii) asymptomatic or mild symptoms;
iii) clinical or diagnostic observations only; and iv) intervention
not indicated. Grade 5 is associated with death. Adverse events
were assessed on the last day before discharge for each
patient.
Statistical analysis
The plasma levels of 5-FU are presented as
box-and-whisker plots (containing the minimum, first quartile,
median, third quartile and maximum values), unless otherwise
stated. The 5-FU plasma levels were measured three times (0 h prior
to the start of infusion, 22 and 40 h after the start of continuous
5-FU infusion) in each patient, calculated as median values of six
admissions, and statistically analyzed. Statistical significance
was determined by one-way ANOVA with Bonferroni's post hoc test
using GraphPad Prism 7 (GraphPad Software, Inc.). Statistical
significance for the different tumor responses (complete response,
partial response, stable disease and progressive disease) as well
as the response rate and disease control rate between mFOLFOX6 and
FOLFIRI was determined by a bivariate analysis using JMP 13
software (SAS Institute, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients
A total of 15 patients (5 female and 10 male) were
enrolled in the present study. The patient characteristics are
summarized in Table I. The mean age
was 69.0±7.3 (mean ± SD, range of 52–79) years and the mean BSA was
1.57±0.18 m2. Based on the ECOG-PS classification
(23), 1 patient was categorized as
PS 0, 11 patients were categorized as PS 1, and 3 patients were
categorized as PS 2. Only 1 patient had a tumor located in the
descending colon, 10 patients had a tumor located in the sigmoid
colon and 4 patients had a tumor located in the rectum. In total, 4
patients had a pathological diagnosis of well differentiated
adenocarcinoma, 10 patients were diagnosed with moderately
differentiated adenocarcinoma and 1 patient was diagnosed with
papillary adenocarcinoma, according to the Japanese Classification
of Colorectal Carcinoma (8th Edition) (26). A total of 9 patients enrolled were
treated for a primary tumor, while 6 patients were treated for a
recurrent tumor. Among the 9 patients, 8 patients were diagnosed as
Stage IV and 1 patient was diagnosed as Stage IIIb, based on the
Japanese Classification of Colorectal Carcinoma (8th Edition)
(26) (data not shown). The 9
patients were diagnosed with resectable tumors, and thus they were
not treated for a neoadjuvant setting. A total of 14 patients had
metastasis. The most frequent metastatic site (6 patients) was the
liver; other metastasis sites included the lung (1 patient) and
peritoneum (2 patients). A total of 5 patients had multiple
metastatic sites. A total of 11 out of 15 patients underwent
operations.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Value |
|---|
| Age (years) | 69.0±7.3a |
| Male/Female | 10/5 |
| BSA
(m2) |
1.57±0.18a |
| ECOG-PS
classification (0/1/2) | 1/11/3 |
| Tumor location
(descending/sigmoid/rectum) | 1/10/4 |
| Pathological
diagnosis (well/moderately/papillary) | 4/10/1 |
| Primary or
recurrent tumor | 9/6 |
| Metastasis
(+/−) | 14/1 |
| Location of
metastasis (liver/lung/peritoneum/multiple) | 6/1/2/5 |
| Operation
(+/−) | 11/4 |
Changes of the plasma level of 5-FU
during infusion
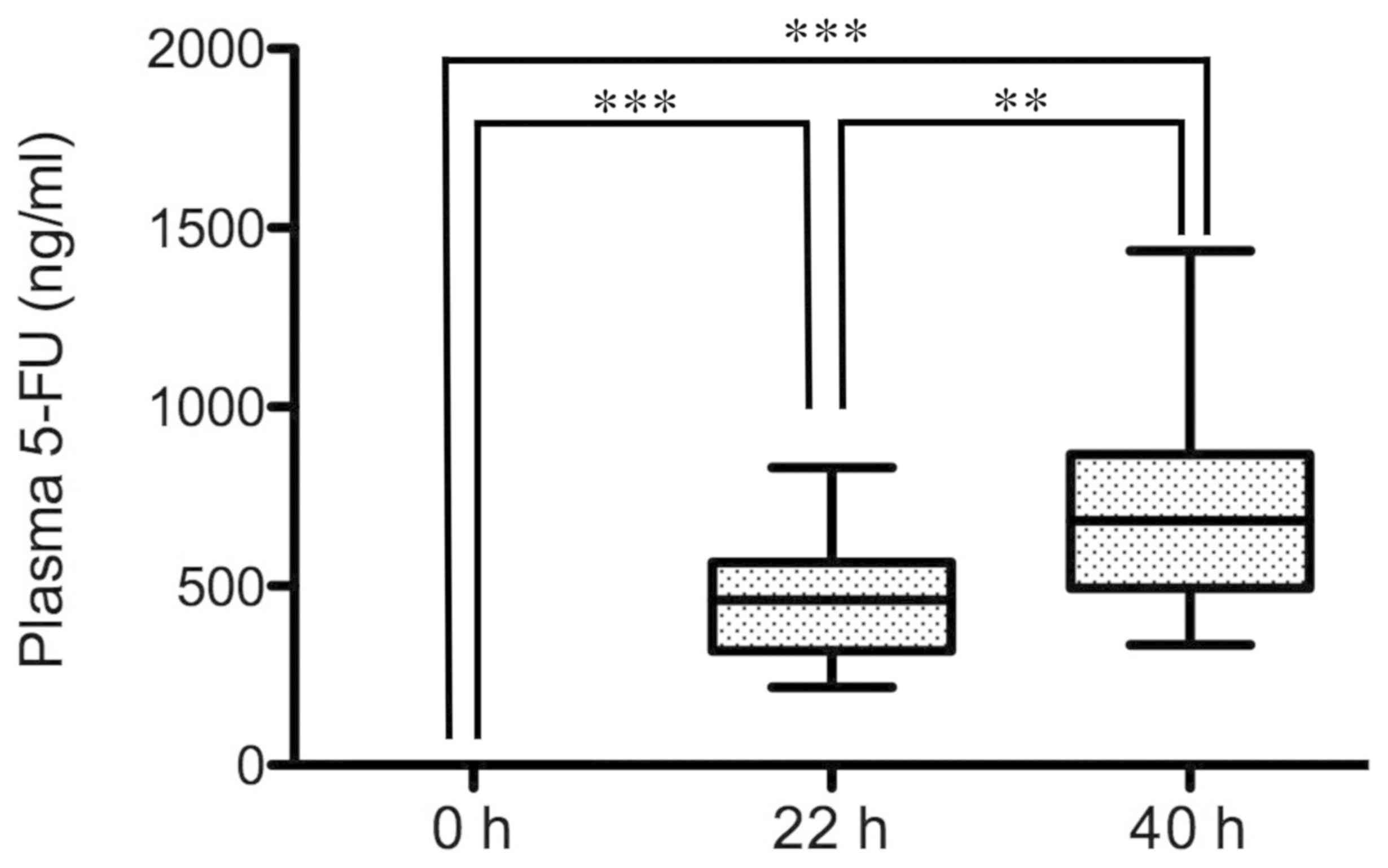
Before the start of infusion, the plasma level of
5-FU was 0 ng/ml in all patients (n=15). During infusion of 5-FU
for 46 h, the level at 22 h was 460.5 ng/ml (median value), while
the level at 40 h reached 682.0 ng/ml. The levels at 22 h and 40 h
were significantly higher than that at 0 h (P<0.001; Fig. 1). Notably, the level at 40 h was
significantly higher than that at 22 h (P<0.01).
Tumor response and regimens
Among the 15 patients, 6 patients underwent
chemotherapy of the mFOLFOX6 regimen and 9 patients underwent
chemotherapy of the FOLFIRI regimen. The tumor response, according
to RECIST 1.1 criteria showed no significant differences between
mFOLFOX6 and FOLFIRI in the categories of complete response,
partial response, stable disease and progressive disease (Table II). The response rate (proportion of
patients who achieved complete or partial response) and the disease
control rate (proportion of patients who achieved complete, partial
response or stable disease) also showed no significant differences
between mFOLFOX6 and FOLFIRI.
 | Table II.Tumor response and 5-fluorouracil
regimens. |
Table II.
Tumor response and 5-fluorouracil
regimens.
| Tumor response | mFOLFOX6 (n=6) | FOLFIRI (n=9) | P-value |
|---|
| Complete
response | 0 | 0 | N/A |
| Partial
response | 2 | 3 | >0.99 |
| Stable disease | 1 | 3 | 0.60 |
| Progressive
disease | 3 | 3 | 0.52 |
| Response rate | 33% | 33% | >0.99 |
| Disease control
rate | 50% | 67% | 0.52 |
Changes of the plasma level of 5-FU in
different tumor response groups
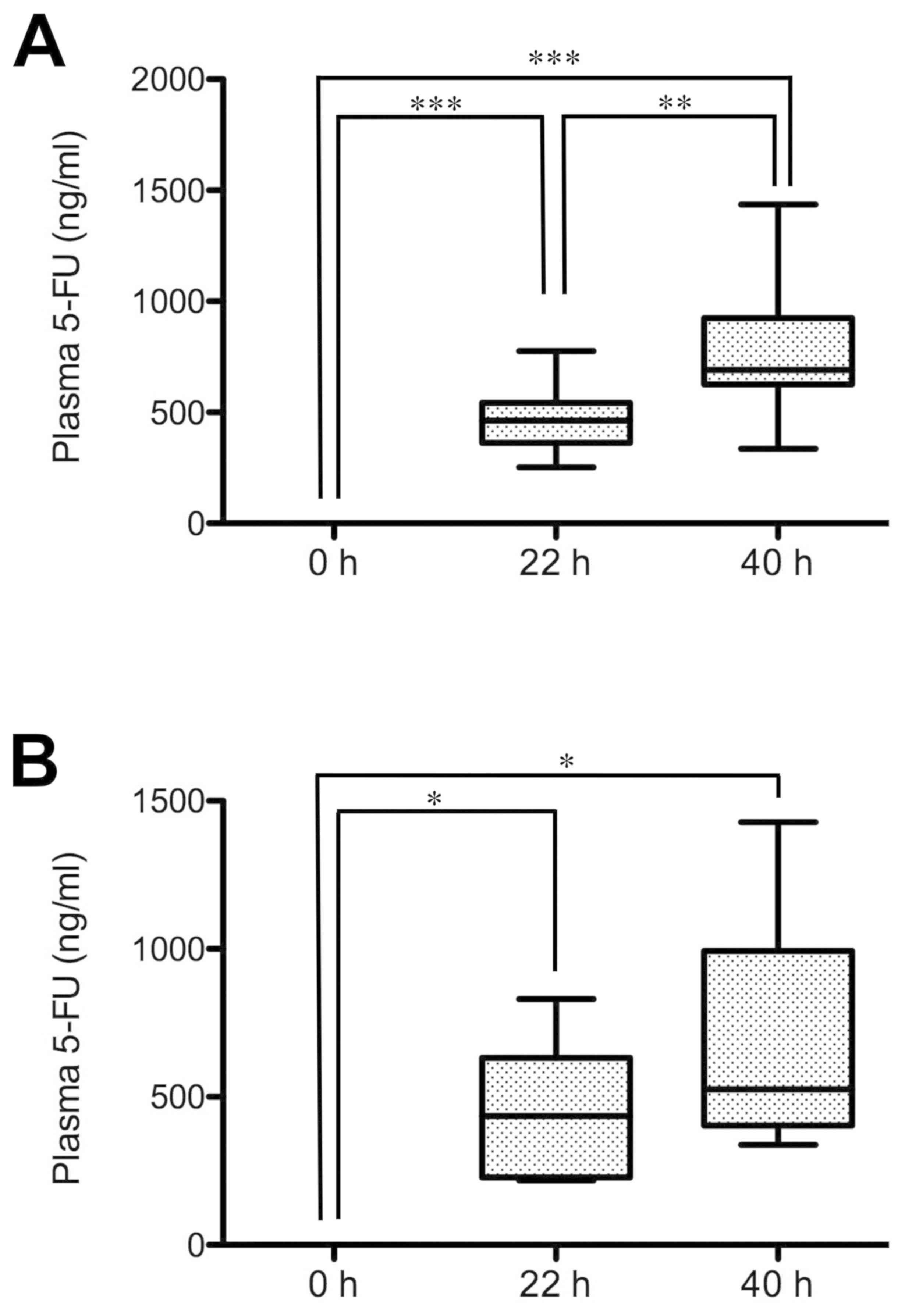
The plasma levels of 5-FU in the patients with
different tumor responses were examined. In the partial
response/stable disease group, the 5-FU plasma level at 22 h after
the start of infusion was 460.5 ng/ml (median value), while the
level at 40 h reached 690.9 ng/ml (Fig.
2A). The levels at 22 and 40 h were significantly higher than
at 0 h (P<0.001). Notably, the level at 40 h was significantly
higher than at 22 h (P<0.01). In the progressive disease group,
the 5-FU plasma level at 22 h after the start of infusion was 435.1
ng/ml, while the level at 40 h reached 525.9 ng/ml (Fig. 2B). The levels at 22 h and 40 h were
significantly higher than at 0 h (P<0.05). However, the level at
40 h was not significantly higher than at 22 h.
Furthermore, the mean 5-FU plasma level at 40 h
after the start of infusion between the partial response/stable
disease group and the progressive disease group was compared
(Fig. 2). The 5-FU plasma level of
the partial response/stable disease group (776.2±286.9 ng/ml; mean
± SD) was higher than that of the progressive disease group
(681.9±369.4 ng/ml; data not shown), although there was no
significant difference between the two groups, as assessed by
one-way ANOVA analysis with Bonferroni's post hoc test.
Adverse events in different tumor
response groups
Table III shows the
adverse events of grade ≥2 in the different tumor response groups;
grade 2, according to CTCAE v5.0, which is defined according to the
following criteria: i) Moderate; ii) minimal, local or noninvasive
intervention indicated; and iii) limiting age-appropriate
instrumental activities of daily living. In the partial response
group, numbness was the most common adverse event (75%). In the
stable disease group, numbness and diarrhea were observed (both
50%). In the progressive disease group, numbness (80%), appetite
loss (60%) and hand foot syndrome (60%) were observed. Adverse
events were evaluated after the infusion of 5-FU for 46 h on the
4th day of admission. Therefore, this suggests that the 5-FU plasma
level at 40 h after the start of infusion may reflect the results
of the adverse events.
 | Table III.Tumor response and severity of
adverse events. |
Table III.
Tumor response and severity of
adverse events.
| Adverse events | Partial response,
% | Stable disease,
% | Progressive
disease, % |
|---|
| Hand foot
syndrome | 50 | 0 | 60 |
| Fatigue | 0 | 25 | 40 |
| Edema | 0 | 25 | 40 |
| Stomatitis | 50 | 25 | 40 |
| Appetite loss | 25 | 25 | 60 |
| Numbness | 75 | 50 | 80 |
| Neutropenia | 0 | 0 | 20 |
| Nausea/Vomit | 25 | 0 | 0 |
| Facial
flushing | 0 | 0 | 20 |
| Diarrhea | 0 | 50 | 20 |
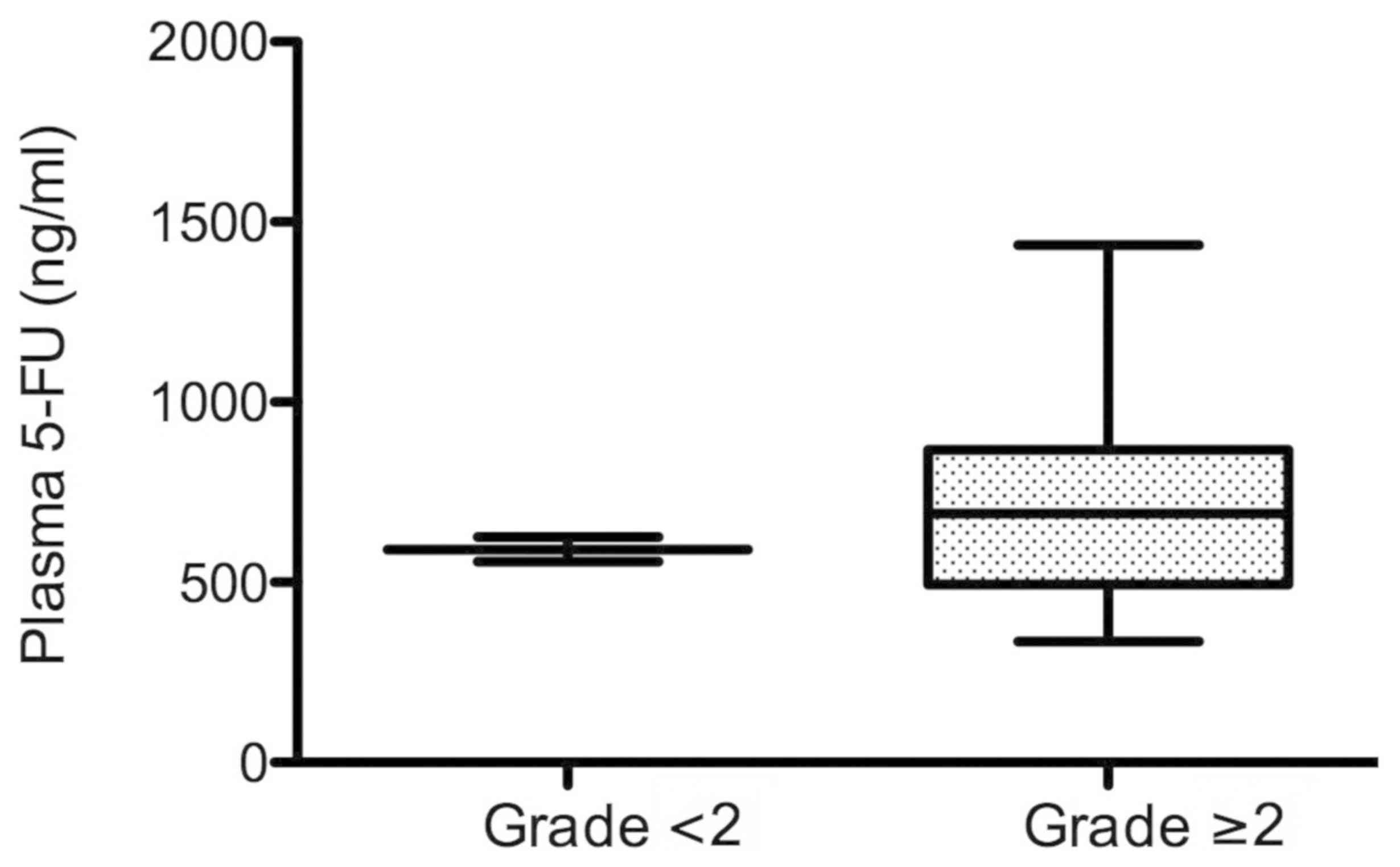
Plasma levels of 5-FU in the patients
with different severities of adverse events
Among the 15 patients, 13 patients were included in
the adverse event level of grade ≥2. In the partial response/stable
disease group, 8 out of 9 patients were included in the adverse
event level of grade ≥2, whereas in the progressive disease group,
5 out of the 6 patients were included to have an adverse event
level of grade ≥2. 5-FU plasma levels at 40 h after the start of
infusion in the adverse event level of grade ≥2 (13 patients in the
partial response/stable disease and progressive disease groups)
reached 690.9 ng/ml (median value), while the 5-FU plasma level for
the adverse event level of grade <2 (2 patients in the partial
response/stable disease and progressive disease groups) was 591.6
ng/ml (Fig. 3). Although there was
no statistical significance between the groups of grades ≥2 and
<2, the 5-FU plasma level was ~100 ng/ml higher in the group of
grades ≥2 than that in the group of grade <2.
Discussion
5-FU dosing has traditionally been based on BSA in
colorectal cancer treatment. However, there is accumulating
evidence that the dosing based on BSA may be of limited use
(13). The purpose of the present
study was to evaluate the changes in 5-FU plasma levels and the
tumor response as well as the severity of adverse events in
patients with cancer treated with 5-FU combined chemotherapy
(mFOLFOX6 and FOLFIRI). The dosing amount of 5-FU was determined
based on the BSA, and the transition of 5-FU plasma levels in 15
patients with colorectal cancer was monitored three times (0,22 and
40 h) before and after the start of infusion during
constant-infusion of 5-FU for 46 h. The present study demonstrated
that 5-FU plasma levels were significantly higher at 22 and 40 h
compared with at 0 h, when all 15 patients were analyzed. Notably,
the partial response/stable disease group showed significant
increases in 5-FU plasma levels at 40 h compared with at 22 h,
although the progressive disease group showed no significant
increase. In addition, the 5-FU plasma level in the adverse event
level of grade ≥2 was higher than that of grade <2 at 40 h after
the start of infusion.
A significant increase in the 5-FU plasma level at
40 h compared with 22 h after the start of infusion was observed.
In order to explain the wide range of increases in 5-FU plasma
levels, a number of previous studies suggested that
dihydropyrimidine dehydrogenase (DPD), an initial rate-limiting
enzyme related to 5-FU metabolism, plays a role in determining the
plasma levels of 5-FU (27–30). Therefore, the significant increase in
5-FU plasma levels after continuous intravenous infusion
(particularly between 22 and 40 h) observed in the partial
response/stable disease group may be due to the decreased
5-FU-metabolizing activity of DPD. However, the non-significant
increase of 5-FU plasma level in the progressive disease group
(between 22 and 40 h) may suggest that the 5-FU-metabolizing
activity of DPD is higher than that of the partial response/stable
disease group. Furthermore, the present results suggested that the
tumor response may be influenced by the increase of 5-FU plasma
level (possibly based on the low DPD activity) after the start of
continuous infusion, as observed in the higher plasma 5-FU level in
the partial response/stable disease group, compared with the
progressive disease group, suggesting that a high DPD activity may
be associated with a poor prognosis in the tumor response. However,
DPD activity was not evaluated in the present study. Therefore, DPD
activity should be evaluated in future studies.
In contrast to a previous study (18), in which the 5-FU plasma level reached
a plateau at 22 h after the continuous infusion with an
electronical pump, the present study showed that the 5-FU plasma
level continued to significantly increase between 22 and 40 h after
infusion. In the previous study (18), 5-FU continuous infusion relied on an
elastomeric infusion pump that is suitable for ambulatory patients
treated in an out-patient clinic. In the present study,
professional staff members constantly monitored the electronical
infusion pump to prevent the discontinuity of 5-FU during
admission. Therefore, the infusion methods used in the present
study may explain a continuous increase of 5-FU plasma levels
during 40 h from the start of admission. Furthermore, the increase
in the 5-FU plasma level may also explain the adverse events
occurring in the partial response/stable disease group. However, it
is not possible to conclude that an elastomeric pump is inferior to
an electronical infusion pump. Previous studies have suggested that
patients prefer elastomeric balloon infusion pump rather than an
electronical infusion pump, as the weight and size of elastomeric
infusion pump is low (31,32). However, it has been reported that the
accuracy in delivery rate of an elastomeric infusion pump is lower
compared with an electronical infusion pump (33,34).
Therefore, the accuracy of the delivery rate in an electronical
infusion pump may contribute to the significant increase of the
plasma level of 5-FU at 40 h in the present study. Furthermore, it
has also been suggested in a previous study utilizing an
electronical infusion pump that acute toxicity was correlated with
a high 5-FU plasma level (35).
Taking these observations into consideration, it was hypothesized
that the high plasma level of 5-FU observed in the partial
response/stable disease group may reflect the severity in adverse
events shown in the present study.
However, the present study has several limitations.
The number of patients enrolled in the present study was limited
and the tumor response was evaluated for only a short term of 3
months. Therefore, future studies should monitor patients for a
longer period to evaluate the prognosis and overall survival.
However, despite these limitations, the present study supports the
accumulating evidence that measuring 5-FU plasma levels may
contribute to the improvement of a positive tumor response as well
as to minimize the risk of severe adverse events (14,20).
Furthermore, it is difficult to discuss the effect of 5-FU for
tumor response and adverse event in the protocol of FOLFOX or
FOLFIRI using oxaliplatin or irinotecan, respectively. However, to
the best of our knowledge, a standardized method has not yet been
developed to evaluate the plasma levels of oxaliplatin or
irinotecan. Notably, a previous study has revealed that a higher
plasma level of 5-FU undergoing a regimen of continuous 5-FU plus
leucovorin with an electronical infusion pump was correlated with
more severe toxicities (35). The
present study also indicated that a higher 5-FU plasma level
results in a positive tumor response. Although the plasma levels of
oxaliplatin and irinotecan were not measured in the present study,
these previous studies (15,17,36)
likely support the hypothesis that a significant increase of 5-FU
plasma at 40 h is associated with the severity of toxicity as well
as a positive tumor response.
In conclusion, during continuous infusion of 5-FU,
the 5-FU plasma level increased significantly. The tumor response
may be influenced by the increase of 5-FU plasma level from the
start of infusion. The 5-FU plasma level may be a predictive factor
for maximizing the tumor response and minimizing the risk of severe
adverse events.
Acknowledgements
The authors would like to thank Mr Hiroshi Yamane
and Mr Makoto Miyazaki (FALCO Biosystems Ltd.) for measuring the
plasma levels of 5-FU in the present study.
Funding
Funding was provided from Takeda Pharmaceutical
Company Ltd. (grant. no. RS2016A001043) and Chugai Pharmaceutical
Co., Ltd. (grant. no. AC-1-20170518213426-569401).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA and TO designed the research. YA, NS, TS, KK,
KNa, AN, MK, TW, KNi and TO performed the clinical study. YA, TO
and IN analyzed the data. YA and IN prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tobu Chiiki Hospital (approved December 21, 2015; IRB
nos. 15 and 4), and performed between January 1, 2017 and March 31,
2017. The present study was conducted in accordance with the
principles of the amended ‘Declaration of Helsinki and Ethical
Guidelines for Epidemiological Research’ (established by the
Japanese Government in 2008). Written informed consent was obtained
from all patients before they were enrolled in the present
study.
Patient consent for publication
All the participants enrolled in the present study
provided written informed consent for the publication of any
associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayer RJ: Moving beyond fluorouracil for
colorectal cancer. N Engl J Med. 343:963–964. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, et al: Irinotecan plus fluorouracil and leucovorin for
metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tournigand C, Andre T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jonker DJ, O'Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et
al: Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III;
Eastern Cooperative Oncology Group Study E3200, : Bevacizumab in
combination with oxaliplatin, fluorouracil, and leucovorin
(FOLFOX4) for previously treated metastatic colorectal cancer:
Results from the Eastern Cooperative Oncology Group Study E3200. J
Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu M, Rivkin A and Pham T: Panitumumab:
Human monoclonal antibody against epidermal growth factor receptors
for the treatment of metastatic colorectal cancer. Clin Ther.
30:14–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peeters M, Price TJ, Cervantes A, Sobrero
AF, Ducreux M, Hotko Y, Andre T, Chan E, Lordick F, Punt CJ, et al:
Randomized phase III study of panitumumab with fluorouracil,
leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as
second-line treatment in patients with metastatic colorectal
cancer. J Clin Oncol. 28:4706–4713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saam J, Critchfield GC, Hamilton SA, Roa
BB, Wenstrup RJ and Kaldate RR: Body surface area-based dosing of
5-fluoruracil results in extensive interindividual variability in
5-fluorouracil exposure in colorectal cancer patients on FOLFOX
regimens. Clin Colorectal Cancer. 10:203–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel JN, O'Neil BH, Deal AM, Ibrahim JG,
Sherrill GB, Olajide OA, Atluri PM, Inzerillo JJ, Chay CH, McLeod
HL and Walko CM: A community-based multicenter trial of
pharmacokinetically guided 5-fluorouracil dosing for personalized
colorectal cancer therapy. Oncologist. 19:959–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saif MW, Choma A, Salamone SJ and Chu E:
Pharmacokinetically guided dose adjustment of 5-fluorouracil: A
rational approach to improving therapeutic outcomes. J Natl Cancer
Inst. 101:1543–1552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gamelin E, Boisdron-Celle M, Delva R,
Regimbeau C, Cailleux PE, Alleaume C, Maillet ML, Goudier MJ, Sire
M, Person-Joly MC, et al: Long-term weekly treatment of colorectal
metastatic cancer with fluorouracil and leucovorin: Results of a
multicentric prospective trial of fluorouracil dosage optimization
by pharmacokinetic monitoring in 152 patients. J Clin Oncol.
16:1470–1478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ychou M, Duffour J, Kramar A, Debrigode C,
Gourgou S, Bressolle F and Pinguet F: Individual 5-FU dose
adaptation in metastatic colorectal cancer: Results of a phase II
study using a bimonthly pharmacokinetically intensified LV5FU2
regimen. Cancer Chemother Pharmacol. 52:282–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaldate RR, Haregewoin A, Grier CE,
Hamilton SA and McLeod HL: Modeling the 5-fluorouracil area under
the curve versus dose relationship to develop a pharmacokinetic
dosing algorithm for colorectal cancer patients receiving FOLFOX6.
Oncologist. 17:296–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Capitain O, Asevoaia A, Boisdron-Celle M,
Poirier AL, Morel A and Gamelin E: Individual fluorouracil dose
adjustment in FOLFOX based on pharmacokinetic follow-up compared
with conventional body-area-surface dosing: A phase II,
proof-of-concept study. Clin Colorectal Cancer. 11:263–267. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gamelin E, Delva R, Jacob J, Merrouche Y,
Raoul JL, Pezet D, Dorval E, Piot G, Morel A and Boisdron-Celle M:
Individual fluorouracil dose adjustment based on pharmacokinetic
follow-up compared with conventional dosage: Results of a
multicenter randomized trial of patients with metastatic colorectal
cancer. J Clin Oncol. 26:2099–2105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho J, Yoon J, Kim Y, Oh D, Kim SJ, Ahn J,
Suh GY, Nam SJ and Mitchell SA: Linguistic validation of the US
national cancer institute's patient-reported outcomes version of
the common terminology criteria for adverse events in Korean. J
Glob Oncol. 5:1–10. 2019. View Article : Google Scholar
|
|
22
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2016 for the treatment of colorectal cancer. Int J Clin
Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Correa GT, Bandeira GA, Cavalcanti BG,
Santos FB, Rodrigues Neto JF, Guimaraes AL, Haikal DS and De Paula
AM: Analysis of ECOG performance status in head and neck squamous
cell carcinoma patients: Association with sociodemographical and
clinical factors, and overall survival. Support Care Cancer.
20:2679–2685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skierka AS and Michels KB: Ethical
principles and placebo-controlled trials-interpretation and
implementation of the Declaration of Helsinki's placebo paragraph
in medical research. BMC Med Ethics. 19:242018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishiguro S: Pathological diagnosis of
colorectal cancer according to Japanese classification of
colorectal carcinoma. Nihon Rinsho. 69 (Suppl 3):S325–S329.
2011.(In Japanese).
|
|
27
|
Pinedo HM and Peters GF: Fluorouracil:
Biochemistry and pharmacology. J Clin Oncol. 6:1653–1664. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu ZH, Zhang R and Diasio RB: Purification
and characterization of dihydropyrimidine dehydrogenase from human
liver. J Biol Chem. 267:17102–17109. 1992.PubMed/NCBI
|
|
29
|
Diasio RB and Lu Z: Dihydropyrimidine
dehydrogenase activity and fluorouracil chemotherapy. J Clin Oncol.
12:2239–2242. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diasio RB and Johnson MR:
Dihydropyrimidine dehydrogenase: Its role in 5-fluorouracil
clinical toxicity and tumor resistance. Clin Cancer Res.
5:2672–2673. 1999.PubMed/NCBI
|
|
31
|
Zahnd D, Aebi S, Rusterholz S, Fey MF and
Borner MM: A randomized crossover trial assessing patient
preference for two different types of portable infusion-pump
devices. Ann Oncol. 10:727–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Capdevila X, Macaire P, Aknin P, Dadure C,
Bernard N and Lopez S: Patient-controlled perineural analgesia
after ambulatory orthopedic surgery: A comparison of electronic
versus elastomeric pumps. Anesth Analg. 96:414–417, table of
contents. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ganapathy S, Amendola A, Lichfield R,
Fowler PJ and Ling E: Elastomeric pumps for ambulatory patient
controlled regional analgesia. Can J Anaesth. 47:897–902. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaye T: Prolonged infusion times with
disposable elastomeric infusion devices. Am J Hosp Pharm.
51:533–534. 1994.PubMed/NCBI
|
|
35
|
Gamelin EC, Danquechin-Dorval EM, Dumesnil
YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH,
Gesta PH and Larra FG: Relationship between 5-fluorouracil (5-FU)
dose intensity and therapeutic response in patients with advanced
colorectal cancer receiving infusional therapy containing 5-FU.
Cancer. 77:441–451. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blaschke M, Blumberg J, Wegner U,
Nischwitz M, Ramadori G and Cameron S: Measurements of 5-FU plasma
concentrations in patients with gastrointestinal cancer: 5-FU
levels reflect the 5-FU dose applied. J Cancer Ther. 3:28–36. 2012.
View Article : Google Scholar
|