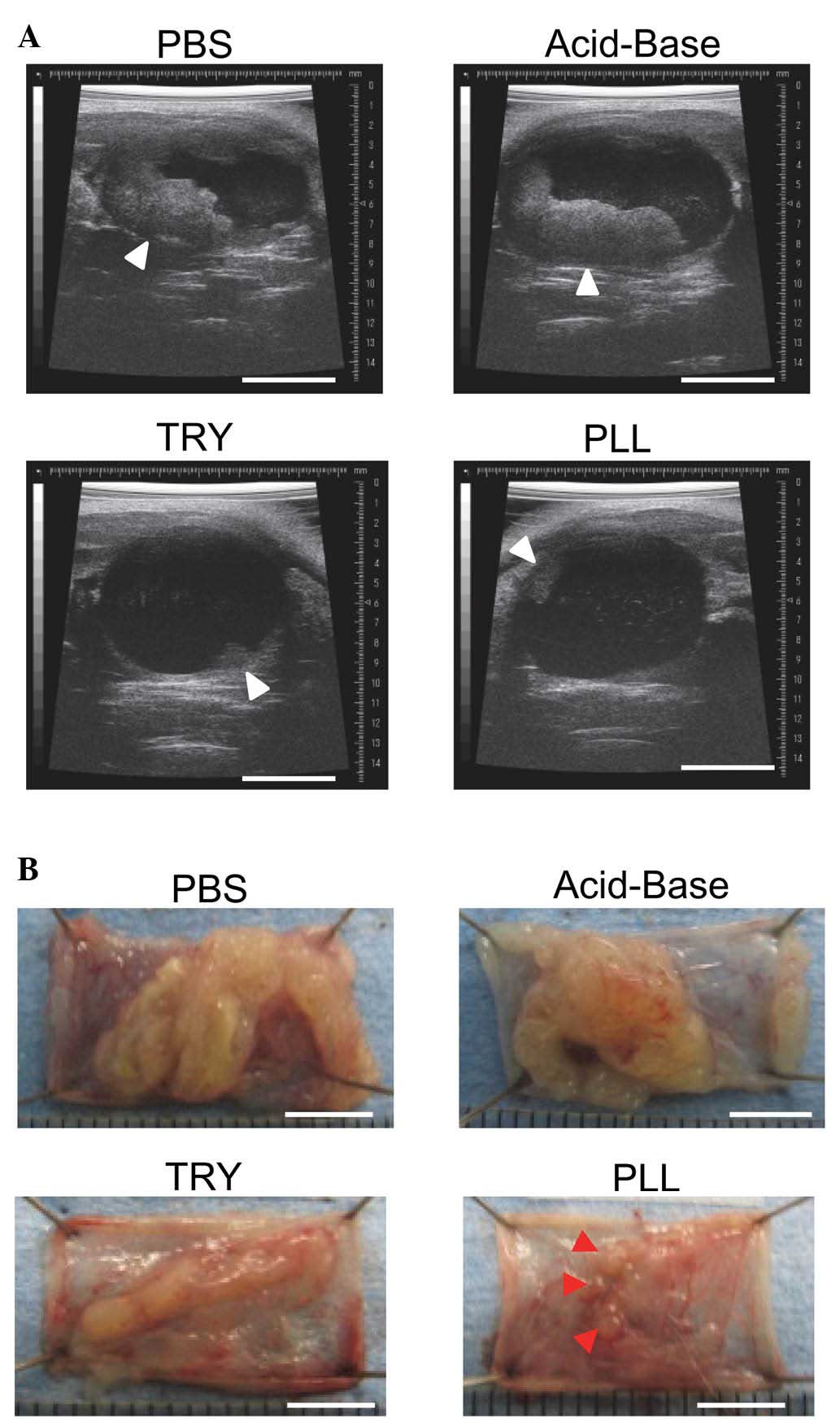
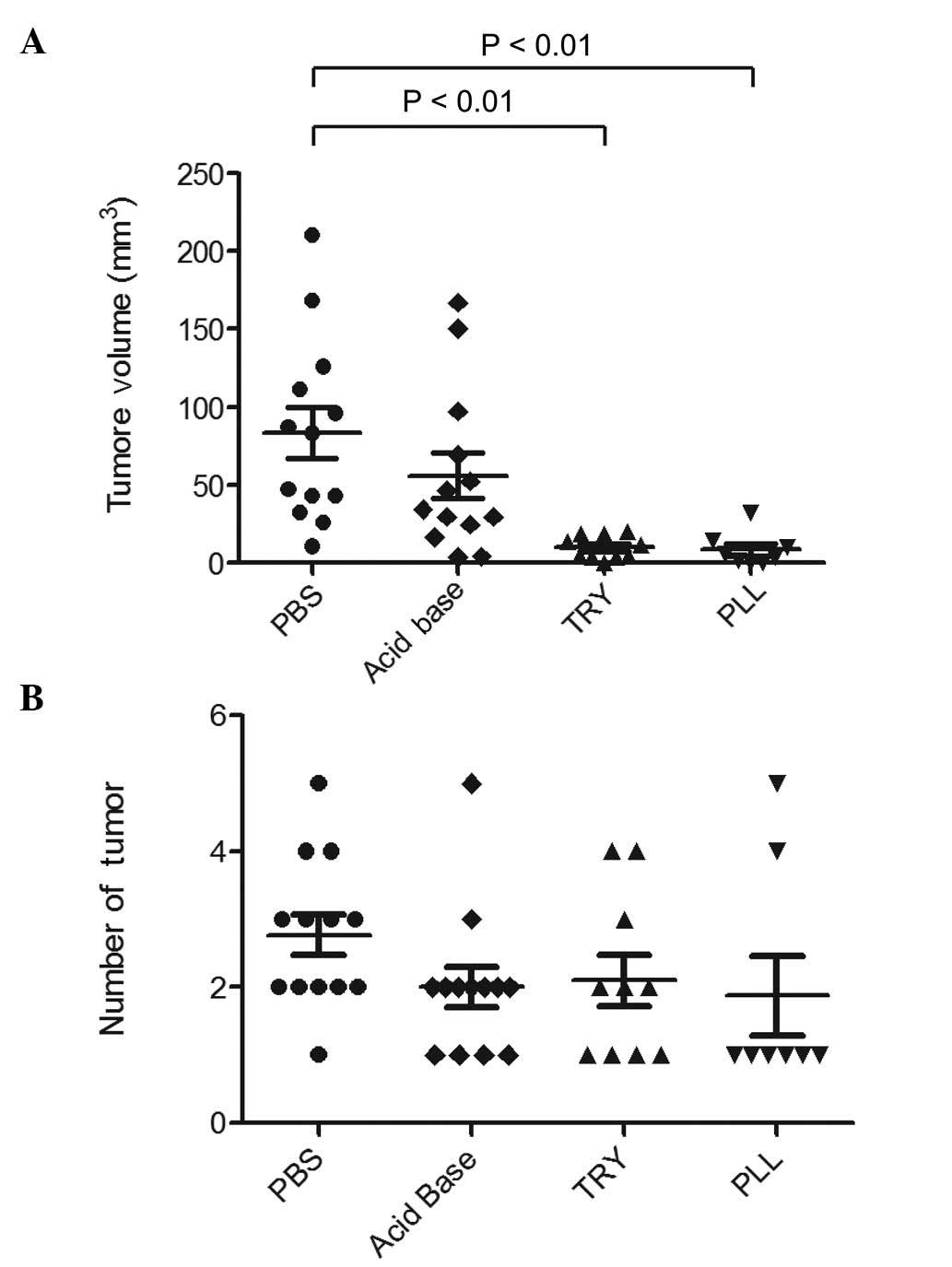
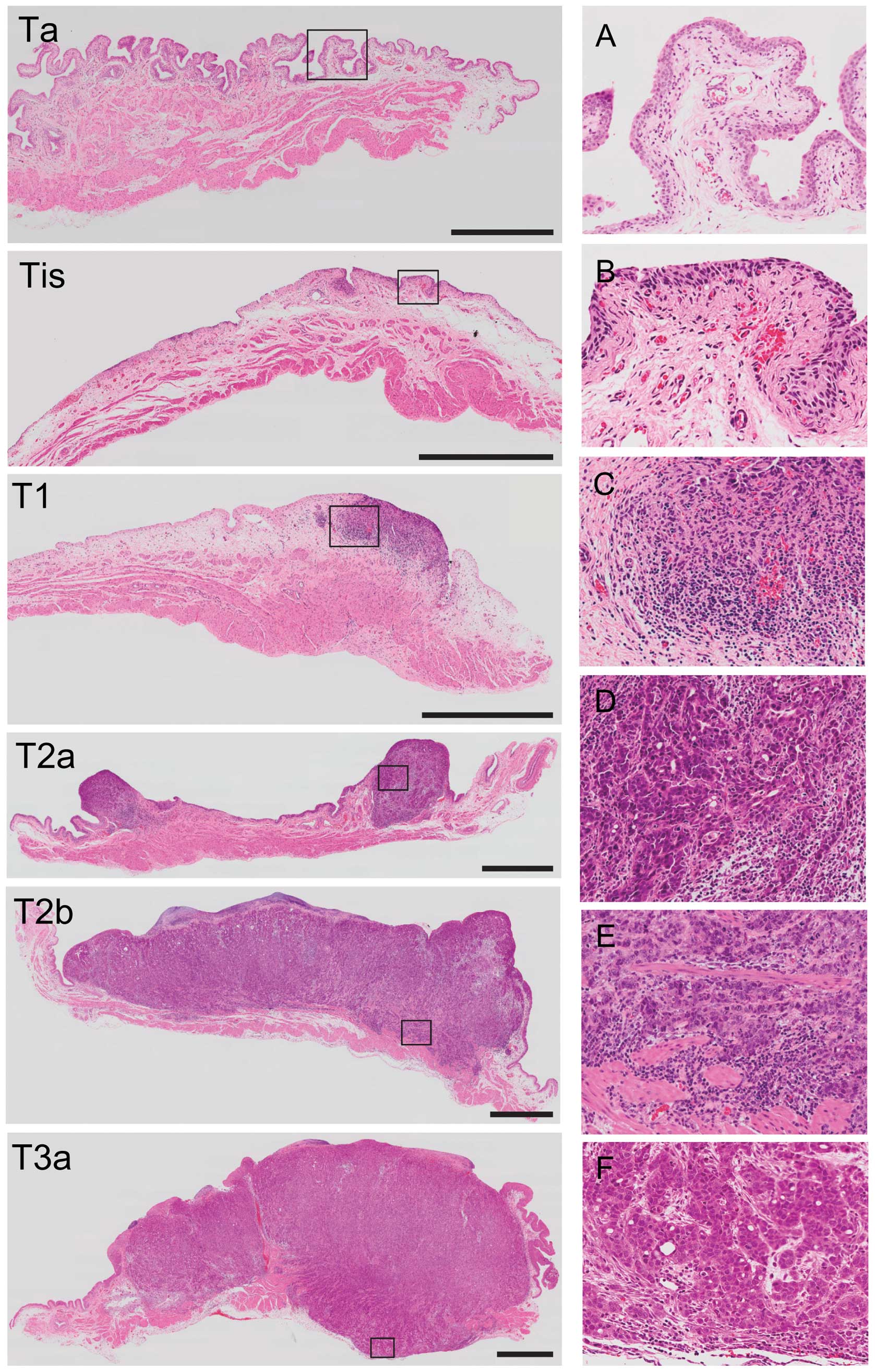
|
1.
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, et al: Predicting recurrence and progression in
individual patients with stage Ta T1 bladder cancer using EORTC
risk tables: a combined analysis of 2596 patients from seven EORTC
trials. Eur Urol. 49:466–475. 2006. View Article : Google Scholar
|
|
2.
|
Lamm DL: Carcinoma in situ. Urol Clin
North Am. 19:499–508. 1992.
|
|
3.
|
Xiao Z, McCallum TJ, Brown KM, et al:
Characterization of a novel transplantable orthotopic rat bladder
transitional cell tumour model. Br J Cancer. 81:638–646. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhou JH, Rosser CJ, Tanaka M, et al:
Visualizing superficial human bladder cancer cell growth in vivo by
green fluorescent protein expression. Cancer Gene Ther. 9:681–686.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Watanabe T, Shinohara N, Sazawa A, et al:
An improved intravesical model using human bladder cancer cell
lines to optimize gene and other therapies. Cancer Gene Ther.
7:1575–1580. 2000. View Article : Google Scholar
|
|
6.
|
Arentsen HC, Hendricksen K, Oosterwijk E
and Witjes JA: Experimental rat bladder urothelial cell carcinoma
models. World J Urol. 27:313–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Loskog A, Ninalga C, Hedlund T,
Alimohammadi M, Malmstrom PU and Totterman TH: Optimization of the
MB49 mouse bladder cancer model for adenoviral gene therapy. Lab
Anim. 39:384–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ramesh N, Ge Y, Ennist DL, et al: CG0070,
a conditionally replicating granulocyte macrophage
colony-stimulating factor - armed oncolytic adenovirus for the
treatment of bladder cancer. Clin Cancer Res. 12:305–313. 2006.
View Article : Google Scholar
|
|
9.
|
Shapiro A, Kelley DR, Oakley DM, Catalona
WJ and Ratliff TL: Technical factors affecting the reproducibility
of intravesical mouse bladder tumor implantation during therapy
with Bacillus Calmette-Guerin. Cancer Res. 44:3051–3054. 1984.
|
|
10.
|
Bisson JF, Parache RM, Droulle P, Notter
D, Vigneron C and Guillemin F: A new method of implanting
orthotopic rat bladder tumor for experimental therapies. Int J
Cancer. 102:280–285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
TNM Classification of Malignant Tumours.
6th edition. (UICC).Hoboken: Wiley-Blackwell; NJ: 2002
|
|
12.
|
Fischer D, Li Y, Ahlemeyer B, Krieglstein
J and Kissel T: In vitro cytotoxicity testing of polycations:
influence of polymer structure on cell viability and hemolysis.
Biomaterials. 24:1121–1131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Arnold LJ Jr, Dagan A, Gutheil J and
Kaplan NO: Antineoplastic activity of poly(L-lysine) with some
ascites tumor cells. Proc Natl Acad Sci USA. 76:3246–3250. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Reuter M, Schwieger C, Meister A, Karlsson
G and Blume A: Poly-l-lysines and poly-l-arginines induce leakage
of negatively charged phospholipid vesicles and translocate through
the lipid bilayer upon electrostatic binding to the membrane.
Biophys Chem. 144:27–37. 2009. View Article : Google Scholar
|
|
15.
|
Yamashita K, Mimori K, Inoue H, Mori M and
Sidransky D: A tumor-suppressive role for trypsin in human cancer
progression. Cancer Res. 63:6575–6578. 2003.PubMed/NCBI
|
|
16.
|
Patel Y, Rahman S, Siddiqua A, Wilkinson
JM, Kakkar VV and Authi KS: Functional characterization of PM6/13,
a beta3-specific (GPIIIa/CD61) monoclonal antibody that shows
preferential inhibition of fibrinogen binding over fibronectin
binding to activated human platelets. Thromb Haemost. 79:177–185.
1998.
|
|
17.
|
Takeichi M: The cadherins: cell-cell
adhesion molecules controlling animal morphogenesis. Development.
102:639–655. 1988.PubMed/NCBI
|
|
18.
|
Hendricksen K, Molkenboer-Kuenen J,
Oosterwijk E, Hulsbergen-van de Kaa CA and Witjes JA: Evaluation of
an orthotopic rat bladder urothelial cell carcinoma model by
cystoscopy. BJU Int. 101:889–893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
El Khatib S, Berrahmoune S, Leroux A,
Bezdetnaya L, Guillemin F and D’Hallewin MA: A novel orthotopic
bladder tumor model with predictable localization of a solitary
tumor. Cancer Biol Ther. 5:1327–1331. 2006.PubMed/NCBI
|
|
20.
|
Pili R, Guo Y, Chang J, Nakanishi H,
Martin GR and Passaniti A: Altered angiogenesis underlying
age-dependent changes in tumor growth. J Natl Cancer Inst.
86:1303–1314. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Gronlund-Pakkanen S, Wahlfors J, Talja M,
et al: The effect of photodynamic therapy on rat urinary bladder
with orthotopic urothelial carcinoma. BJU Int. 92:125–130. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Gunther JH, Jurczok A, Wulf T, et al:
Optimizing syngeneic orthotopic murine bladder cancer (MB49).
Cancer Res. 59:2834–2837. 1999.PubMed/NCBI
|