Introduction
Colorectal cancer is the third most common cancer
among men and women in the USA and Australia (1). Approximately 15% of the patients
present with synchronous and a further 15–20% develop metachronous
metastasis (2). The treatment of
liver metastasis has radically changed with the advent of novel
chemotherapeutic drugs and advances in resection techniques.
Despite comprehensive genetic characterization of human colon and
rectal cancer (3), the exact
mechanisms underlying the metastatic process remain to be
elucidated. Recent expansions in the field of metabolomics and the
effect on genomics has led to a reinforcement of the idea that the
hepatic cellular environment is a crucial factor affecting the
behaviour of colorectal liver metastases (4).
Pre-existing liver disease may modify the local
environment, thereby altering the ability of circulating tumour
cells to form metastatic deposits within the liver. For example,
colorectal liver metastases rarely occur in patients with liver
cirrhosis (3, 5). Systemic chemotherapy may result in
liver damage, which may vary from steatosis, to sinusoidal injury,
to hepatic fibrosis (6–10). Indeed, a recent study demonstrated
that sinusoidal injury was associated with poor survival and early
recurrence in patients treated with oxaliplatin-based neoadjuvant
therapy (11). The histological
changes induced in the liver by chemotherapy have been well
documented. However, the local cellular processes resulting in
these histopathological characteristics and the altered propensity
for hepatic metastasis, are poorly understood.
The liver plays a central role in protein,
carbohydrate and lipid metabolism and a number of these metabolic
pathways are tightly regulated by receptors. Peroxisome
proliferator-activated receptors (PPARs) constitute one such family
of nuclear receptors, which regulate lipid metabolism as well as
inflammation and response to injury (12). These receptors also play an
important role in tumourigenesis (13). Given the abovementioned functions,
we hypothesised that PPARs may be involved in chemotherapy-induced
liver injury and may affect tumour progression and patient
survival. Therefore, the aim of this study was to investigate the
association of PPAR expression with the histopathological
characteristics of chemotherapy-related liver injury and clinical
outcomes.
Materials and methods
Patients
A total of 145 patients underwent hepatectomy for
colorectal liver metastases at the North Shore campus of the
University of Sydney between June, 1998 and August, 2007. Among
those patients, 103 non-consecutive and non-selected patients had
adequate tissue blocks for PPAR staining and histological
re-evaluation; this cohort formed the basis of the present study.
Basic demographic, clinical and pathological data were collected
from hospital records, while survival data were collected from
ongoing clinical follow-up. Patients were excluded from the study
if they had undergone prior liver resection, whereas those who
underwent subsequent resection were included. Synchronous liver
metastases were defined as those presenting within 4 months of the
primary colorectal cancer diagnosis; metachronous liver metastases
were defined as those identified >4 months after the primary
colorectal cancer diagnosis.
Liver resection
All the patients underwent a standard preoperative
assessment, which included a multiphase computed tomography (CT)
scan of the abdomen and thorax. A positron emission tomography scan
was also performed from January, 2004 onwards. All the cases were
discussed at a multidisciplinary group meeting prior to liver
resection. The operative criteria included the likelihood of
achieving an R0 resection, along with preservation of vascular
inflow and outflow and an adequate post-resection residual liver
volume. Patients with limited extrahepatic intra-abdominal disease
were not excluded from resection. Liver transection was performed
using the Cavitron Ultrasonic Surgical Aspirator dissection device
(Integra, Plainsboro, NJ, USA), under low central venous pressure
conditions, with intermittent inflow occlusion. The postoperative
complications were classified according to the Clavien-Dindo
classification, with major complications graded as ≥3 (14). The follow-up regime included
3-monthly clinical evaluations, serum tumour markers and CT scans
of the abdomen and thorax for the first year, 6-monthly in the
second year and annually thereafter.
Pathological protocol
The histological analyses were based on hematoxylin
and eosin, Masson trichrome and reticulin stains. The following
characteristics were evaluated and scored: sinusoidal dilatation,
perisinusoidal fibrosis, ballooning and steatosis and were graded
semi-quantitatively as follows: 0, absent; 1, mild (centrilobular
involvement limited to one-third of the lobule); 2, moderate
(centrilobular involvement involving two-thirds of the lobule); and
3, severe (complete lobular involvement). Steatosis was estimated
as the percentage of involved hepatocytes and was categorized as
follows: 0, absent; 1, mild (steatosis in <30% of the
hepatocytes); 2, moderate (steatosis in 30–60% of the hepatocytes);
and 3, severe (steatosis in >60% of the hepatocytes). These
variables were scored by a single pathologist (A.K.) who was
blinded as to all other data.
For PPAR- γand-α staining, the slides were processed
with an automated staining system, namely the Bond-Max Autostainer
(Vision Biosystems, Mount Waverley, Victoria, Australia) used
according to the manufacturer's protocol and with the
manufacturer's retrieval solutions. For PPAR- α, a rabbit
polyclonal antibody (cat. no. 8934; Abcam, Cambridge, UK) was used
at a dilution of 1 : 600. For PPAR- γ, a mouse monoclonal antibody
(clone E8; cat. no. SC-7273; Santa Cruz Biotechnology, Santa Cruz,
CA, USA) was used at a dilution of 1 : 100. For both antibodies,
heat-induced epitope retrieval was performed for 30 min in the
manufacturer's alkaline retrieval solution ER2 (VBS part no.
AR9640). A biotin-free detection system was employed (VBS part no.
DS 9713).
The PPAR- γand-α slides were scored
semi-quantitatively as follows: 0, no positive cells (<1%); 1+,
focal positive staining (<10% of cells); 2+, diffuse weak
staining (>10% of cells staining weakly positive); and 3+,
diffuse strong positive staining (>10% of cells staining
diffusely strongly positive). PPAR- γand-α staining were scored by
a single pathologist (A.G.) who was blinded as to all other
data.
Statistical analysis
Descriptive statistics were reported using means
(standard deviation) and medians (interquartile ranges), depending
on the distribution. Inferential statistical comparisons between
groups were performed using the Fisher's exact test and Student's
t-test for categorical and parametric data, respectively. A
multivariate logistic analysis of covariates with P<0.2 in the
univariate analysis was performed. Hosmer and Lemeshow's
goodness-of-fit test was used.
Overall survival was defined as survival censored by
either last follow-up or death from any cause. Disease-free
survival was censored at the first event of recurrence in any area,
last follow-up or death. Kaplan-Meier curves were constructed. A
univariate survival analysis was performed using the log-rank test
and a multivariate analysis was performed by constructing Cox
proportional hazards models from covariates with P<0.2 in the
univariate analysis. The purposeful selection of covariates method
was used to select variables for the final model. The final model
was then assessed for validity of the proportional hazards
assumption using Shoenfeld residuals and goodness-of-fit using
Cox-Snell residuals. All the statistical analyses were performed
using Stata/SE software, version 11.2 for Windows (StataCorp,
College Station, TX, USA).
In order to investigate factors affecting long-term
survival, a survival analysis was performed excluding early
postoperative deaths (90-day mortality); i.e., conditional upon
survival to 90 days.
Results
Clinicopathological
characteristics
The demographic, histopathological and clinical
characteristics of the study cohort are summarized in Table I. Briefly, the mean age of the
patients was 63 years and there were 68 (66%) men and 35 (34%)
women. Lobular inflammation and sinusoidal dilatation were the most
common histopathological abnormalities, with >70% of the
patients exhibiting some degree of lobular inflammation and
approximately the same proportion exhibiting some degree of
sinusoidal dilatation. The least common abnormality was ballooning
(only 21% of the patients). In terms of tumour characteristics, the
majority (52%) were solitary metastases and most of the tumours
(76%) were moderately differentiated. The mean tumour size was 46
mm and 84% of the patients underwent an R0 resection.
 | Table I.Summary of patient characteristics for
the entire cohort and according to PPAR- α and- γstaining. |
Table I.
Summary of patient characteristics for
the entire cohort and according to PPAR- α and- γstaining.
|
|
| PPAR-αa | PPAR- γb |
|---|
|
|
|
|
|
|---|
| Covariates | Overall | Negative | Positivec | P-value | Negative | Positiveb | P-value |
|---|
| Patient
characteristics |
| Age,
years [mean (95% CI)] | 63 (11) | 63 (60–66) | 63 (59–66) | 0.96 | 61 (58–65) | 64 (61–66) | 0.31 |
| Gender
(n, %) | 0.046 |
|
Female | 35 (34) | 19 (37) | 16 (31) | 0.68 | 7(21) | 28 (42) | |
|
Male | 68 (66) | 32 (63) | 35 (69) | | 27 (79) | 39 (58) | |
| Pathological
characteristics (n, %) |
|
Steatosis | 0.92 | 0.59 |
|
0 | 54 (52) | 28 (55) | 25 (49) | 15 (44) | 37 (56) |
|
1 | 25 (24) | 12 (24) | 13 (25) | 11 (32) | 14 (21) |
|
2 | 21 (20) | 10 (20) | 11 (22) | 7 (21) | 14 (21) |
| 3 | 3 (3) | 1 (2) | 2 (4) | 1 (3) | 2 (3) |
| Lobular
inflammation |
0.023b | 0.15 |
|
0 | 30 (29) | 20 (39) | 10 (20) | 6 (18) | 24 (36) |
|
1 | 38 (37) | 19 (37) | 18 (35) |
| 16 (47) | 20 (30) |
|
|
2 | 24 (23) | 6 (12) | 18 (35) |
| 7 (21) | 17 (25) |
|
|
3 | 11 (11) | 6 (12) | 5 (10) |
| 5 (15) | 6 (9) |
|
|
Ballooning | 1.00 |
|
| 0.93 |
|
0 | 81 (79) | 40 (78) | 40 (78) |
| 27 (79) | 52 (78) |
|
|
1 | 17 (17) | 9 (18) | 8 (16) |
| 6 (18) | 11 (16) |
|
|
2 | 5 (5) | 2 (4) | 3 (6) |
| 1 (3) | 4 (6) |
|
|
Fibrosis | 0.36 |
|
| 0.23 |
|
0 | 52 (51) | 27 (53) | 24 (47) |
| 15 (44) | 35 (52) |
|
|
1 | 22 (21) | 13 (25) | 9 (18) |
| 5 (15) | 17 (25) | 0/1 vs.
2/3 |
|
2 | 21 (20) | 7 (14) | 14 (27) |
| 10 (29) | 11 (16) | P=0.042 |
|
3 | 8 (8) | 4 (8) | 4 (8) |
| 4 (12) | 4 (6) |
|
|
Sinusoidal dilatation | 0.65 |
|
| 0.24 |
|
0 | 29 (28) | 17 (33) | 12 (24) |
| 6 (18) | 22 (33) |
|
|
1 | 32 (31) | 14 (27) | 17 (33) |
| 10 (29) | 21 (31) |
|
|
2 | 17 (17) | 7 (14) | 10 (20) |
| 6 (18) | 11 (16) |
|
|
3 | 25 (24) | 13 (26) | 12 (24) |
| 12 (35) | 13 (19) |
| PPAR
staining (n, %) PPAR-α |
|
0 | 51 (50) |
|
|
|
|
|
|
|
1+ | 24 (24) |
|
|
|
|
|
|
|
2+ | 11 (11) |
|
|
|
|
|
|
|
3+ | 16 (16) |
|
|
|
|
|
|
|
PPAR-γ |
|
0 | 34 (34) |
|
|
|
|
|
|
|
1+ | 52 (51) |
|
|
|
|
|
|
|
2+ | 14 (14) |
|
|
|
|
|
|
|
3+ | 1 (1) |
|
|
|
|
|
|
| Clinical
characteristics (n, %) |
|
Resection type | 1.00 |
|
| 0.40 |
|
Minor | 45 (44) | 22 (43) |
| 22 (43) | 12 (35) | 31 (46) |
|
|
Major | 58 (56) | 29 (57) | 29 (57) |
| 22 (65) | 36 (54) |
|
Resection margin (mm) | 0.93 |
|
| 0.015 |
|
R1 | 17 (16) | 9 (18) | 8 (16) |
| 9 (26) | 8 (12) |
|
R0 |
|
<1 | 14 (14) | 8 (16) | 6 (12) |
| 8 (24) | 6 (9) |
|
1-10 | 48 (47) | 23 (45) | 24 (47) |
| 9 (26) | 3 7 (55) |
|
>10 | 24 (23) | 11 (22) | 13 (25) |
| 8 (24) | 16 (24) |
| Preoperative
chemotherapy | 1.00 |
|
| 0.39 |
| No | 40 (39) | 20 (39) | 20 (40) |
| 15 (45) | 24 (36) |
|
|
Yes | 62 (61) | 31 (61) | 30 (60) |
| 18 (55) | 43 (64) |
| Preoperative
oxaliplatin | 0.64 |
| 0.023 |
| No | 80 (78) | 38 (75) | 41 (80) |
| 31 (91) | 47 (70) |
|
|
Yes | 23 (22) | 13 (25) | 10 (20) |
| 3 (9) | 20 (30) |
|
| Postoperative
chemotherapy | 1.00 |
|
| 1.00 |
| No | 50 (50) | 24 (49) | 26 (51) |
| 17 (50) | 33 (51) |
|
|
Yes | 51 (51) | 25 (51) | 25 (49) |
| 17 (50) | 32 (49) |
|
| Complications
(Clavien-Dindo) | 0.086 |
|
| 0.54 |
|
<3 | 87 (84) | 46 (90) | 40 (78) |
| 29 (85) | 56 (84) |
| ≥3 | 16 (16) | 5 (10) | 11 (22) |
| 5 (15) | 11 (16) |
| Perioperative
death | 0.31 |
|
| 0.59 |
| No | 98 (96) | 50 (98) | 48 (94) |
| 33 (97) | 64 (96) |
|
|
Yes | 4 (4) | 1 (2) | 3 (6) |
| 1 (3) | 3 (4) |
| Primary tumour
characteristics (n, %) |
|
Metachronous | 0.53 |
|
| 0.53 |
|
No | 49 (48) | 23 (45) | 26 (52) |
| 14 (42) | 34 (51) |
|
|
Yes | 53 (52) | 28 (55) | 24 (48) |
| 19 (58) | 33 (49) |
|
| Lymph
node + | 0.55 |
|
| 0.53 |
|
No | 44 (44) | 20 (40) | 24 (48) |
| 13 (39) | 31 (47) |
|
|
Yes | 57 (56) | 30 (60) | 26 (52) |
| 20 (61) | 35 (53) |
|
| Tumour
characteristics |
| No. of
metastases (n, %) | 0.077 0.92 |
|
|
|
1 | 54 (52) | 29 (57) | 24 (47) |
| 19 (56) | 34 (51) |
|
|
2-3 | 34 (33) | 12 (23) | 22 (43) |
| 10 (29) | 23 (34) |
|
|
>3 | 15 (15) | 10 (20) | 5 (10) |
| 5 (15) | 10 (15) |
|
| Grade
of differentiation (n, %) | 0.24 |
|
| 0.25 |
|
High | 10 (11) | 3 (6) | 7 (15) |
| 5 (17) | 4 (6) |
|
|
Moderate | 76 (81) | 39 (83) | 36 (80) |
| 24 (80) | 52 (84) |
|
|
Poor | 8 (8.5) | 5 (11) | 2 (4) |
| 1 (3) | 6 (10) |
|
| Size,
mm [mean (range)] | 46 (28) | 46 (37–55) | 45 (38–52) | 0.86 | 49 (39–59) | 44 (37–50) | 0.40 |
The PPAR- α and-γstaining characteristics are shown
in Table I. A total of 50% of the
patients in this study were PPAR- α -negative and 34% were PPAR-
γ-negative. When comparing patients whose livers stained for PPAR-
α to those who did not (Table I),
there was a significant difference in the proportion of patients
with lobular inflammation, with more patients exhibiting lobular
inflammation in the PPAR- α -positive group (P=0.023). As regards
PPAR- γstaining, there were more patients with moderate to severe
fibrosis (score 2 or 3) in the PPAR- γ-negative group compared to
the PPAR- γ-positive group. On multivariate analysis (Table II), PPAR- α staining was
significantly associated with lobular inflammation (odds ratio =
2.9). There was a non-significant trend with regards to fibrosis,
whilst the association with oxaliplatin use exhibited marginal
statistical significance.
 | Table II.Multivariate logistic regression
model for the expression of PPAR -α and -γ. |
Table II.
Multivariate logistic regression
model for the expression of PPAR -α and -γ.
| Covariates | OR | SE | 95% CI | P-value |
|---|
| PPAR- α |
| Lobular
inflammation | 2.9 | 1.3 | 1.2-7.0 | 0.015 |
| Clavien-Dindo
≥3 | 3.0 | 1.8 | 0.92-9.7 | 0.067 |
| PPAR- γ |
| Oxaliplatin
use | 3.9 | 2.7 | 1.0-15 | 0.048 |
| Margin (≥1 vs.
<1 mm) | 3.3 | 1.6 | 1.3-8.6 | 0.015 |
| Fibrosis (score 3/4
vs. 0/1) | 0.40 | 0.20 | 0.15-1.1 | 0.07 0 |
| Male gender | 0.41 | 0.22 | 0.15-1.2 | 0.091 |
The median follow-up period for the study cohort was
48 months (range, 0.4-142 months). The results of the univariate
survival analysis demonstrated improved survival associated with
PPAR- α negativity (median survival, 79 vs. 36 months; P=0.037),
minor resection and the absence of major complications (Table III).
 | Table III.Influence of various patient factors
and PPAR status on survival. |
Table III.
Influence of various patient factors
and PPAR status on survival.
| Covariates | No. | Overall
survival | Disease-free
survival |
|---|
| Median survival,
months (95% CI) | P-value | Median survival,
months (95% CI) | P-value |
|---|
| Overall | 103 |
| 48(36–42) | 15(11–24) |
|
| Demographic
characteristics |
|
|
|
|
|
| Age(years) |
|
| 0.44 |
| 0.82 |
| <65 | 55 | 65(38–82) |
| 20(7–31) |
|
| ≥65 | 48 | 39(32–57) |
| 13(8–24) |
|
| Gender |
|
| 0.26 |
| 0.19 |
| Female | 35 | 81(32-NR) |
| 12(7–82) |
|
| Male | 68 | 46(35–60) |
| 15(11–23) |
|
| PPAR staining |
|
|
|
|
|
| PPAR-α |
|
| 0.037 |
| 0.12 |
| 0 | 51 | 79(48-NR) |
| 24(12–32) |
|
| 1+to 3+ | 51 | 36(31–56) |
| 11(7–20) |
|
| PPAR-γ |
|
| 0.44 |
| 0.39 |
| 0 | 34 | 43(33–60) |
| 15(7–25) |
|
| 1+to 3+ | 67 | 56(32–82) |
| 19(9–27) |
|
| Pathological
characteristics |
|
|
|
|
|
| Steatosis |
|
| 0.29 |
| 0.72 |
| 0-1 | 79 | 53(37–80) |
| 14(8–23) |
|
| 2-3 | 24 | 44(18–83) |
| 25(7–32) |
|
| Lobular
inflammation |
|
| 0.60 |
| 0.42 |
| 0-1 | 68 | 53(37–81) |
| 15(9–24) |
|
| 2-3 | 35 | 40(31–82) |
| 20(8–26) |
|
| Ballooning |
|
| 0.92 |
| 0.73 |
| 0 | 81 | 51(36–79) |
| 15(8–23) |
|
| 1-2 | 22 | 72(19–101) |
| 17(8–34) |
|
| Fibrosis |
|
| 0.87 |
| 0.90 |
| 0-1 | 74 | 48(35–72) |
| 15(7–24) |
|
| 2-3 | 29 | 57(33–82) |
| 21(10–31) |
|
| Sinusoidal
dilatation |
|
| 0.81 |
| 0.88 |
| 0-1 | 61 | 46(34–82) |
| 15(11–24) |
|
| 2-3 | 42 | 53(34–81) |
| 15(7–31) |
|
| Clinical
characteristics |
|
|
|
|
|
| Resection type |
|
| 0.048 |
| 0.060 |
| Minor | 45 | 65(38-NR) |
| 20(8–35) |
|
| Major | 58 | 39(26–72) |
| 15(8–24) |
|
| Resection
margin(mm) |
|
| 0.19 |
| 0.090 |
| <1 | 31 | 36(24–79) |
| 8(5–24) |
|
| ≥1 | 72 | 57(38–82) |
| 20(12–30) |
|
| Preoperative
chemotherapy |
|
| 0.52 |
| 0.21 |
| No | 40 | 60(37–101) |
| 20(11–38) |
|
| Yes | 62 | 48(31–72) |
| 13(7–23) |
|
| Postoperative
chemotherapy |
|
| 0.56 |
|
0.043a |
| No | 50 | 57(34-NR) |
| 23(10–54) |
|
| Yes | 51 | 46(34–80) |
| 14(7–21) |
|
| Complications |
|
| 0.0008 |
| 0.0098a |
| Dindo≤2 | 87 | 56(40–96) |
| 20(12–25) |
|
| Dindo≥3 | 16 | 17(2–72) |
| 5(1–13) |
|
| Complications(excl.
deaths) |
|
| 0.042 |
| 0.17 |
| Dindo≤2 | 87 | 56(40–96) |
| 20(12–25) |
|
| Dindo≥3 | 12 | 24(5–81) |
| 7(2–54) |
|
| Primary tumour
characteristics |
|
|
|
|
|
| Metachronous |
|
| 0.77 |
| 0.46 |
| No | 49 | 51(35–81) |
| 15(7–25) |
|
| Yes | 53 | 56(26–101) |
| 20(10–30) |
|
| Lymph node + |
|
| 0.50 |
| 0.19 |
| No | 44 | 53(37–101) |
| 20(10–35) |
|
| Yes | 57 | 48(31–81) |
| 14(6–23) |
|
| Tumour
characteristics |
|
|
|
|
|
| No. of
metastases |
|
| 0.16 |
| 0.029 |
| Solitary | 54 | 57(40-NR) |
| 23(12–35) |
|
| Multiple | 49 | 39(26–72) |
| 11(6–17) |
|
| Grade of
differentiation |
|
| 0.41 |
| 0.41 |
| High-moderate | 86 | 53(36–79) |
| 15(8–25) |
|
| Poor | 8 | 34(2-NR) |
| 14(1–25) |
|
| Size(mm) |
|
| 0.070 |
| 0.033 |
| <40 | 49 | 65(45–101) |
| 25(13–35) |
|
| ≥40 | 54 | 34(25–53) |
| 11(7–20) |
|
The median disease-free survival was 15 months. The
factors associated with prolonged disease-free survival on
univariate analysis included having a solitary tumour, tumour size
<40 mm, no postoperative chemotherapy and absence of major
postoperative complications. Neither PPAR- α nor PPAR- γpositivity
were associated with disease-free survival. Following exclusion of
early postoperative deaths, the P-value associated with major
complications increased to 0.042 and 0.17 for overall and
disease-free survival, respectively, suggesting that early deaths
may have accounted for the apparent association.
The multivariate analysis confirmed an independent
association between PPAR- α staining and overall survival. However,
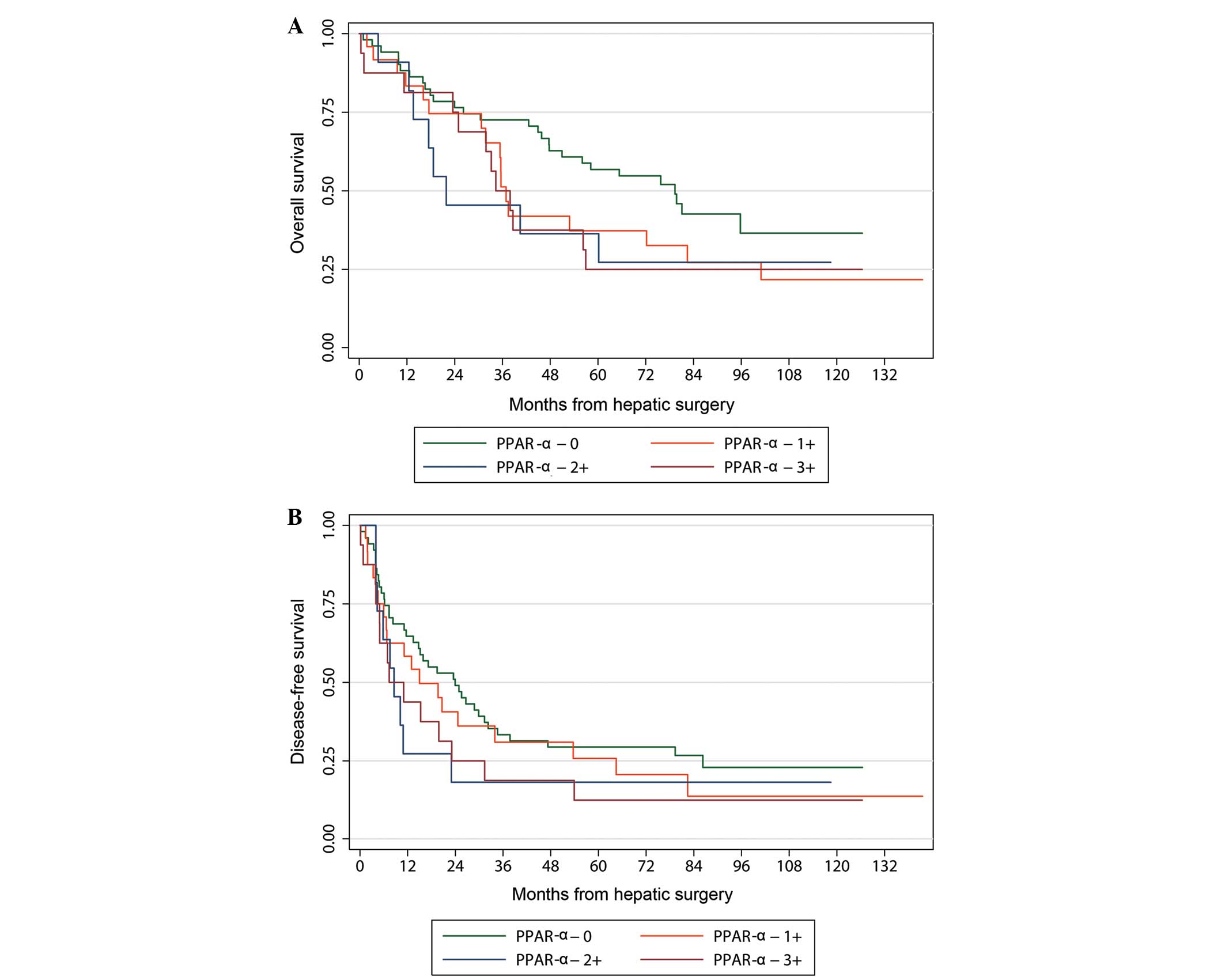
this was only of marginal statistical significance (Table IV). The Kaplan-Meier curve
comparing different levels of PPAR- α staining demonstrated that
survival was similar in each of the PPAR- α groups. However, when
PPAR- α expression was not detected, survival was significantly
better (Fig. 1). PPAR- γstaining
exhibited no significant association with survival. A multivariate
analysis of the determinants of disease-free survival confirmed the
conventional poor prognostic factors, such as multiple metastases
and large tumour size. In addition, patients who had received
postoperative chemotherapy exhibited worse disease-free survival
compared to those who did not.
 | Table IV.Multivariate models for long-term
survival overall survival and disease-free survival.a |
Table IV.
Multivariate models for long-term
survival overall survival and disease-free survival.a
| Covariates | HR (95% CI) | P-value |
|---|
| Overall
survival |
| PPAR-α + | 1.68
(1.03-2.8) | 0.039 |
| Margin ≥1 mm | 0.6 (0.4-1.0) | 0.06 |
| Disease-free
survival |
| Multiple
metastases | 1.7 (1.1-2.7) | 0.027 |
| Size ≥40 mm | 1.7 (1.04-2.7) | 0.034 |
| Postop
chemotherapy | 1.7 (1.1-2.8) | 0.022 |
Discussion
Two major interrelated issues are raised by this
study. First, the effect of chemotherapy on non-neoplastic liver
parenchyma and the role of PPARs play in modulating this effect;
and second, the role of PPAR- α and-γon tumour progression and,
therefore, long-term patient outcomes.
It is well known that preoperative chemotherapy
damages the liver parenchyma surrounding the tumour.
Microscopically, this damage variously causes steatosis,
steatohepatitis, fibrosis and sinusoidal injury (9). In addition, different types of
chemotherapy cause different patterns of injury. Specifically,
oxaliplatin causes sinusoidal obstruction, whereas irinotecan
causes steatohepatitis (8,
15, 16). However, the effect of
chemotherapy-related liver injury on perioperative morbidity and
mortality remains unclear, with certain studies reporting an
association (15, 17), unlike others (8, 16).
The effects on long-term mortality have been even less extensively
investigated. A recent retrospective study of 196 patients who
underwent resection of liver metastases reported that severe
sinusoidal dilatation, related to oxaliplatin use, was associated
with poor overall and recurrence-free survival (11). This has not been previously
described. Contrary to that study, we did not observe any
association between the histopathological characteristics of
chemotherapy-associated liver injury (CALI) and overall survival.
Furthermore, we did not identify an association between CALI and
perioperative morbidity or mortality (data not shown).
PPAR- α and-γare nuclear receptors. Following ligand
binding, heterodimerisation occurs with retinoid X receptor to form
a transcription factor with resultant downstream effects (18). PPAR- α is expressed in a wide
variety of tissue cells involved in lipid metabolism, including
hepatocytes. By contrast, PPAR- γis mainly expressed in adipose
tissue and is generally poorly expressed in the liver, although its
expression increases with lipid accumulation (19–21).
PPAR- α is associated with lipid metabolism and inflammation. In
addition, the two receptors play a role in the modulation of
chemical and ischaemic-reperfusion mediated hepatic damage
(18).
The role of these receptors in
chemotherapy-associated damage has not been established; however,
our hypothesis is that they are likely to be significant. However
we did not, in general, observe an association between different
types of liver injury and the expression of PPAR- α and-γas
detected by immunohistochemistry, with the exception of the
association between PPAR- α expression and the presence of lobular
inflammation. Of note, PPAR- α generally exerts an
anti-inflammatory effect, e.g., in the amelioration of ethanol- and
diet-induced steatohepatitis, as well as ischaemic-reperfusion
injury in rats (22–25). We therefore hypothesised that PPAR-
α expression may be a response to lobular inflammation rather than
a causative factor.
In the liver, decreased expression of PPAR- γin
hepatic stellate cells is associated with activation and
transdifferentiation; this, in turn, leads to hepatic fibrosis
(12). However, the role of PPAR-
γin hepatocytes is less clear. Indeed, we found that PPAR-
γexpression was associated with a non-significant trend (P=0.07)
for milder (or absent) hepatic fibrosis. We also noted an
unexpected association with good surgical margins, perhaps as a
result of differences in liver texture.
Furthermore, we observed that the expression of
PPAR- α was associated with worse long-term overall survival
compared to tumours that do not express this receptor. This was
unexpected, as PPAR- α activity is considered to exert a negative
effect on tumourigenesis. This inhibitory effect is likely mediated
by at least three different pathways: inhibition of endothelial
cell proliferation, anti-inflammatory action and inhibition of the
Warburg effect (13, 26, 27). However, the effect of PPAR- α on
tumour growth is likely to be more complex, as PPAR- α-deficient
hosts (PPAR- α knockout rats) may prevent tumour growth by
inhibition of angiogenesis through excessive inflammation (28). Although such an effect has never
been demonstrated in human hepatocytes, it confirms a complex
interaction between the tumour microenvironment and tumour growth
and progression. Larger studies involving molecular analysis are
required to confirm the role of these receptors in colorectal liver
metastases.
The major weakness of this study is the lack of
mechanistic explanation for these associations. Further studies
with more mechanistic assays or metabolomic studies are required to
investigate this issue.
In summary, we found that PPAR- α is associated with
the presence of lobular inflammation. Apart from this finding,
neither PPAR- α nor PPAR- γwere found to be associated with any
specific patterns of chemotherapy-related liver damage. There was
also an association between PPAR- α expression and worse overall
survival. However, there is no obvious mechanistic explanation for
these findings. This study has raised several issues regarding the
role PPAR- α plays in tumour progression. The exact underlying
mechanisms remain unclear; specifically, it has not been elucidated
whether inflammation causes tumour progression and increased PPAR-
α expression in the liver, or the increased PPAR expression results
in increased inflammatory response and tumour progression. Further
research into the diagnostic and therapeutic implications of our
findings is warranted.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramia JM, Lopez-Andujar R, Torras J, et
al: Multicentre study of liver metastases from colorectal cancer in
pathological livers. HPB (Oxford). 13:320–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez-Outschoorn UE, Lin Z, Trimmer C,
et al: Cancer cells metabolically ‘fertilize’ the tumor
microenvironment with hydrogen peroxide, driving the Warburg
effect: implications for PET imaging of human tumors. Cell Cycle.
10:2504–2520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamaya K, Hashimoto H and Maeda Y:
Metastatic carcinoma in cirrhotic liver - statistical survey of
autopsies in Japan. Acta Pathol Jpn. 25:153–159. 1975.PubMed/NCBI
|
|
6
|
King PD and Perry MC: Hepatotoxicity of
chemotherapy. Oncologist. 6:162–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rubbia-Brandt L, Audard V, Sartoretti P,
et al: Severe hepatic sinusoidal obstruction associated with
oxaliplatin-based chemotherapy in patients with metastatic
colorectal cancer. Ann Oncol. 15:460–466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kandutsch S, Klinger M, Hacker S, Wrba F,
Gruenberger B and Gruenberger T: Patterns of hepatotoxicity after
chemotherapy for colorectal cancer liver metastases. Eur J Surg
Oncol. 34:1231–1236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cleary JM, Tanabe KT, Lauwers GY and Zhu
AX: Hepatic toxicities associated with the use of preoperative
systemic therapy in patients with metastatic colorectal
adenocarcinoma to the liver. Oncologist. 14:1095–1105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryan P, Nanji S, Pollett A, et al:
Chemotherapy-induced liver injury in metastatic colorectal cancer:
semiquantitative histologic analysis of 334 resected liver
specimens shows that vascular injury but not steatohepatitis is
associated with preoperative chemotherapy. Am J Surg Pathol.
34:784–791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamandl D, Klinger M, Eipeldauer S, et al:
Sinusoidal obstruction syndrome impairs long-term outcome of
colorectal liver metastases treated with resection after
neoadjuvant chemotherapy. Ann Surg Oncol. 18:421–430. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizzo G and Fiorucci S: PPARs and other
nuclear receptors in inflammation. Curr Opin Pharmacol. 6:421–427.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peters JM, Shah YM and Gonzalez FJ: The
role of peroxisome proliferator-activated receptors in
carcinogenesis and chemoprevention. Nat Rev Cancer. 12:181–195.
2012.PubMed/NCBI
|
|
14
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: a new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vauthey JN, Pawlik TM, Ribero D, et al:
Chemotherapy regimen predicts steatohepatitis and an increase in
90-day mortality after surgery for hepatic colorectal metastases. J
Clin Oncol. 24:2065–2072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehta NN, Ravikumar R, Coldham CA, et al:
Effect of preoperative chemotherapy on liver resection for
colorectal liver metastases. Eur J Surg Oncol. 34:782–786. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakano H, Oussoultzoglou E, Rosso E, et
al: Sinusoidal injury increases morbidity after major hepatectomy
in patients with colorectal liver metastases receiving preoperative
chemotherapy. Ann Surg. 247:118–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Paola R and Cuzzocrea S: Peroxisome
proliferator-activated receptors ligands and ischemia-reperfusion
injury. Naunyn Schmiedebergs Arch Pharmacol. 375:157–175. 2007.(In
Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braissant O, Foufelle F, Scotto C, Dauca M
and Wahli W: Differential expression of peroxisome
proliferator-activated receptors (PPARs): tissue distribution of
PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology.
137:354–366. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vidal-Puig AJ, Considine RV, Jimenez-Linan
M, et al: Peroxisome proliferator-activated receptor gene
expression in human tissues. Effects of obesity, weight loss, and
regulation by insulin and glucocorticoids. J Clin Invest.
99:2416–2422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naishadham D, Lansdorp-Vogelaar I, Siegel
R, Cokkinides V and Jemal A: State disparities in colorectal cancer
mortality patterns in the United States. Cancer Epidemiol
Biomarkers Prev. 20:1296–1302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong L, Ren W, Li W, Zhao S, Mi H, Wang R,
Zhang Y, Wu W, Nan Y and Yu J: Activation of peroxisome
proliferator activated receptor alpha ameliorates ethanol induced
steatohepatitis in mice. Lipids Health Dis. 10:2462011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akiyama TE, Nicol CJ, Fievet C, et al:
Peroxisome proliferator-activated receptor-alpha regulates lipid
homeostasis, but is not associated with obesity: studies with
congenic mouse lines. J Biol Chem. 276:39088–39093. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stienstra R, Mandard S, Patsouris D, Maass
C, Kersten S and Muller M: Peroxisome proliferator-activated
receptor alpha protects against obesity-induced hepatic
inflammation. Endocrinology. 148:2753–2763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okaya T and Lentsch AB: Peroxisome
proliferator-activated receptor-alpha regulates postischemic liver
injury. Am J Physiol Gastrointest Liver Physiol. 286:G606–G612.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pozzi A, Ibanez MR, Gatica AE, et al:
Peroxisomal proliferator-activated receptor-alpha-dependent
inhibition of endothelial cell proliferation and tumorigenesis. J
Biol Chem. 282:17685–17695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grau R, Diaz-Munoz MD, Cacheiro-Llaguno C,
Fresno M and Iniguez MA: Role of peroxisome proliferator-activated
receptor alpha in the control of cyclooxygenase 2 and vascular
endothelial growth factor: involvement in tumor growth. PPAR Res.
2008:3524372008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaipainen A, Kieran MW, Huang S, et al:
PPA Ralpha deficiency in inflammatory cells suppresses tumor
growth. PLoS One. 2:e2602007. View Article : Google Scholar : PubMed/NCBI
|