Introduction
In 2012, prostate cancer was the most common cancer
among European men, comprising 12% of all new cancer cases and 5%
of all cancer deaths. This incidence was the highest in high-income
countries (1). Treatment of
localized disease varies, as there is no evidence that one
treatment is more effective compared with another (2). According to the current guidelines of
the American Urological Association (3) and the European Urological Association
(4), the treatment of low-risk,
localized prostate cancer may include active surveillance,
prostatectomy, or either interstitial or external-beam radiation
therapy. Permanent seed brachytherapy (BT) is mostly used for
patients with low prostate-specific antigen (PSA) concentrations
and low Gleason scores. For these patients, studies have shown no
significant difference in clinical effectiveness among treatments
(5). According to previous studies,
the 5-year disease-free survival is ~80–90% (5,6).
The aim of this study was to evaluate risk factors
for biochemical failure (BF) after permanent seed 125I
BT for prostate cancer among patients treated in Oulu University
Hospital. The study consisted of a retrospective chart review
conducted to evaluate clinical characteristics at diagnosis,
treatment-related details and follow-up data.
Materials and methods
Chart review
Between March, 2001 and December, 2014, 607 patients
received treatment for early-stage (T1/2N0M0), histologically
confirmed prostate cancer using 125I seed BT. Study data
were collected retrospectively from their medical records at Oulu
University Hospital (Oulou, Finland) and at other hospitals for
those who were followed up elsewhere, including local hospitals in
Kajaani, Kemi, Kokkola, Oulainen and Rovaniemi. The following data
were recorded: Patient age, former diagnosis, Gleason score, TNM
stage, PSA concentration prior to treatment, PSA nadir
concentration, possible increase in PSA concentration during
follow-up, all additional treatments for prostate cancer before or
after BT, and possible radiological progression. Treatment outcome
was defined in terms of time to PSA nadir concentration, BF,
defined as PSA concentration progression >2 µg/l from the PSA
nadir concentration according to the Phoenix criteria (7), treatment of BF, and overall and
prostate-cancer specific survival. PSA nadir concentration was
defined as the lowest PSA concentration observed after BT. After
treatment, the patients were followed up at 3, 6 and 12 months,
then biannually for 4 years, and annually thereafter. More frequent
follow-ups were possible based on the decisions of the
urologists/doctors responsible for the follow-ups. There were no
strict treatment protocols following BF.
Ethics
According to Finnish legislation and directions from
Finnish Ethics Committees, this chart review was exempted from
formal approval by the Institutional Review Board. However, the
study was conducted according to the principles of the Helsinki
Declaration.
BT technique
The dose plan was based on ultrasonic (US) images
taken at 0.5-cm intervals and used stranded seeds (125I
IsoCord®; Bebig GmbH, Berlin, Germany). The implantation
technique was intraoperative and used sagittal images. VariSeed 8.0
(Varian; Palo Alto, CA, USA) was used for dose planning and for
calculating the dose-volume histogram. The actual dose plan was
performed using 125I IsoCord S06 seeds (Bebig GmbH). The
prescribed dose was 145 Gy using seed activity of 17.46 MBq (0.472
mCi).
Statistical analysis
Unless otherwise stated, the summary statistics
included the mean, range and standard deviation (SD) or, if biased,
the median with the 25–75th percentile. Survival analyses and
analyses of time to BF were conducted using the Kaplan-Meier
method, and the statistical significance of the differences between
groups was analysed using the log-rank test. The data were analyzed
using SPSS statistical software, version 22.0 (IBM SPSS; Armonk,
NY, USA). Two-tailed P-values are reported, and P-values <0.05
were considered to indicate statistically significant
differences.
Results
Patient characteristics
Follow-up details were available for 605 patients,
and survival data were available for 606 patients. The mean age at
BT was 64 years (range, 44–78; SD, 6.1). The median PSA
concentration prior to BT was 7.7 (25–75th percentiles, 5.5–10.0).
The median prostate volume was 29.0 cm3 (25–75th
percentiles, 23.3–35.9). The median number of seeds used was 55
(25–75th percentiles, 47–63). The median number of needles used was
21 (25–75th percentiles, 19–23). Of the 607 patients, 537 (88.5%),
69 (11.4%) and 1 (0.2%) had Gleason scores of 6, 7 and 8,
respectively, on prostate biopsy. The clinical T-stage according to
the TNM classification (8) was cT1
and cT2 in 400 (65.9%) and 207 (34.1%) patients, respectively.
Prior to BT, 91 patients (15%) received neoadjuvant hormonal
therapy with the intention of decreasing the prostate volume to
<50 cm3. The median duration of this neoadjuvant
hormonal therapy was 5 months (25–75th percentiles, 3–9).
The median follow-up time was 81 months (range,
2–161; SD, 39). After a mean of 46 months (range, 8–136; SD, 32),
117 (19.3%) patients developed BF and 91 (15.0%) were treated for
BF. For those patients, the mean time to the first treatment after
BT was 57 months (range, 12–149; SD, 34). The first treatment
selected was antiandrogens (n=47, 51.6%), chemical castration
(n=29, 31.9%), external-beam radiation therapy (n=11, 12.1%) or
radical prostatectomy (n=2, 2.2%). In addition, as an initial
treatment for BF, 2 patients (2.2%) were treated with a 5-α
reductase inhibitor. During follow-up, 32 (5.3%) patients received
external-beam radiation therapy. Eventually, 21 (3.5%) developed
metastatic disease, the mean time to which was 66 months (range,
18–149; SD, 43). Of the 606 patients. 12 succumbed to prostate
cancer and 47 to other causes. The overall survival and
prostate-cancer specific survival rates were 90.3 and 98.0%,
respectively. The mean overall survival time and prostate-cancer
specific survival time were 147 and 158 months, respectively.
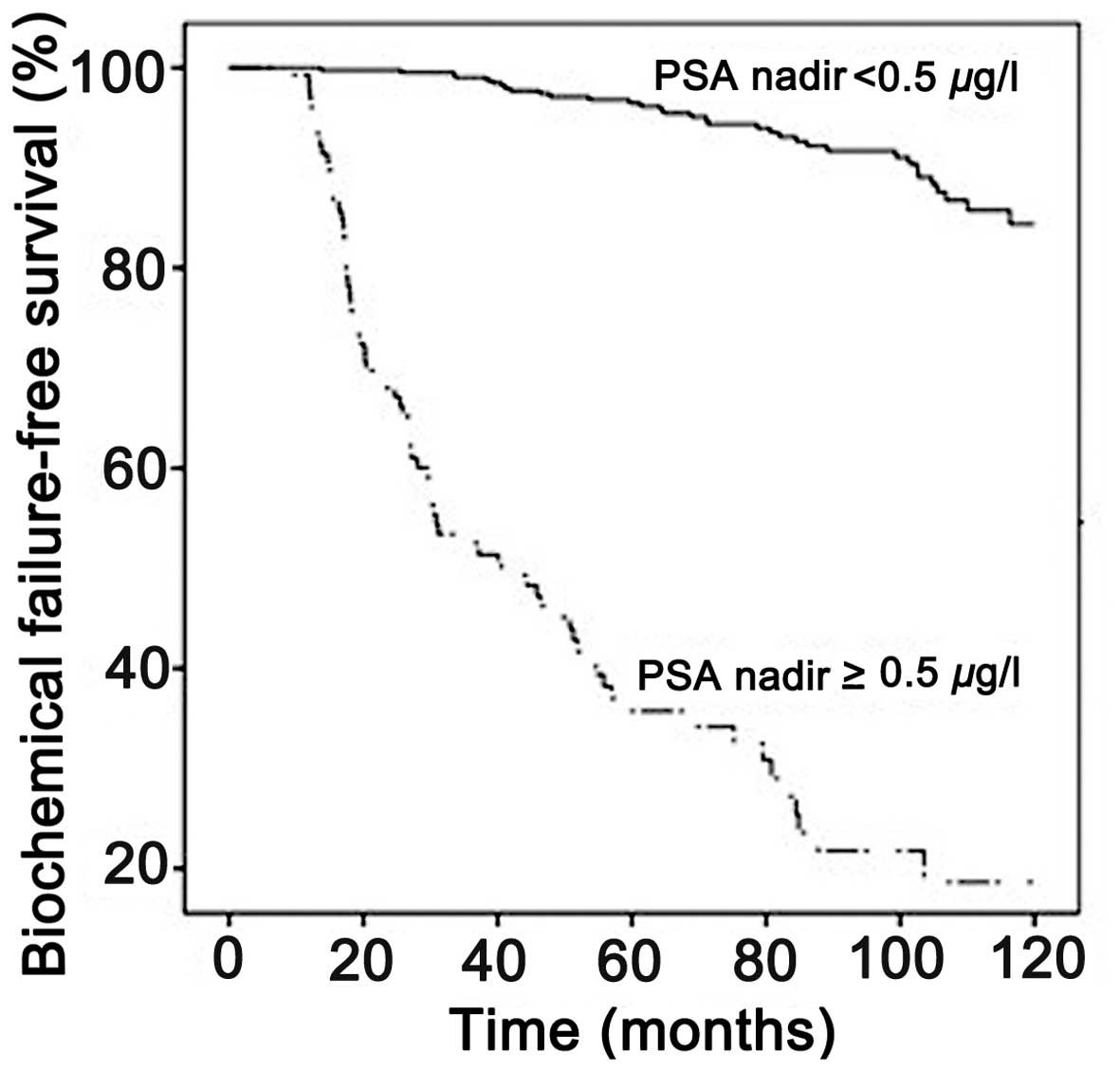
BF was significantly more common among patients with
PSA nadirs ≥0.5 µg/l compared with those with PSA nadirs <0.5
µg/l (Fig. 1). The mean time to BF
was 55 [95% confidence interval (CI): 47–63] and 114 (95% CI:
112–116) months for patients with PSA nadirs ≥0.5 µg/l and <0.5
µg/l, respectively (P<0.001).
We further evaluated the potential association of
underlying diseases with BF. BF tended to develop more rapidly
among patients with hypertension or diabetes at diagnosis. For
patients without and with hypertension, the mean time to BF was 104
(95% CI: 100–107) and 98 (95% CI: 93–103) months, respectively
(P=0.035). For patients without and with diabetes, the mean time to
BF was 103 (95% CI: 100–106) and 89 (95% CI: 77–102) months,
respectively (P=0.006). Other comorbidities, including coronary
artery disease, previous myocardial infarctions, other cancers, or
obstructive pulmonary disease, did not affect the time to BF. Due
to the small number of deaths, further survival analyses for
overall survival and prostate-cancer specific survival were not
performed.
Discussion
BT is an option for treating low-risk prostate
cancer, and it has achieved promising results in terms of disease
recurrence (9–18); however, certain patients still
develop disease recurrence. In search of the risk factors for BF
after BT, PSA nadir was identified as a strong predictor of BF. For
patients with PSA nadirs ≥0.5 µg/l, the mean time to BF was <6
years, whereas it was >9 years for patients with PSA nadirs
<0.5 µg/l. Recently, McLaren et al (19) published their institutional results,
reporting that PSA concentrations of >0.4 µg/l over the PSA
nadir predict disease recurrence. In addition, their analyses
demonstrated that, if their PSA nadir was >0.8 µg/l (19), approximately half of the patients
experienced disease relapse within 4 years, a finding that is in
line with the results of the present study.
Comorbidities, including hypertension and diabetes,
have previously been associated with decreased overall survival
following BT (20,21). Despite the promising results of lower
mortality among prostate cancer patients exposed to metformin
(22), an analysis of 270 men with
diabetes, with and without metformin use, revealed no association
between metformin use and progression-free, disease-free, or
overall survival following treatment with BT (23). Similar results were found in another
cohort of 199 diabetes patients, in which diabetes did not affect
cancer-specific survival or biochemical progression following BT
(24). However, increased blood
glucose levels have been associated with increased risk of disease
recurrence following radical prostatectomy or radiation therapy as
a radical treatment for prostate cancer (25). The finding of the present study
regarding a tendency toward more rapid BF among patients with
underlying diabetes or hypertension is partly supported by
previously published results in cases with diabetes (25). To the best of our knowledge,
hypertension has not been associated with progression-free survival
in prostate cancer. However, the findings of the present study
require evaluation in other cohorts. In addition, the presence of a
clinically meaningful association with BF remains obscure, as the
mean time to BF was long (89–104 months).
Our study was limited by its retrospective,
single-center nature. In addition, the study did not evaluate
diabetes medications or the use of statins, although they may have
a positive prognostic effect (26).
Furthermore, the small number of deaths did not enable reliable
survival analyses. Moreover, BF was defined according to the
Phoenix criteria, which have been shown to be more sensitive and
specific in defining BF in patients treated with BT. However, it
has been demonstrated that the ASTRO and Phoenix criteria (7) have a ~8% difference in the rate of
biochemical control, with the latter achieving lower values
(27). Finally, comparing among
various studies is difficult due to the differing definitions
used.
However, the results of the present study indicate
that low PSA nadir reliably predicts BF after BT. Patients with
underlying hypertension or diabetes tended to exhibit shorter times
to BF, emphasizing the need for more attentive follow-up of such
patients after BT. However, these findings require further
investigation.
Acknowledgements
We are grateful to Leena Heikkilä for assisting with
data collection, and to Pasi Ohtonen, MSc, for assisting with
statistical analyses.
References
|
1
|
Prostate (PRC) cancer factsheet.
http://www.encr.eu/images/docs/factsheets/ENCR_Factsheet_Prostate_2014.pdf04
17–2016
|
|
2
|
Wilt TJ, MacDonald R, Rutks I, Shamliyan
TA, Taylor BC and Kane RL: Systematic review: Comparative
effectiveness and harms of treatments for clinically localized
prostate cancer. Ann Intern Med. 148:435–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guideline for the management of clinically
localized prostate cancer (2007). https://www.auanet.org/education/guidelines/prostate-cancer.cfmUpdated
2007. 04 17–2016
|
|
4
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
treatment of advanced, relapsing and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Norderhaug I, Dahl O, Høisaeter PA,
Heikkilä R, Klepp O, Olsen DR, Kristiansen IS, Waehre H and
Johansen TE Bjerklund: Brachytherapy for prostate cancer: A
systematic review of clinical and cost effectiveness. Eur Urol.
44:40–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zelefsky MJ, Hollister T, Raben A,
Matthews S and Wallner KE: Five-year biochemical outcome and
toxicity with transperineal CT-planned permanent I-125 prostate
implantation for patients with localized prostate cancer. Int J
Radiat Oncol Biol Phys. 47:1261–1266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roach M III, Hanks G, Thames H Jr,
Schellhammer P, Shipley WU, Sokol GH and Sandler H: Defining
biochemical failure following radiotherapy with or without hormonal
therapy in men with clinically localized prostate cancer:
Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int
J Radiat Oncol Biol Phys. 65:965–974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin L and Wittekind C: Union for
international cancer control: TNM classification of malignant
tumours. 6th. Wiley; New York: 2002
|
|
9
|
Grimm PD, Blasko JC, Sylvester JE, Meier
RM and Cavanagh W: 10-year biochemical (prostate-specific antigen)
control of prostate cancer with (125)I brachytherapy. Int J Radiat
Oncol Biol Phys. 51:31–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zelefsky MJ, Kuban DA, Levy LB, Potters L,
Beyer DC, Blasko JC, Moran BJ, Ciezki JP, Zietman AL, Pisansky TM,
et al: Multi-institutional analysis of long-term outcome for stages
T1-T2 prostate cancer treated with permanent seed implantation. Int
J Radiat Oncol Biol Phys. 67:327–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Potters L, Morgenstern C, Calugaru E,
Fearn P, Jassal A, Presser J and Mullen E: 12-year outcomes
following permanent prostate brachytherapy in patients with
clinically localized prostate cancer. J Urol. 179(5 Suppl):
S20–S24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aaltomaa SH, Kataja VV, Lahtinen T,
Palmgren JE and Forsell T: Eight years experience of local prostate
cancer treatment with permanent I125 seed brachytherapy-morbidity
and outcome results. Radiother Oncol. 91:213–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hinnen KA, Battermann JJ, van Roermund JG,
Moerland MA, Jürgenliemk-Schulz IM, Frank SJ and van Vulpen M:
Long-term biochemical and survival outcome of 921 patients treated
with I-125 permanent prostate brachytherapy. Int J Radiat Oncol
Biol Phys. 76:1433–1438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Henry AM, Al-Qaisieh B, Gould K, Bownes P,
Smith J, Carey B, Bottomley D and Ash D: Outcomes following
iodine-125 monotherapy for localized prostate cancer: The results
of leeds 10-year single-center brachytherapy experience. Int J
Radiat Oncol Biol Phys. 76:50–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crook J, Borg J, Evans A, Toi A,
Saibishkumar EP, Fung S and Ma C: 10-year experience with I-125
prostate brachytherapy at the Princess Margaret Hospital: Results
for 1,100 patients. Int J Radiat Oncol Biol Phys. 80:1323–1329.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marshall RA, Buckstein M, Stone NN and
Stock R: Treatment outcomes and morbidity following definitive
brachytherapy with or without external beam radiation for the
treatment of localized prostate cancer: 20-year experience at mount
sinai medical center. Urol Oncol. 32(38): e1-72014.
|
|
17
|
Dickinson PD, Malik J, Mandall P, Swindell
R, Bottomley D, Hoskin P, Logue JP and Wylie JP: Five-year outcomes
after iodine-125 seed brachytherapy for low-risk prostate cancer at
three cancer centres in the UK. BJU Int. 113:748–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi N, Izumi K, Sano F, Miyoshi Y,
Uemura H, Kasuya T, Mukai A, Hata M and Inoue T: Ten-year outcomes
of I125 low-dose-rate brachytherapy for clinically
localized prostate cancer: A single-institution experience in
japan. World J Urol. 33:1519–1526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McLaren DB, Kerr G, Law AB, Brush JP,
Keanie J, Malik J, Keough W, Ronaldson T, Lee J and Kehoe T: The
importance of prostate-specific antigen (PSA) nadir and early
identification of PSA relapse after 10 years of prostate iodine 125
seed brachytherapy in edinburgh. Clin Oncol (R Coll Radiol).
27:519–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taira AV, Merrick GS, Butler WM, Galbreath
RW, Lief J, Adamovich E and Wallner KE: Long-term outcome for
clinically localized prostate cancer treated with permanent
interstitial brachytherapy. Int J Radiat Oncol Biol Phys.
79:1336–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nanda A, Chen MH, Moran BJ, Braccioforte
MH and D'Amico AV: Cardiovascular comorbidity and mortality in men
with prostate cancer treated with brachytherapy-based radiation
with or without hormonal therapy. Int J Radiat Oncol Biol Phys.
85:e209–e215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Margel D, Urbach DR, Lipscombe LL, Bell
CM, Kulkarni G, Austin PC and Fleshner N: Metformin use and
all-cause and prostate cancer-specific mortality among men with
diabetes. J Clin Oncol. 31:3069–3075. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taira AV, Merrick GS, Galbreath RW, Morris
M, Butler WM and Adamovich E: Metformin is not associated with
improved biochemical free survival or cause-specific survival in
men with prostate cancer treated with permanent interstitial
brachytherapy. J Contemp Brachytherapy. 6:254–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shetti MB, Merrick GS, Butler WM,
Galbreath R, Torlone A, Lief JH, Adamovich E and Wallner KE: The
impact of diabetes mellitus on survival in men with clinically
localized prostate cancer treated with permanent interstitial
brachytherapy. Am J Clin Oncol. 35:572–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wright JL, Plymate SR, Porter MP, Gore JL,
Lin DW, Hu E and Zeliadt SB: Hyperglycemia and prostate cancer
recurrence in men treated for localized prostate cancer. Prostate
Cancer Prostatic Dis. 16:204–208. 2013.PubMed/NCBI
|
|
26
|
Meng Y, Liao YB, Xu P, Wei WR and Wang J:
Statin use and mortality of patients with prostate cancer: A
meta-analysis. Onco Targets Ther. 9:1689–1696. 2016.PubMed/NCBI
|
|
27
|
Kuban DA, Levy LB, Potters L, Beyer DC,
Blasko JC, Moran BJ, Ciezki JP, Zietman AL, Zelefsky MJ, Pisansky
TM, et al: Comparison of biochemical failure definitions for
permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys.
65:1487–1493. 2006. View Article : Google Scholar : PubMed/NCBI
|