Introduction
The proposed model for the development of cervical
cancer involves the involvement of phenotypes, including
glycolytic, hypoxic and acidosis. Previous experimental and
clinical studies have shown that cervical cancer tumors are
characterized by a highly hypoxic metabolism (1–5) and a
high rate of glycolysis (2,6–8).
Additionally, these high hypoxic, glycolytic and acidosis
metabolisms are implicated in resistance to treatments, including
radiotherapy and chemotherapy. These phenotypes are associated with
genetic instability, which may be reflected by the increased
expression of certain proteins, including glucose transporter 1
(GLUT1), carbonic anhydrase 9 (CAIX) and hexokinase 1 (HKII)
(3,8–10). These
proteins are considered as potential prognostic markers of disease
progression, metastasis and survival. GLUT1, also termed SLC2A, is
part of a family composed of 14 GLUT proteins (glucose
transporters). Their expression is dually controlled via hypoxia
inducible factor (HIF)-1 in response to reduced oxidative
phosphorylation (11) and by the
Akt/PI3K signaling pathway, which is activated by insulin and
growth factors induced by glucose metabolism (12,13).
CAIX is considered an endogenous marker of hypoxia, a condition
that increases its expression levels, thus leading to acidosis
(decreased extracellular pH). CAIX is regulated by HIF-1 (14,15) and
may also depend on factors including low levels of glucose, which
prevents its expression (14,16,17),
low levels of bicarbonate and cellular density (14,18).
HKII is involved in the conversion of glucose to
glucose-6-phosphate for glycolysis (19,20). It
serves a role in apoptosis and inhibition of cell death by binding
and stabilizing the mitochondrial membrane. Therefore, it is
hypothesized that its increased activity assists with the
maintenance of the malignant cell phenotype (9,10,21,22).
The presence of hypoxia in solid tumors is a concern
in clinical practice as a result of its negative impact on the
prognosis and treatment response in cancer. Both experimental and
clinical studies suggest a direct association between the decrease
in hemoglobin (Hgb) levels and decreased oxygenation in the tumor
(23). In squamous cell carcinoma,
as with cervical cancer, it is noted that the maximum level of
oxygenation occurs when the Hgb range is between 12 and 14 g/dl.
Hgb levels <11 g/dl are directly associated with tumor hypoxia
(24–26). Under this condition, in cervical
cancer, low levels of hemoglobin (anemia) have been associated with
poor local control of the disease (16,25) and
low survival rates (4,27). However, it is controversial whether
Hb is a prognostic factor in cancer; a significant correlation with
tumor hypoxia (pO2 <5 mg) prior to radiotherapy or
radiochemotherapy in cervical cancer remains to be established
(25).
Certain previous studies reportd that GLUT1, CAIX,
HKII and Hgb level can be considered as biomarkers, suggesting they
can be used as prognostic markers for improved therapeutic
management of cervical cancer (1,6). The
purpose of the present study was to determine whether baseline
expression of GLUT1, CAIX and HKII, as well as pre- and
post-treatment Hgb levels are associated with treatment response
and survival in cancer patients with locally advanced cervical
carcinoma.
Materials and methods
Study design, selection and patient
characteristics
The present study was a retrospective study in a
prospective data bank. Between January 2001 and December 2007, 66
patients were selected with locally advanced cervical carcinoma
staged IIB (n=24) and IIIB (n=42) according to the International
Federation of Gynecology and Obstetrics. These patients were
treated at the National Cancer Institute (Bogota, Colombia). The
histological types were squamous cell carcinoma in all cases. The
median age was 47 years, ranging between 26 and 72. A performance
status of 0–1 was observed in 65/66 patients. The protocols
followed in the present study were consistent with medical
standards of practice and administrative techniques for health
research from the Ministry of Health Colombia. Each patient was
first informed of the objectives of the study and voluntarily
agreed to take part by signing the informed consent, previously
approved by the Ethics Committee of Neuwirth Cancer Institute
(Saint Priest En Jarez, France).
The clinicopathological characteristics of the
patients, as conditions and parameters considered for the making
and reading of the immunohistochemistry to GLUT1, CAIX and HKII,
were previously described (1). With
regards to the expression of GLUT1, CAIX and HKII, a greater
increase was observed for the expression of GLUT-1 (74%), followed
by CAIX (41%) and HKII (18%).
Treatment
All 66 patients included in the present study
underwent radiotherapy. Treatment consisted of pelvic external beam
radiotherapy (EBRT) using 6–18 MV photons with a standard
four-field technique, delivering a total dose of 45 Gy in 25
fractions (1.8 Gy/fraction on 5 consecutive days/week with an
overall EBRT treatment time of 5 weeks). Following the initial
EBRT, an intracavitary brachytherapy-boost of 35 Gy at point A was
delivered, according the International Commission on Radiation
Units and Measurements (ICRU). A total of 44 patients received
weekly concomitant chemotherapy (cisplatin, 40 mg/m2)
and 22 patients treated with radiotherapy alone.
Assessment of response
Follow-up was scheduled 6 weeks following the
completion of EBRT, and then every 3 months during the subsequent 5
years. Complete response was defined as an absence of residual
disease at clinical examination and radiological imaging 3 months
after the completion of treatment. The responder group was defined
as the group of patients who presented complete response and the
non-responder group was the patients that presented partial
response, stable disease or tumor progression.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(IBM SPSS, Chicago, IL, USA) and Stata 11.1 software (National
Cancer Institute, Bogota, Colombia). The present study calculated
measures of central tendency and dispersion for continuous
variables, and proportions for categorical variables. Correlations
between the levels of Hgb, the expression levels of GLUT1, CAIX and
HKII, and the outcome during follow-up were analyzed using
Kaplan-Meier survival tests and the differences were calculated
using the log-rank test. To determine which variables were
associated with survival, a Cox regression model was constructed,
calculating crude and adjusted hazard ratio. Variables with
significant results in the test log-rank test and those with
biological plausibility were used for adjustment. Analyses were
two-tailed. P≤0.05 were considered to indicate a statistically
significant difference.
Results
A total of 53 patients (80.3%) exhibited a complete
response. Non-responders, based on concomitant chemotherapy or not,
were as follows: 6/22 (27.2%) and 7/44 (15.9%) patients revealed no
response with exclusive radiotherapy and radiochemotherapy,
respectively.
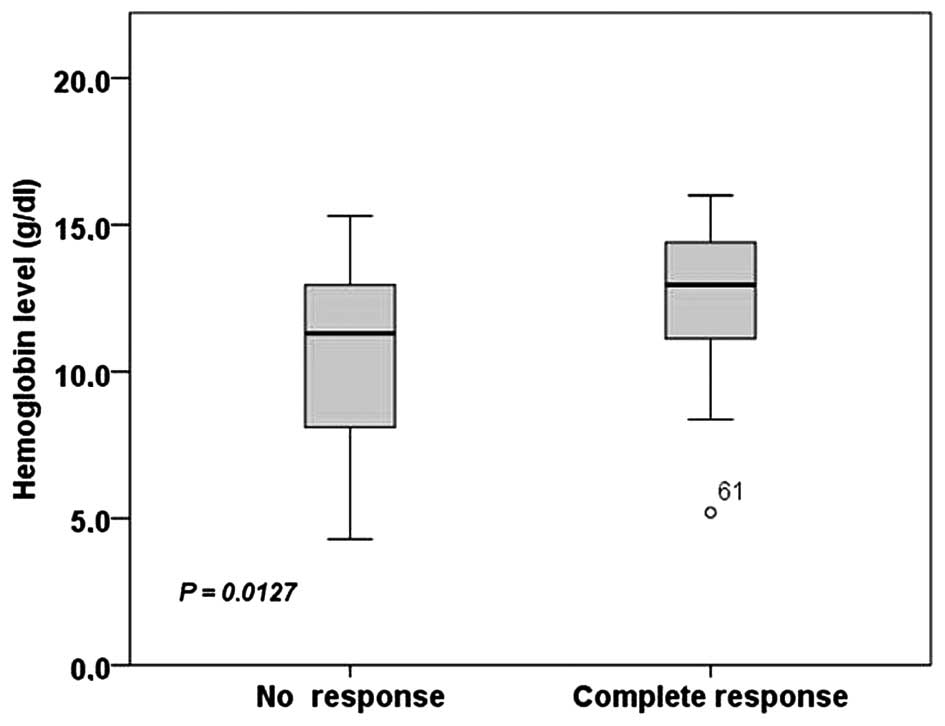
When comparing the Hgb levels, a significantly
higher average Hgb level was observed in the complete response
group compared with the no response group (Fig. 1). The average levels of Hgb were:
12.7 g/dl (range, 5.2–16.0) for the complete response group and
10.6 g/dl (range, 4.3–15.3) for the no response group.
Pre-treatment Hgb ≤11 g/dl was associated with non-response to
treatment in univariate logistic regression analysis [odds ratio
(OR)=3.99; 95% confidence intervals (CI)=1.13–14.14; P=0.032]. In
multivariate analyses, the risk was close to significance (OR=4.31;
95% CI=0.89–20.93; P=0.05). No significant difference between
post-treatment hemoglobin levels and response to treatment (data
not shown).
There was no significant difference was observed for
the expression levels of GLUT, HKII and CAIX. The following
characteristics appeared to have a tendency to no response: Stage
IIIB (OR=2.19), keratinizing tumors (OR=1.42), poorly
differentiated tumors-G3 (OR=1.33), tumors >4 cm (OR=2.13),
bilateral involvement of parametrium (OR=1.48), receive exclusive
radiotherapy (OR=1.98) and high dose rate brachytherapy
(OR=1.69).
Cox-model analysis on factors influencing
disease-free survival (DFS) and overall survival (OS) are presented
in Table I. Only two factors were
associated with a decrease of DFS: Low levels of hemoglobin ≤11
g/dl (OR=4.33) and no response to treatment (OR=34.7). No response
to treatment was associated with an unfavorable outcome on OS
(OR=40.6). Other factors appeared to have a tendency towards OS:
Stage IIIB, keratinizing tumors, tumors >4 cm, bilateral
involvement of parametrium, management with exclusive radiotherapy,
moderate G2 and poor G3 differentiation.
 | Table I.Cox analysis of disease-free survival
and global survival. |
Table I.
Cox analysis of disease-free survival
and global survival.
|
| Multivariate
analysisa |
|---|
|
|
|
|---|
|
| Disease-free
survival | Global survival |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HRa | 95% CI | P-value |
|---|
| Treatment
response |
|
| Complete
response | 1.00 |
|
| 1.00 |
|
|
|
| No
response | 34.70 | 7.95–151.45 | 0.00 | 40.6 | 8.10–203.5 | 0.00 |
| FIGO |
|
|
IIB | 1.00 |
|
| 1.00 |
|
|
|
IIIB | 0.31 | 0.75–1.33 | 0.12 | 0.22 | 0.04–1.13 | 0.07 |
| Differentiation
grade |
|
| G1 | 1.00 |
|
| 1.00 |
|
|
| G2 | 5.07 | 0.38–68.28 | 0.22 | 2.57 | 0.15–44.9 | 0.52 |
| G3 | 1.72 | 0.70–42.11 | 0.74 | 1.49 | 0.051–43.23 | 0.82 |
| Tumor
keratinization |
|
|
Presence | 1.00 |
|
| 1.00 |
|
|
|
Absence | 2.48 | 0.42–14.63 | 0.32 | 2.37 | 0.29–19.44 | 0.42 |
| Parametrial
commitment |
|
|
Unilateral | 1.00 |
|
| 1.00 |
|
|
|
Bilateal | 2.49 | 0.63–9.78 | 0.19 | 2.04 | 0.47–8.84 | 0.33 |
| Tumor size >4
cm |
|
| No | 1.00 |
|
| 1.00 |
|
|
|
Yes | 1.22 | 0.12–12.06 | 0.86 | 1.01 | 0.097–10.59 | 0.99 |
| Hgb ≤11g/dl |
|
| No | 1.00 |
|
| 1.00 |
|
|
|
Yes | 4.33 | 1.22–15.44 | 0.02 | 4.33 | 0.88–21.33 | 0.05 |
| Treatment type |
|
|
Concurrent
radiochemotherapy | 1.00 |
|
| 1.00 |
|
|
|
Exclusive radiotherapy | 3.02 | 0.63–14.45 | 0.17 | 4.58 | 0.67–31.50 | 0.12 |
| Brachytheraphy |
|
|
Low-dose rate | 1.00 |
|
| 1.00 |
|
|
|
High-dose rate | 0.74 | 0.17–3.25 | 0.69 | 1.07 | 0.19–6.09 | 0.94 |
| Protein
expression |
|
|
Negative | 1.00 |
|
| 1.00 |
|
|
|
GLUT1 | 1.50 | 0.37–2.48 | 0.12 | 1.12 | 0.411–3.04 | 0.16 |
| GLUT1
and CAIX | 0.89 | 0.21–3.65 | 0.87 | 0.89 | 0.18–4.28 | 0.88 |
| GLUT1
and HKII | 1.60 | 0.19–4.24 | 0.14 | 0.90 | 0.25–5.60 | 0.90 |
| GLUT1,
CAIX and HKII | 0.15 | 0.01–2.01 | 0.15 | 0.013 | 0.00–0.59 | 0.03 |
A significantly positive impact on the OS (OR=0.013)
and DFS (OR=0.15) was observed with the expression levels of GLUT1,
CAIX and HKII when all three markers were expressed (Table I). No significant influence on the OS
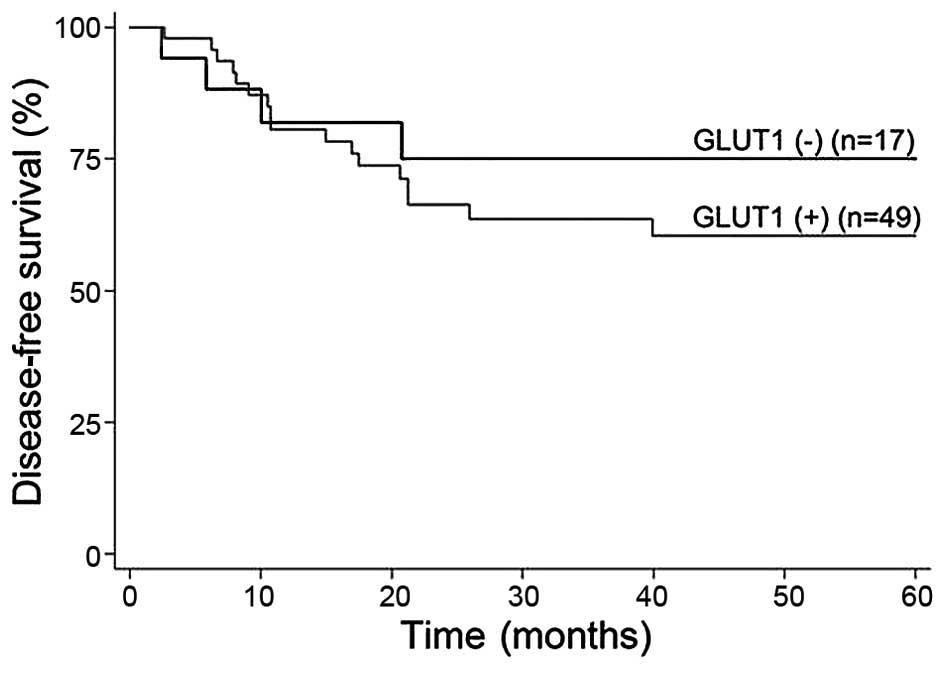
and DFS was observed from individual marker expression. The 5-year
DFS and OS rates were 60 and 62.5%, respectively, among patients
with GLUT1 expression. By contrast, the DFS and OS were 75 and 60%,
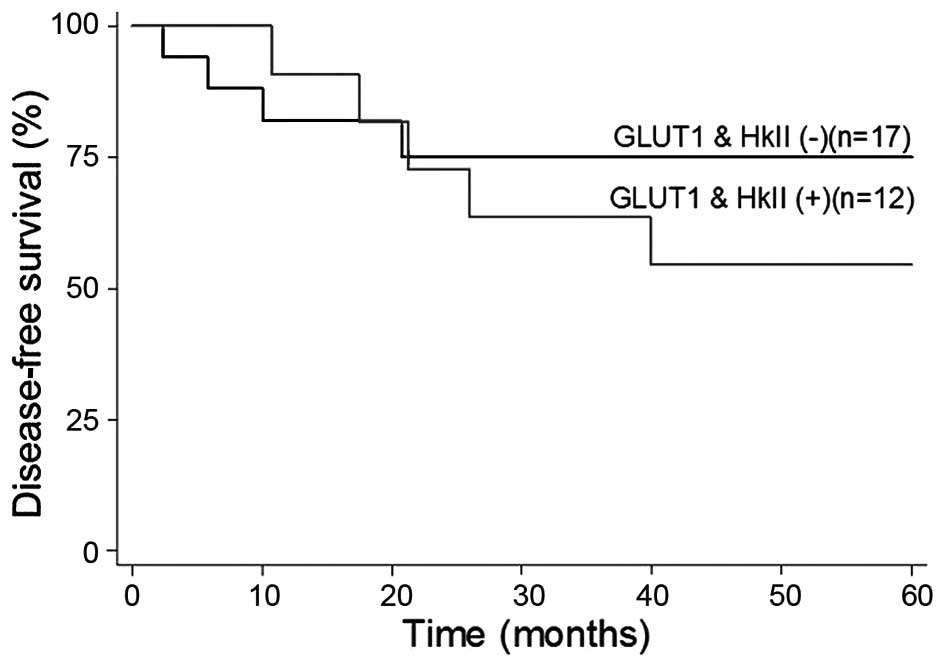
respectively, among those with no GLUT1 expression (Fig. 2). The DFS and OS rates were both 55%
among patients with GLUT1 and HKII co-expression, whereas the DFS
and OS were 75 and 62.5%, respectively, among those with no GLUT1
and HKII co-expression (Fig. 3). The
DFS and OS were 75 and 62.5%, respectively, among those without
co-expression of GLUT1 and HKII.
Discussion
The present study evaluated whether baseline
expression levels of GLUT1, CAIX and HKII, and Hgb levels, were
associated with treatment response and survival in patients with
locally advanced cervical carcinoma.
A number of patients do not respond adequately in
the management of invasive cervical cancer, and this number
increases when treated with exclusive radiotherapy compared with
radiochemotherapy, a situation observed in the present study. A
potential survival benefit of 12% is attributable to the use of
chemoradiotherapy (28). This
confirms the recommendation made by the American Society of
Clinical Oncology, which advises to privilege concomitant
radiochemotherapy for patients with locally advanced cervical
cancer.
No response to treatment, as in the 13 reported
cases, may be explained by two reasons: The first is based on
squamous cell carcinoma, including cervical cancer, and are
characterized by having a marked ability to repopulate with rapidly
dividing cells replacing those who die by radiation or chemical
agents (29). The other possible
explanation is related to the involvement of different pre-existing
factors that may be involved in the response to treatment,
including low Hgb levels (anemia), low immune function, tumor
status (e.g. the degree of differentiation), the tumor
micro-environment that meets tumor hypoxia, increased glycolysis
and extracellular acidosis (10,30–33).
The presence of hypoxia in solid tumors is a concern
at the clinical level due to its negative impact on the prognosis
and treatment response. Previous experimental and clinical studies
suggest that there is a direct association between the decrease in
Hgb levels and decreased oxygenation in a tumor (23). In squamous cell carcinoma, like that
of the cervix, the prognostic impact of anemia is well-established
(23). The present study revealed
that Hgb levels <11 g/dl pretreatment, were observed in the
group of patients who did not present complete response to
exclusive radiotherapy. The results of the comparative analysis
demonstrated a significant difference (P=0.0127) between the levels
of Hgb in patients with no response compared with the complete
response group. Multivariate analysis revealed a close risk to the
significance for patients with anemia that failed to respond to
exclusive radiotherapy. This finding was consistent with reports
that it is considered that anemia is a risk factor predictive of
treatment outcome (23), since it
has been associated with an unfavorable local control of disease
(16,25,26) and
low survival rates (4,25,27).
Retrospective studies, similar to the present study, show that
those patients with Hgb levels <11 g/dl have a high risk of
reducing DFS, which can be improved with the correction of the
anemia (25).
Direct measurement of oxygen levels in tumor tissues
presents technical limitations, which has promoted the use of
endogenous and exogenous markers associated with tumor hypoxia,
endogenous markers including hypoxia-related proteins (HIF-1α,
GLUT-1, CAIX) and exogenous markers including bio-reductive drugs
(34–36). In the evaluation of GLUT 1 and CAIX
in this study, tumor hypoxia was measured indirectly. We observed
differences in their levels of expression, the expression of GLUT-1
was higher compared to CAIX; This result suggests that the tumors
showed episodic hypoxia possibly due to intratumoral heterogeneity.
These findings suggested an effect of GLUT1 associated with
response to treatment. These results supported the hypothesis that
a combination of biomarkers is more robust compared with a single
marker, so further work is required to confirm the usefulness of
incorporating multiple biomarkers to identify patients with hypoxic
tumors for future targeting (37).
Further prospective studies are required to confirm the present
results. The study and detection of these markers may contribute to
determining the metabolic and hypoxic state of tumors, allowing the
optimization of the therapeutic management by considering such
markers as predictive markers and/or molecular targets.
References
|
1
|
Moreno-Acosta P, Carrillo S, Gamboa O,
Acosta Y, Balart-Serrad J, Magne N, Melo-Uribef MA and Romero-Rojas
AE: Expresión de marcadores hipóxicos y glucolíticos CAIX, GLUT-1,
HKII y su relación con la respuesta temprana al tratamiento en
carcinoma escamocelular de cuello uterino. Progresos de Obstetricia
y Ginecología. 56:404–413. 2013. View Article : Google Scholar
|
|
2
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms and cellular response.
Oncologist. 9:(Suppl 5). S4–S9. 2004. View Article : Google Scholar
|
|
5
|
Pili R and Donehower RC: Is HIF-1 alpha a
valid therapeutic target? J Natl Cancer Inst. 95:498–499. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gatenby RA and Gillies RJ: Glycolysis in
cancer: A potential target for therapy. Int J Biochem Cell Biol.
39:1358–1366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamkin DM, Spitz DR, Shahzad MM, Zimmerman
B, Lenihan DJ, Degeest K, Lubaroff DM, Shinn EH, Sood AK and
Lutgendorf SK: Glucose as a prognostic factor in ovarian carcinoma.
Cancer. 115:1021–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan Y and Zong WX: Hacking hexokinase
halts tumor growth. Cancer Biol Ther. 7:1136–1138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee WY, Huang SC, Hsu KF, Tzeng CC and
Shen WL: Roles for hypoxia-regulated genes during cervical
carcinogenesis: Somatic evolution during the
hypoxia-glycolysis-acidosis sequence. Gynecol Oncol. 108:377–384.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue F, Lin LL, Dehdashti F, Miller TR,
Siegel BA and Grigsby PW: F-18 fluorodeoxyglucose uptake in primary
cervical cancer as an indicator of prognosis after radiation
therapy. Gynecol Oncol. 101:147–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Behrooz A and Ismail-Beigi F: Dual control
of glut1 glucose transporter gene expression by hypoxia and by
inhibition of oxidative phosphorylation. J Biol Chem.
272:5555–5562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatzivassiliou G, Andreadis C and Thompson
CB: Akt-directed metabolic alterations in cancer. Drug Discovery
Today: Disease Mechanisms. 2:255–262. 2005. View Article : Google Scholar
|
|
13
|
Gottlob K, Majewski N, Kennedy S, Kandel
E, Robey RB and Hay N: Inhibition of early apoptotic events by
Akt/PKB is dependent on the first committed step of glycolysis and
mitochondrial hexokinase. Genes Dev. 15:1406–1418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayer A, Höckel M and Vaupel P: Endogenous
hypoxia markers: Case not proven! Adv Exp Med Biol. 614:127–136.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lal A, Peters H, St Croix B, Haroon ZA,
Dewhirst MW, Strausberg RL, Kaanders JH, van der Kogel AJ and
Riggins GJ: Transcriptional response to hypoxia in human tumors. J
Natl Cancer Inst. 93:1337–1343. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rafajová M, Zatovicová M, Kettmann R,
Pastorek J and Pastoreková S: Induction by hypoxia combined with
low glucose or low bicarbonate and high posttranslational stability
upon reoxygenation contribute to carbonic anhydrase IX expression
in cancer cells. Int J Oncol. 24:995–1004. 2004.PubMed/NCBI
|
|
17
|
Vordermark D, Kaffer A, Riedl S, Katzer A
and Flentje M: Characterization of carbonic anhydrase IX (CA IX) as
an endogenous marker of chronic hypoxia in live human tumor cells.
Int J Radiat Oncol Biol Phys. 61:1197–1207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pastorek J, Pastoreková S, Callebaut I,
Mornon JP, Zelník V, Opavský R, Zat'ovicová M, Liao S, Portetelle
D, Stanbridge EJ, et al: Cloning and characterization of MN, a
human tumor-associated protein with a domain homologous to carbonic
anhydrase and a putative helix-loop-helix DNA binding segment.
Oncogene. 9:2877–2888. 1994.PubMed/NCBI
|
|
19
|
Lyshchik A, Higashi T, Hara T, Nakamoto Y,
Fujimoto K, Doi R, Imamura M, Saga T and Togashi K: Expression of
glucose transporter-1, hexokinase-II, proliferating cell nuclear
antigen and survival of patients with pancreatic cancer. Cancer
Invest. 25:154–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase II: Cancer's double-edged sword acting as both
facilitator and gatekeeper of malignancy when bound to
mitochondria. Oncogene. 25:4777–4786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh JM, Ryoo IJ, Yang Y, Kim HS, Yang KH
and Moon EY: Hypoxia-inducible transcription factor (HIF)-1 alpha
stabilization by actin-sequestering protein, thymosin beta-4 (TB4)
in Hela cervical tumor cells. Cancer Lett. 264:29–35. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dunst J, Kuhnt T, Strauss HG, Krause U,
Pelz T, Koelbl H and Haensgen G: Anemia in cervical cancers: Impact
on survival, patterns of relapse, and association with hypoxia and
angiogenesis. Int J Radiat Oncol Biol Phys. 56:778–787. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang CC, Murphy SP and Ferrone S:
Differential in vivo and in vitro HLA-G expression in melanoma
cells: Potential mechanisms. Hum Immunol. 64:1057–1063. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Winter WE III, Maxwell GL, Tian C, Sobel
E, Rose GS, Thomas G and Carlson JW: Association of hemoglobin
level with survival in cervical carcinoma patients treated with
concurrent cisplatin and radiotherapy: A gynecologic oncology group
study. Gynecol Oncol. 94:495–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayer A, Höckel M and Vaupel P: Carbonic
anhydrase IX expression and tumor oxygenation status do not
correlate at the microregional level in locally advanced cancers of
the uterine cervix. Clin Cancer Res. 11:7220–7225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haensgen G, Krause U, Becker A, Stadler P,
Lautenschlaeger C, Wohlrab W, Rath FW, Molls M and Dunst J: Tumor
hypoxia, p53, and prognosis in cervical cancers. Int J Radiat Oncol
Biol Phys. 50:865–872. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Green J, Kirwan J, Tierney J, Vale C,
Symonds P, Fresco L, Williams C and Collingwood M: Concomitant
chemotherapy and radiation therapy for cancer of the uterine
cervix. Cochrane Database Syst Rev: CD002225. 2005. View Article : Google Scholar
|
|
29
|
Symonds R and Foweraker K: Principles of
chemotherapy and radiotherapy. Current Obstetrics &
Gynaecology. 16:100–106. 2006. View Article : Google Scholar
|
|
30
|
Airley RE, Loncaster J, Raleigh JA, Harris
AL, Davidson SE, Hunter RD, West CM and Stratford IJ: GLUT-1 and
CAIX as intrinsic markers of hypoxia in carcinoma of the cervix:
Relationship to pimonidazole binding. Int J Cancer. 104:85–91.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bachtiary B, Schindl M, Pötter R, Dreier
B, Knocke TH, Hainfellner JA, Horvat R and Birner P: Overexpression
of hypoxia-inducible factor 1alpha indicates diminished response to
radiotherapy and unfavorable prognosis in patients receiving
radical radiotherapy for cervical cancer. Clin Cancer Res.
9:2234–2240. 2003.PubMed/NCBI
|
|
32
|
Moreno-Acosta P, Gamboa O, de Gomez M
Sanchez, Cendales R, Diaz GD, Romero A, Serra J Balart, Conrado Z,
Levy A, Chargari C and Magné N: IGF1R gene expression as a
predictive marker of response to ionizing radiation for patients
with locally advanced HPV16-positive cervical cancer. Anticancer
Res. 32:4319–4325. 2012.PubMed/NCBI
|
|
33
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 60:4693–4696.
2000.PubMed/NCBI
|
|
34
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yen TC, See LC, Lai CH, Yah-Huei CW, Ng
KK, Ma SY, Lin WJ, Chen JT, Chen WJ, Lai CR and Hsueh S: 18F-FDG
uptake in squamous cell carcinoma of the cervix is correlated with
glucose transporter 1 expression. J Nucl Med. 45:22–29.
2004.PubMed/NCBI
|
|
36
|
Kim BW, Cho H, Chung JY, Conway C, Ylaya
K, Kim JH and Hewitt SM: Prognostic assessment of hypoxia and
metabolic markers in cervical cancer using automated digital image
analysis of immunohistochemistry. J Transl Med. 11:1852013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le QT and Courter D: Clinical biomarkers
for hypoxia targeting. Cancer Metastasis Rev. 27:351–362. 2008.
View Article : Google Scholar : PubMed/NCBI
|