Introduction
Gastric cancer continues to be a major cause of
morbidity and mortality worldwide (1). According to the International Agency
for Research on Cancer (IARC), one million new cases of gastric
cancer are diagnosed each year, and gastric cancer causes over
700,000 deaths worldwide each year (2). Despite an improvement in survival over
recent years due to the development of better endoscopic and
imaging techniques, surgical procedures and skills, and oncological
treatments, its prognosis is still unfavorable.
Recently, there has been a growing interest in the
host inflammatory response to tumors, and the systemic inflammatory
response has been shown to reflect the promotion of angiogenesis,
DNA damage, and tumor invasion through the over-production of
cytokines (3–5). Based on these findings, a number of
inflammation-based prognostic markers such as the Glasgow
Prognostic Score (GPS) and the platelet to lymphocyte ratio (PLR)
have been studied (6,7). In addition, there is increasing
evidence that the neutrophil to lymphocyte ratio (NLR) can be an
effective prognostic indicator in various types of malignant
diseases (8–18).
In cancer patients, lymphopenia reflects an impaired
cell-mediated immunity, while neutrophilia is acknowledged as a
response to systemic inflammation. The NLR has been suggested to be
a marker for general immune responses to various stress stimuli.
The NLR has also been reported to correlate with the severity of
clinical progress in severely ill patients in the intensive care
unit; emerging evidence has shown that the NLR has a prognostic
value in patients with solid tumors (19–21).
To the best of our knowledge, no studies of NLR
regarding responses to chemotherapy against gastric cancer, or its
correlation with patients' nutritional status have been published.
In this study, we therefore evaluated the clinical utility of the
NLR in patients with stage IV gastric cancer under pre-treatment
conditions.
S-1 (TS-1, Taiho Pharmaceutical Co., Ltd) is an oral
fluoropyrimidine agent that is designed to have strong anti-cancer
activity and to have reduced gastrointestinal toxicity compared to
other anti-cancer drugs. It consists of tegafur, 5-chloro-2,
4-dehydroxypyrimidine (an inhibitor of dihydropyrimidine
dehydrogenase), and potassium oxonate dehydrogenase (an inhibitor
of phosphoribosyl transferase) in a molar ratio of 1:0.4:1
(22). S-1 plus cisplatin has been
reported have significant effectivity for unresectable or recurrent
gastric cancer and is recognized as a standard therapy in Japan
(23–26).
Cancer cachexia, another important problem in cancer
treatment and care, is associated with nutritional impairment and
immune suppression (27,28). We previously reported that
malnutrition or hypoalbuminemia shows a good correlation with
immune suppression, as well as with systemic inflammation (29–33).
Malnutrition has also been reported to show a good correlation with
the suppression of cell-mediated immunity. Systemic inflammation
may underlie these important conditions that are prominent in
patients with advanced cancer. Relationships between the NLR and
nutritional status or immune function were therefore also assessed
in the present study.
Patients and methods
Patients
We enrolled 110 patients with stage IV gastric
cancer who received chemotherapy of S-1 plus cisplatin between May
2013 and June 2015 at Saitama Medical University International
Medical Center. Details of these patients are listed in Table I. The study was approved by the
institutional review board of Saitama Medical University
International Medical Center (14–107). All patients provided
written informed consent. This retrospective study was performed on
all consecutive patients referred for chemotherapy treatment for
gastric cancer after invasive diagnostic and staging workup at our
unit. Data were extracted from electronic medical files. Patients
entered in this study were aged 35–80 (66.2 median) years and had
an Eastern Cooperative Study Group (ECOG) performance status (PS)
of 0 or 1.
 | Table I.Study participant characteristics. |
Table I.
Study participant characteristics.
| Characteristics | Values |
|---|
| Sex
(male:female) | 56:54 |
| Age (years) | 66.2 (35–80) |
| Primary tumor stage
(T1-3:T4) | 24:86 |
| Nodal involvement
(N0:N1-3) | 27:83 |
| Distant metastasis
(M0:M1) | 22:88 |
| Serum levels of CEA
(<5.0:>5.0) | 36:74 |
Markers for nutrition, immune reaction
and prognosis
The NLR was defined as follows: NLR=peripheral
neutrophil count/peripheral lymphocyte count. Peripheral blood was
drawn and was used for a PHA-lymphocyte-proliferation assay for
measurement of the activity of cell-mediated immunity. The serum
concentrations of prealbumin (PA) and retinol binding protein
(RBP), which are both rapid turnover proteins (RTPs), were measured
as nutritional parameters, using immunoturbidimetry and latex
flocculation turbidimetry, respectively. The serum levels of
c-reactive protein (CRP) were measured as an inflammatory
indicator, using latex flocculation turbidimetry.
Chemotherapy with S-1 plus
cisplatin
All of the patients received 80 mg/m2
oral S-1 in two 40 mg/m2 doses daily after meals on days
1–21 and cisplatin (60 mg/m2) as an i.v. infusion on day
8, repeated every 5 weeks. The dose of S-1 was assigned according
to body surface area (BSA) as follows: BSA<1.25 m2,
80 mg/day; BSA 1.25 to 1.50 m2, 100 mg/day, and BSA 1.5
m2 or higher, 120 mg/day. This combination chemotherapy
was repeatedly administered for a total of 4 cycles and the
responses were then evaluated according to the Response Evaluation
Criteria in Solid Tumors (RECIST), version 1.0. The patients who
responded to this therapy (with stable disease or partial
remission) received the same chemotherapy regimen, and those who
did not respond to this treatment (with progressive diseases)
received ramucirumab combined with paclitaxel and/or irinotecan as
second and third line chemotherapy.
Statistical analysis
SAS software version 9.2 (SAS Institute Inc, Cary,
NC, USA) was used for statistical analysis. The significance of the
correlations between parameters including the NLR was analyzed by
the χ2 test and a t-test. Survival curves were estimated
using the Kaplan-Meier method and the log-rank test was used to
compare the survival curves. P=0.042 was considered statistically
significant. The patients were divided into 2 groups based on a
cut-off NLR level of 3.0. The survival and the response to
chemotherapy of the 2 groups were compared. Overall survival (OS)
was defined as the time from the starting date of chemotherapy
until death.
The results of Cox proportional hazards model
analyzing factors associated with OS are shown in Table III.
 | Table III.Cox proportional hazards model of
overall survival in patients with gastric cancer with receiving
chemotherapy |
Table III.
Cox proportional hazards model of
overall survival in patients with gastric cancer with receiving
chemotherapy
|
|
| Univariable | Multivariable |
|---|
|
|
|
|
|
|---|
| Marker | No. | P-value | HR | 95% LB | 95% UB | P-value | HR | 95% LB | 95% UB |
|---|
| NLR |
|
|
|
|
|
|
|
|
|
| ≤3 | 53 |
| Ref. |
|
|
| Ref. |
|
|
|
>3 | 47 | 0.013 | 1.224 | 1.072 | 1.393 | 0.017 | 1.493 | 1.054 | 2.138 |
| PA (mg/dl) |
|
|
|
|
|
|
|
|
|
|
≤18 | 52 |
| Ref. |
|
|
| Ref. |
|
|
|
>18 | 48 | 0.136 | 1.145 | 0.961 | 1.366 | 0.910 | 1.091 | 0.810 | 1.230 |
| RBP (mg/dl) |
|
|
|
|
|
|
|
|
|
|
≤2.3 | 51 |
| Ref. |
|
|
| Ref. |
|
|
|
>2.3 | 49 | 0.248 | 1.131 | 0.925 | 1.407 | 0.906 | 1.187 | 0.798 | 1.220 |
| WBC (/µ) |
|
|
|
|
|
|
|
|
|
|
≤5,960 | 56 |
| Ref. |
|
|
| Ref. |
|
|
|
>5,960 | 44 | 0.337 | 0.970 | 0.650 | 1.160 | 0.530 | 1.132 | 0.684 | 1.220 |
| CRP (mg/ml) |
|
|
|
|
|
|
|
|
|
| ≤1 | 83 |
| Ref. |
|
|
| Ref. |
|
|
|
>1 | 17 | 0.739 | 1.050 | 0.970 | 1.130 | 0.814 | 0.996 | 0.866 | 1.030 |
| SI |
|
|
|
|
|
|
|
|
|
|
≤367 | 57 |
| Ref. |
|
|
| Ref. |
|
|
|
>367 | 43 | 0.534 | 1.040 | 0.920 | 1.180 | 0.623 | 0.847 | 0.733 | 1.298 |
Results
NLR and chemotherapy outcome
Among the 110 patients with Stage IV gastric cancer
enrolled in the study, 11 patients did not complete 4 cycles of the
S-1 plus cisplatin chemotherapy because of grade 3 gastrointestinal
adverse effects and a decrease in PS. The responses to chemotherapy
based on RECIST criteria of the high and low NLR patient groups are
shown in Table II. The percentage
of patients with a partial response (PR) was significantly higher
in the group of patients with a lower NLR than in the high NLR
group (38.5 vs. 19.1%, respectively; P=0.031) and the percentage of
patients with progressive diseases (PD) was higher in the group
with a higher NLR (57.4 vs. 25.0%, high vs. low NLR group,
respectively; P=0.044).
 | Table II.Summary of response rate after
chemotherapy. |
Table II.
Summary of response rate after
chemotherapy.
| NLR | CR | PRa | SD | PDa |
|---|
| >3.0 | 0 | 19.1 | 23.4 | 57.4 |
| <3.0 | 0.2 | 38.5 | 34.6 | 25 |
NLR and nutrition, immune response and
inflammation
Correlations of the NLR with patients' nutritional
condition, inflammatory status, and cellular immune responses are
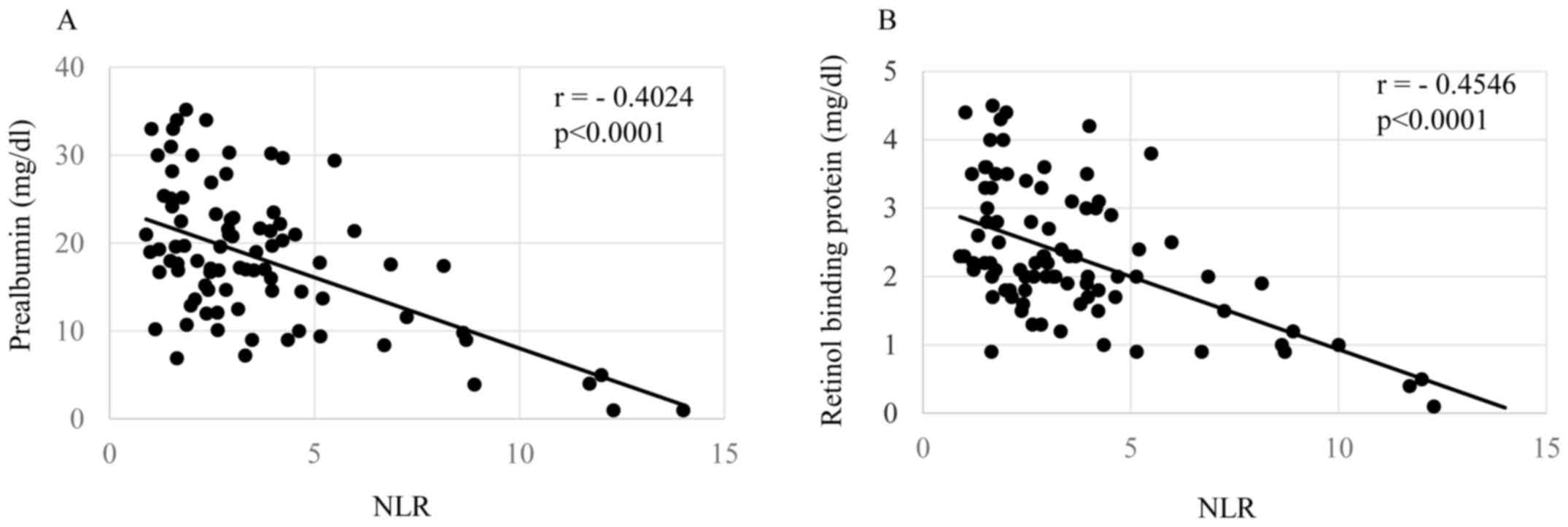
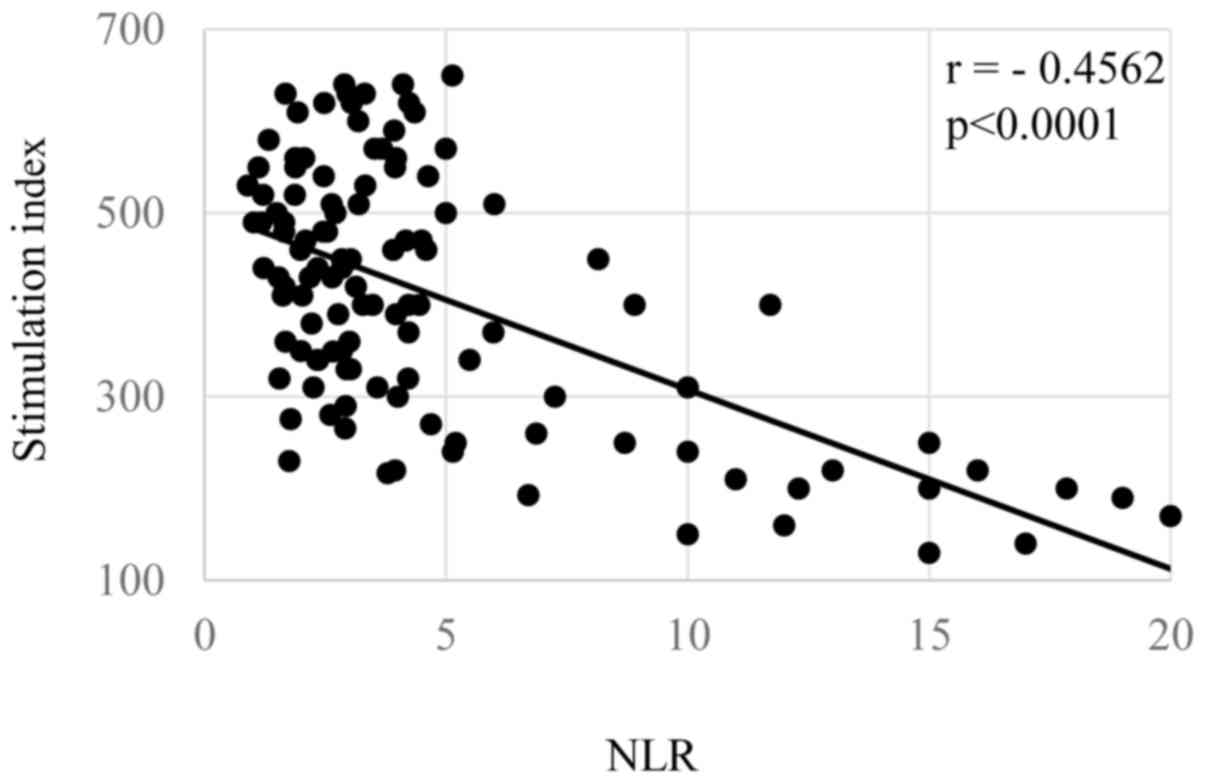
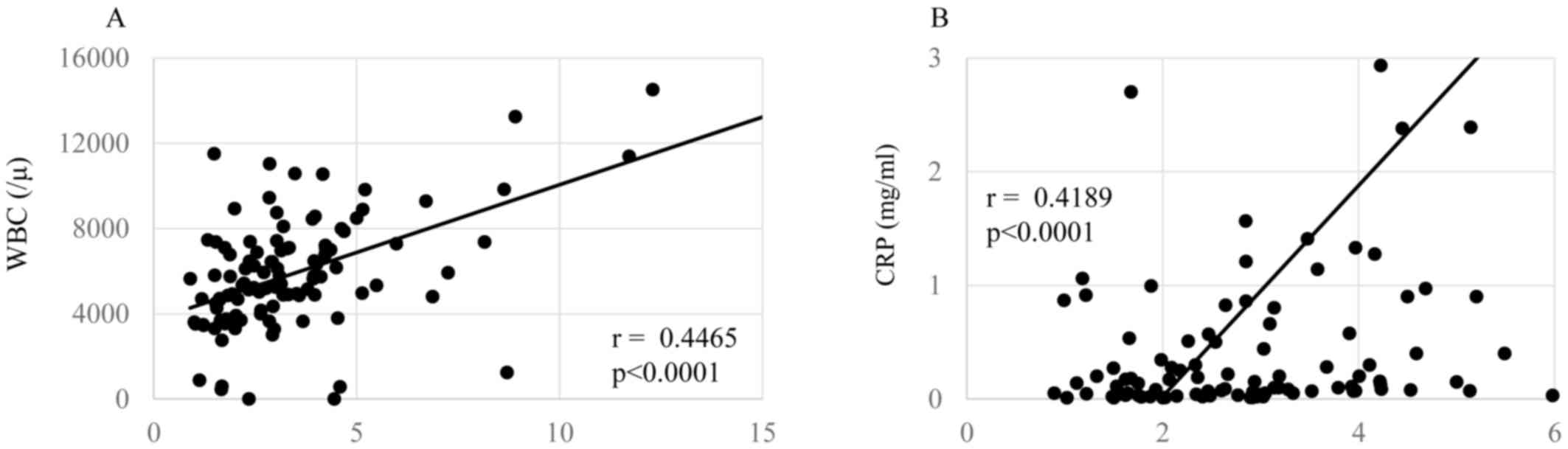
shown in Figs. 1–3, respectively. The levels of the NLR were
significantly inversely correlated with the serum levels of
prealbumin (Fig. 1A, P=0.00006) and
retinol binding protein (Fig. 1B,
P=0.00004). The NLR levels were also significantly correlated with
the levels of CRP (Fig. 2A,
P=0.00007), white blood cell count (Fig.
2B, P=0.00009), and inversely did with SI (Fig. 3, P=0.00003).
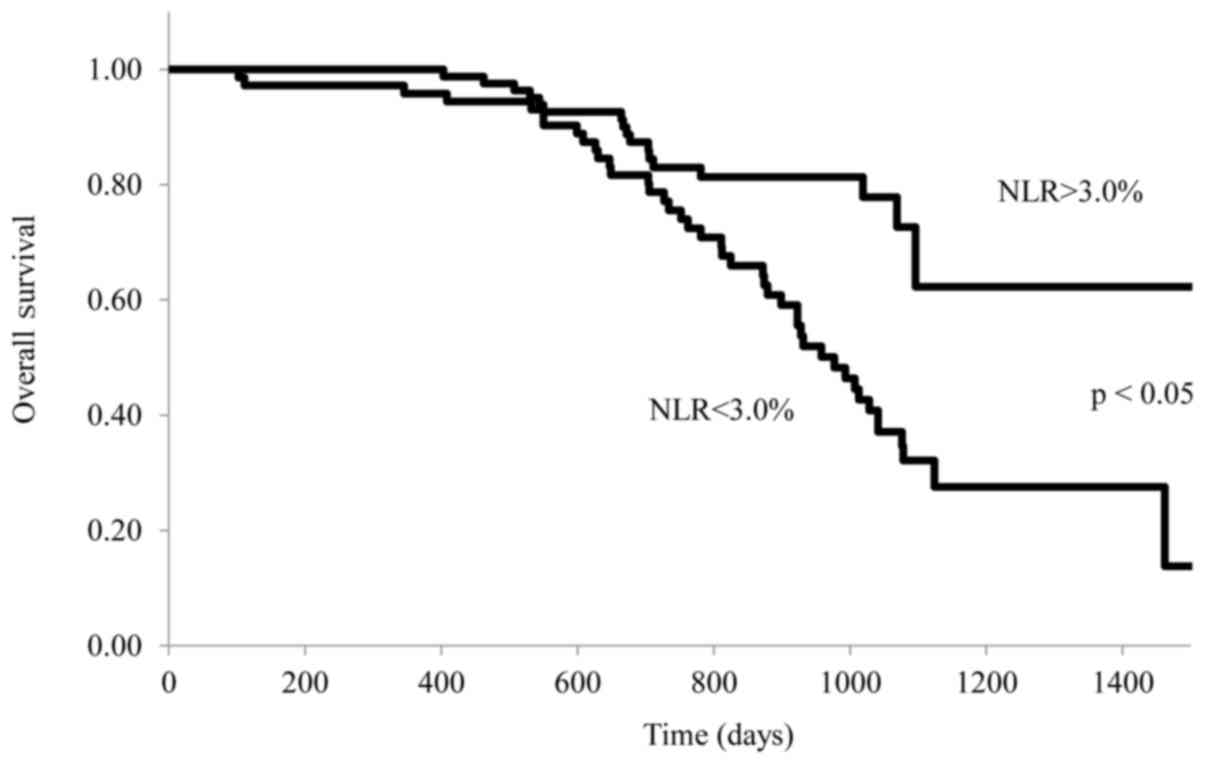
NLR and prognosis
The relationship between the NLR and survival of
stage IV patients with gastric cancer treated with S-1 plus
cisplatin is shown in Fig. 4. OS was
significantly longer in patients with a lower NLR (≤3.0) than in
those with a higher NLR (>3.0) (P=0.042). Range duration of
follow-up was 1–1,514 days in the NLR>3 arm and 1–1533 days in
the NLR≤3. Median OS was 931 vs. 1,034 days for NLR>3 vs. NLR≤ 3
[stratified hazard ratio (HR), 0.831; 95% CI, 0.513 to 0.972; log
rank P=0.042].
Table III shows
that patients with a lower NLR (≤3.0) was associated with a
statistically significant improvement in OS compared with higher
NLR (>3.0) (hazard ratio, 1.493; 95% CI, 1.054–2.138;
P=0.017).
Discussion
The levels of the NLR were analyzed along with
several clinical indicators in patients with Stage IV gastric
cancer who were treated with an S-1 plus CDDP regimen as a
first-line therapy. The present study has demonstrated that the NLR
is a useful marker of malnutrition, systemic inflammation and
immune suppression. Moreover, the NLR was demonstrated to be a
prognostic indicator in these patients.
It has been reported that various
inflammation-related markers such as CRP, the NLR or the GPS are
influenced by the production of pro- and anti-inflammatory
cytokines and are associated with a poor prognosis in various types
of cancer (19–21). In the present study, immune
suppression appeared to be closely associated with an increased NLR
since the levels of the SI were significantly inversely correlated
with an increased NLR. We have previously reported that
myeloid-derived suppressor cells (MDSC), which are suggested to
increase with cancer or inflammation, are increased, and their
circulating levels are related to a decreased SI, malnutrition and
increased vascular endothelial growth factor (VEGF) in patients
with cancer (32,33). We have speculated that immune
suppression may also occur with increased MDSC that are activated
by inflammation or with tumor-produced VEGF.
The reason for the associaton of tumor responses to
chemotherapy with the NLR or systemic inflammation. It may be that
because malnutrition, immune suppression and PS (performance
status) are closely associated with each other in general, patients
with a fair PS may have received a higher dose of chemotherapeutic
drugs or, alternatively, more chemotherapy, or chemotherapy at a
greater rate might have been administered to these patients after 4
cycles of S-1 plus cisplatin.
Another possibility, that of immunogenic cell death
(ICD) after chemotherapy or radiotherapy, has been reported
(34–36). Moreover, recent studies of
immune-checkpoint inhibitors have raised the possibility that
lymphocytes of a cancer-bearing host can recognize neoantigens or
tumor-specific mutation antigens (37). Since a patient's immune function
depends on systemic inflammation, the anti-tumor activity of
cytotoxic T lymphocytes that recognize tumor cells may be
suppressed in patients with high inflammation, and the response of
these patients to chemotherapy and their prognosis might be
poor.
In conclusion, the NLR, which is an inflammatory
marker, is considered to be a useful marker for tumor response to
chemotherapy, immune suppression, malnutrition and poor prognosis.
However, because the NLR is thought to be easily influenced by
infection or stress that is not caused by a tumor, ideally an
appropriate NLR cut-off level needs to be investigated for its use
as a marker in clinical practice. Furthermore, it is possible that
the combined use of anti-inflammatory agents with chemotherapeutic
agents may be more effective. Further investigations into these
points are warranted.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaiswal M, LaRusso NF, Burgart LJ and
Gores GJ: Inflammatory cytokines induce DNA damage and inhibit DNA
repair in cholangiocarcinoma cells by a nitric oxide-dependent
mechanism. Cancer Res. 60:184–190. 2000.PubMed/NCBI
|
|
6
|
McMilan DC: The systemic
inflammation-based Glasgow prognostic score: A decade of experience
in patients with cancer. Cancer Treat Rev. 39:534–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith RA, Bosonnet L, Raraty M, Sutton R,
Neoptolemos JP, Campbell F and Ghaneh P: Preoperative
platelet-lymphocyte ratio is an independent significant prognostic
marker in resected pancreatic ductal adenocarcinoma. Am J Surg.
197:466–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomita M, Shimizu T, Ayabe T, Yonei A and
Onitsuka T: Preoperative neutrophil to lymphocyte ratio as a
prognostic predictor after curative resection of non-small lung
cancer. Anticancer Res. 31:2995–2998. 2011.PubMed/NCBI
|
|
9
|
Pinato DJ, Shiner RJ, Seckl MJ, Stebbing
J, Sharma R and Mauri FA: Prognostic performance of
inflammation-based prognostic indices in primary operable non-small
cell lung cancer. Br J Cancer. 110:1930–1935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu
J, Yue D, Zhang B and Wang C: Prognostic significance of
combination of preoperative platelet count and
neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small
cell lung cancer: Based on a large cohort study. PLoS One.
10:e01264962015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Iseki Y, Ikeya T, Sugano K and Hirakawa K: The prognostic
significance of a postoperative systemic inflammatory response in
patients with colorectal cancer. World J Surg Oncol. 13:1942015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albayrak S, Zengin K, Tanik S, Atar M,
Unal SH, Imamoglu MA and Gurdal M: Can the neutrophil-to-lymphocyte
ratio be used to predict recurrence and progression of
non-muscle-invasive bladder cancer? Kaohsiung J Med Sci.
32:327–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong YW, Shi YQ, He LW and Su PZ:
Prognostic significance of neutrophil-to-lymphocyte ratio in rectal
cancer: A meta-analysis. Onco Targets Ther. 9:3127–3134.
2016.PubMed/NCBI
|
|
14
|
Nakamura K, Nagasaka T, Nishida T, Haruma
T, Ogawa C, Kusumoto T, Seki N and Hiramatsu Y: Neutrophil to
lymphocyte ratio in the pre-treatment phase of final-line
chemotherapy predicts the outcome of patients with recurrent
ovarian cancer. Oncol Lett. 11:3975–3981. 2016.PubMed/NCBI
|
|
15
|
Aino H, Sumie S, Niizeki T, Kuromatsu R,
Tajiri N, Nakano M, Satani M, Okamura S, Shimose S, Miyahara K and
Torimura T: The systemic inflammatory response as a prognostic
factor for advanced hepatocellular carcinoma with extrahepatic
metastasis. Mol Clin Oncol. 5:83–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding PR, An X, Zhang RX, Fang YJ, Li LR,
Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, et al: Elevated preoperative
neutrophil to lymphocyte ratio predicts risk of recurrence
following curative resection of stage IIA colon cancer. Int J
Colorectal Dis. 25:1427–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung MR, Park YK, Jeong O, Seon JW, Ryu
SY, Kim DY and Kim YJ: Elevated preoperative neutrophil to
lymphocyte ratio predicts poor survival following resection in late
stage gastric cancer. J Surg Oncol. 104:504–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stotz M, Gerger A, Eisner F, Szkandera J,
Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner
C, et al: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with primary operable and inoperable
pancreatic cancer. Br J Cancer. 109:416–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zahorec R: Ratio of neutrophil to
lymphocyte count-rapid and simple parameter of systemic
inflammation and stress in critically ill. Bratisl Lek Listy.
102:5–14. 2001.(In English, Slovak). PubMed/NCBI
|
|
22
|
Shirasaka T, Shimamoto Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugimachi K, Maehara Y, Horikoshi N,
Shimada Y, Sakata Y, Mitachi Y and Taguchi T: An early phase II
study of oral S-1, a newly developed 5-fluorouracil derivative foe
advanced and recurrent gastrointestinal cancers. S-1
Gastrointestinal Cancer Study Group. Oncology. 57:202–210. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koizumi W, Kurihara M, Nakano S and
Hasegawa K: Phase II study of S-1, a novel oral derivative of
5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative
Gastric Cancer Study Group. Oncology. 58:191–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shibata M and Gonda K: Cachexia,
immunological participationIn: Horizons in Cancer Research. 58.
Nova Science Publishers Inc; New York: 2015
|
|
28
|
Tisdale MJ: Biology of cachexia. J Natl
Cancer Inst. 89:1763–1773. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shibata M, Nezu T, Kanou H, Abe H,
Takekawa M and Fukuzawa M: Decreased production of interleukin-12
and type 2 immune responses are marked in cachectic patients with
colorectal and gastric cancer. J Clin Gastroenterol. 34:416–420.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shibata M, Nagata Y, Kimura T, Kanou H,
Nezu T, Takekawa M and Fukuzawa M: Elevated serum concentration of
interleukin-1 receptor antagonist (IL-1ra) is correlated to
interleukin-6 and to hypoalbuminemia in cachectic patients with
colorectal cancer. Int J Clin Oncol. 5:116–120. 2000. View Article : Google Scholar
|
|
31
|
Shibata M and Takekawa M: Increased serum
concentration of circulating soluble receptor for interleukin-2 and
its effect as a prognostic indicator in cachectic patients with
gastric and colorectal cancer. Oncology. 56:54–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohki S, Shibata M, Gonda K, Machida T,
Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H and
Takenoshita S: Circulating myeloid-derived suppressor cells are
increased and correlated to immune suppression, inflammation and
hypoproteinemia in patients with cancer. Oncol Rep. 28:453–458.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura I, Shibata M, Gonda K, Yazawa T,
Shimura T, Anazaa T, Suzuki S, Sakurai K, Koyama Y, Ohto H, et al:
Serum levels of vascular endothelial growth factor are increased
and correlated with malnutrition, immunosuppression involving MDSCs
and systemic inflammation in patient with cancer of the digestive
system. Oncol Lett. 5:1682–1686. 2013.PubMed/NCBI
|
|
34
|
Suzuki Y, Mimura K, Yoshimoto Y, Watanabe
M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T and Kono K:
Immunogenic tumor cell death induced by chemoradiotherapy in
patients with esophageal squamous cell carcinoma. Cancer Res.
72:3967–3976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshimoto Y, Kono K and Suzuki Y:
Anti-tumor immune responses induced by radiotherapy: A review.
Fukushima J Med Sci. 61:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garg AD, Galluzzi L, Apetoh L, Baert T,
Birge RB, Pedro Bravo-San JM, Breckpot K, Brough D, Chaurio R,
Cirone M, et al: Molecular and translational classifications of
DAMPs in immunogenic cell death. Front Immunol. 6:5882015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang RF and Wang HY: Immune targets and
neoantigens for cancer immunotherapy and precision medicine. Cell
Res. 27:11–37. 2017. View Article : Google Scholar : PubMed/NCBI
|