Spandidos Publications style
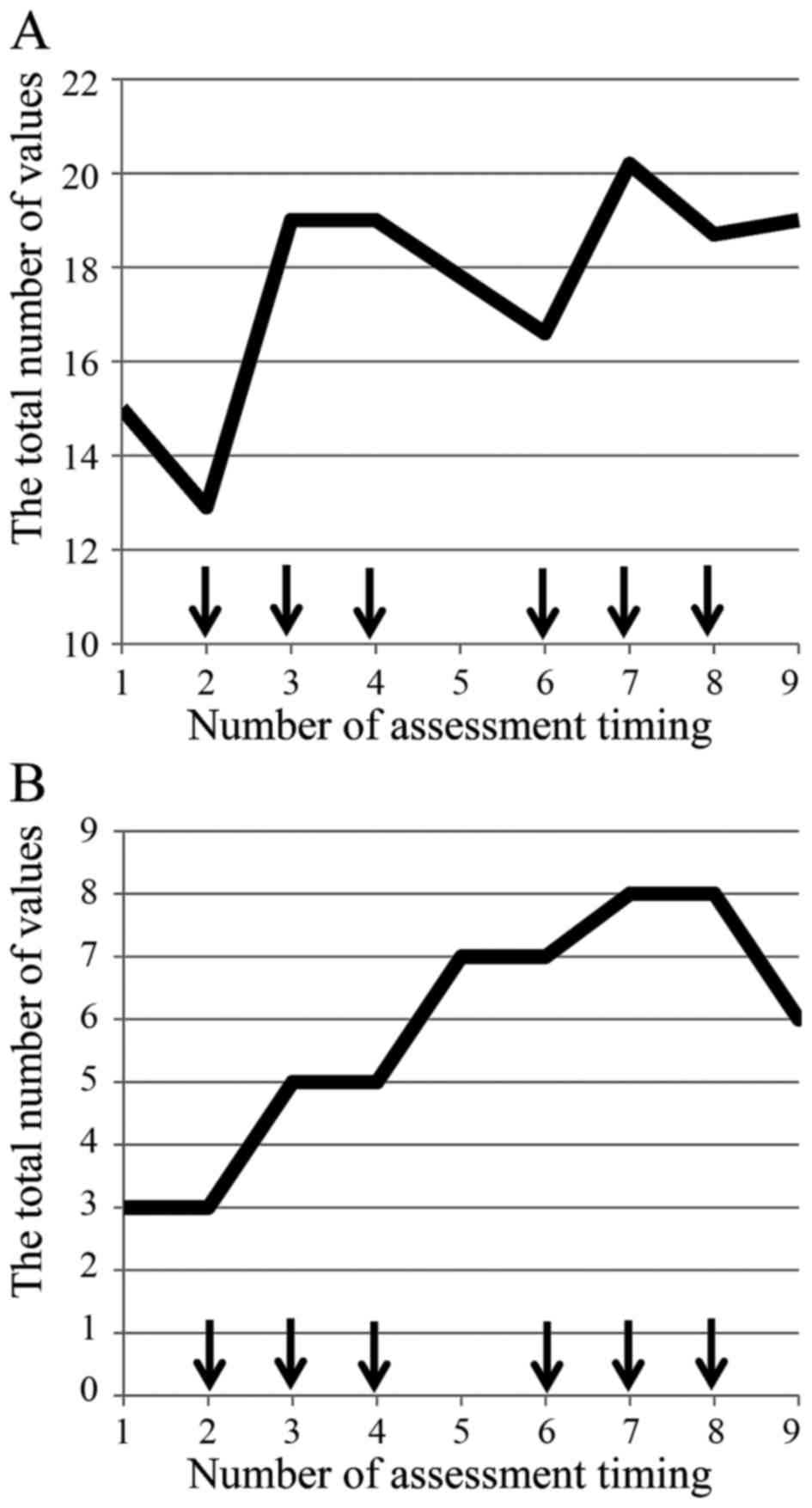
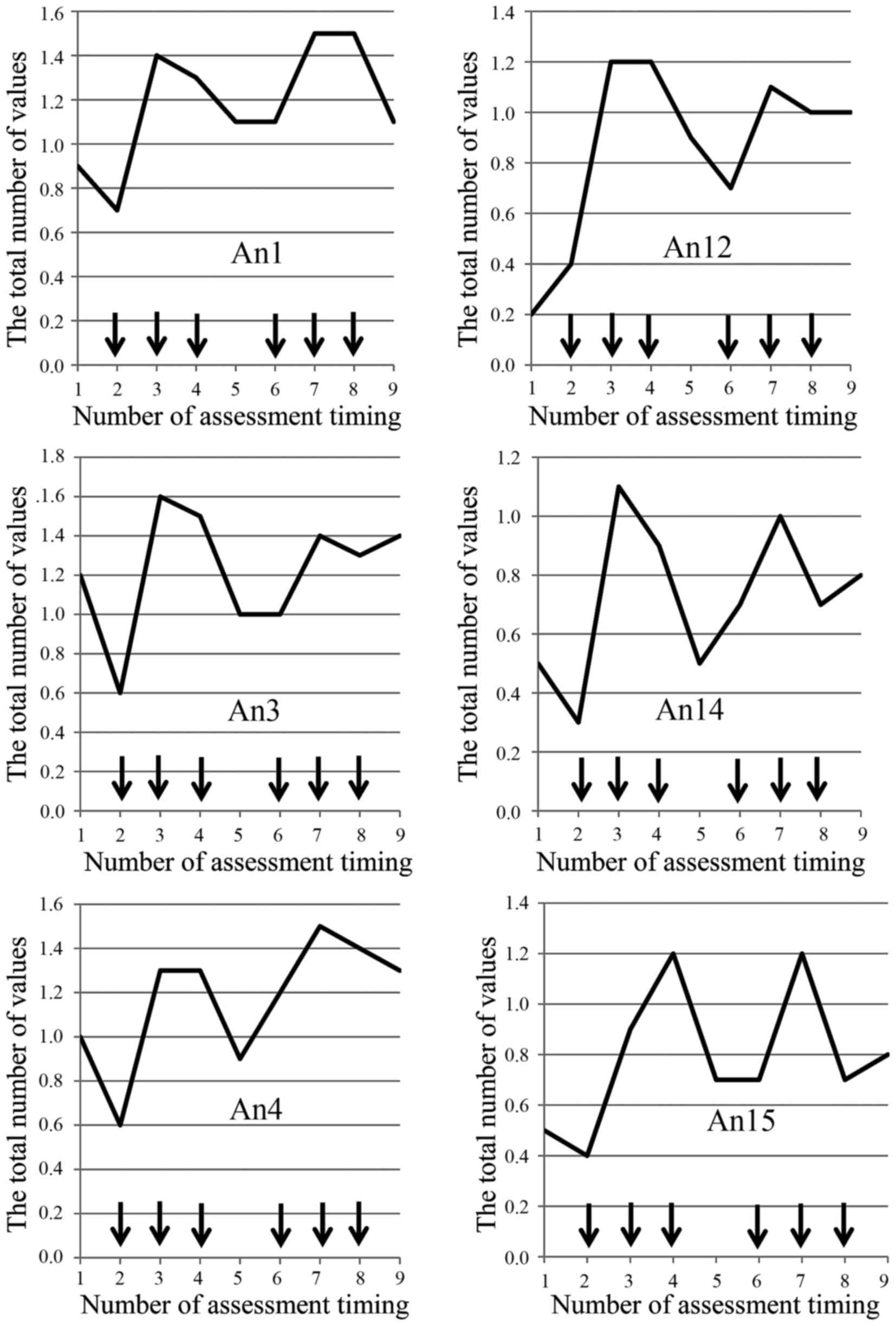
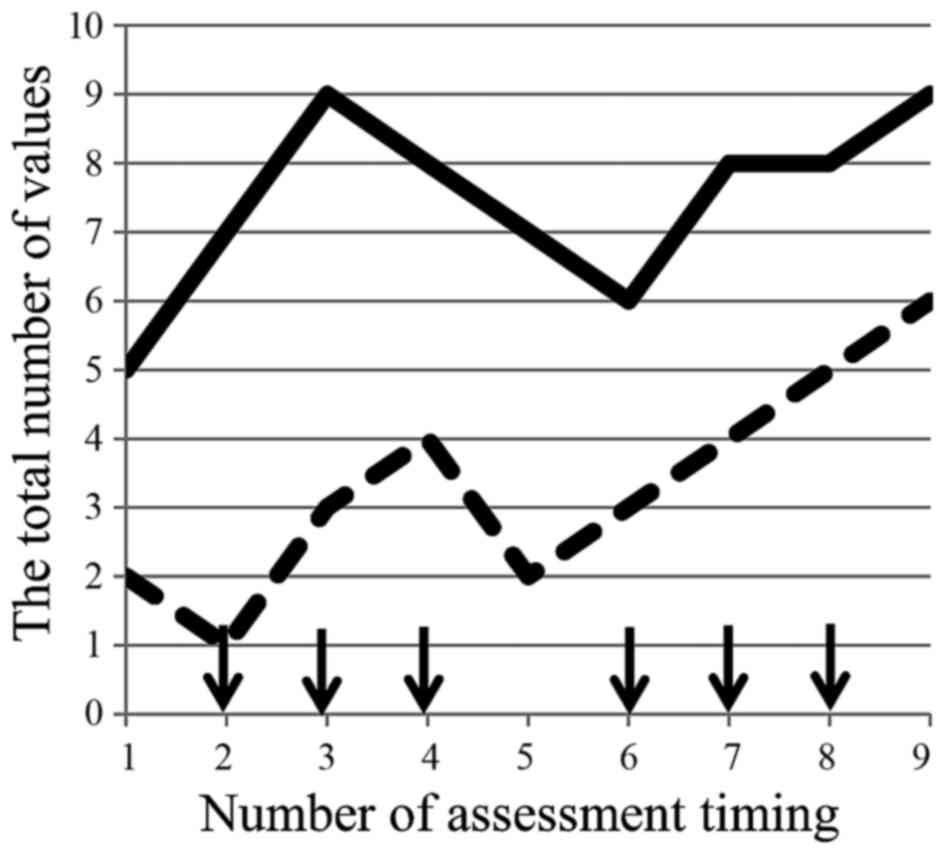
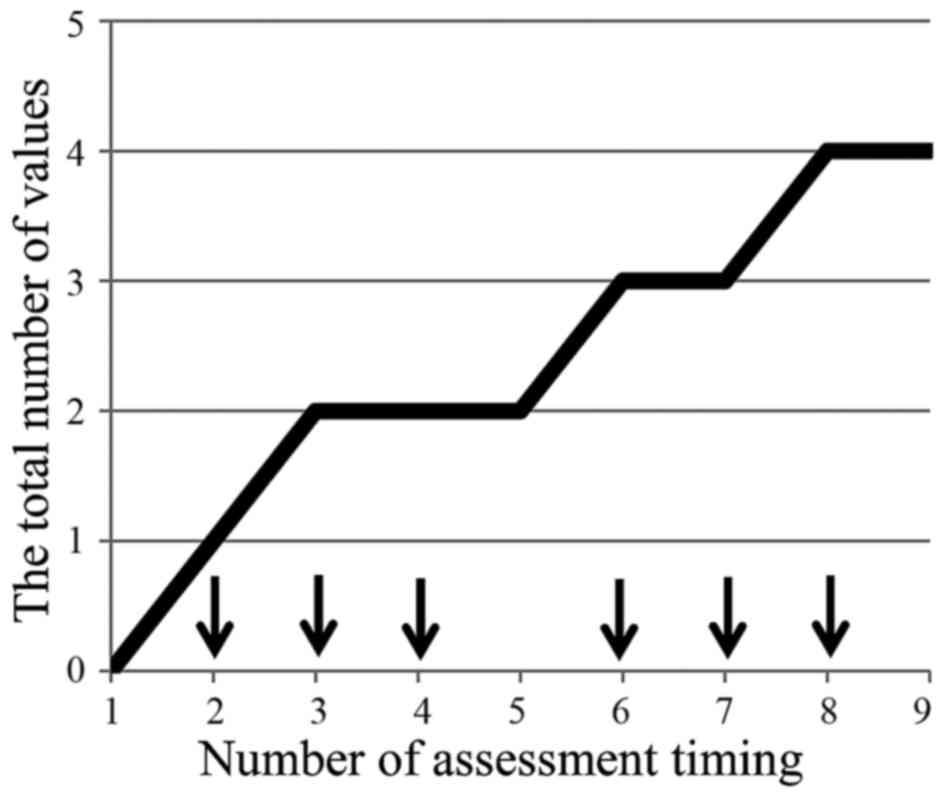
Okada KI, Hirono S, Kawai M, Miyazawa M, Shimizu A, Kitahata Y, Ueno M, Hayami S, Shimokawa T, Yamaue H, Yamaue H, et al: Prospective validation of patient fatigue questionnaire (FACIT‑F) for fatigue assessment in nab‑paclitaxel plus gemcitabine therapy. Mol Clin Oncol 8: 121-126, 2018.
APA
Okada, K., Hirono, S., Kawai, M., Miyazawa, M., Shimizu, A., Kitahata, Y. ... Yamaue, H. (2018). Prospective validation of patient fatigue questionnaire (FACIT‑F) for fatigue assessment in nab‑paclitaxel plus gemcitabine therapy. Molecular and Clinical Oncology, 8, 121-126. https://doi.org/10.3892/mco.2017.1485
MLA
Okada, K., Hirono, S., Kawai, M., Miyazawa, M., Shimizu, A., Kitahata, Y., Ueno, M., Hayami, S., Shimokawa, T., Yamaue, H."Prospective validation of patient fatigue questionnaire (FACIT‑F) for fatigue assessment in nab‑paclitaxel plus gemcitabine therapy". Molecular and Clinical Oncology 8.1 (2018): 121-126.
Chicago
Okada, K., Hirono, S., Kawai, M., Miyazawa, M., Shimizu, A., Kitahata, Y., Ueno, M., Hayami, S., Shimokawa, T., Yamaue, H."Prospective validation of patient fatigue questionnaire (FACIT‑F) for fatigue assessment in nab‑paclitaxel plus gemcitabine therapy". Molecular and Clinical Oncology 8, no. 1 (2018): 121-126. https://doi.org/10.3892/mco.2017.1485