Introduction
Hepatoblastoma is the most common malignant liver
tumor in children and usually presents as a large abdominal mass.
The standard management of hepatoblastoma is multidisciplinary,
usually consisting of surgery and chemotherapy, and is based on the
risk classification systems developed by The International Society
of Pediatric Oncology and the Children's Oncology Group (1). The most commonly used classification of
hepatoblastoma is the pretreatment tumor extension (PRETEXT)
staging system, which classifies tumors based on imaging studies
performed prior to surgical resection. Tumor rupture is considered
an independent high-risk factor in most studies, regardless of
whether rupture occurs within the hepatic capsule or into the
abdominal cavity (2). Ruptured
hepatoblastomas usually require emergency transarterial
embolization (TAE) or even hepatic resection to control the
bleeding. The recommended chemotherapy regimen for the subgroup
into which our patient fell is a dose-dense cisplatin-based
regimen, as described in the International Childhood Liver Tumours
Strategy Group (SIOPEL)-4 protocol (3).
The usual protocol for managing a ruptured
hepatoblastoma is TAE followed by neoadjuvant chemotherapy, surgery
and postoperative chemotherapy. TAE is effective in occluding the
feeding artery of the hepatic tumor (4), particularly in tumors where rupture
occurs within the hepatic capsule. Moreover, TAE plus local
chemotherapy is used to shrink the tumor in patients with advanced
hepatoblastoma who do not respond to systemic chemotherapy. To the
best of our knowledge, no previous study has reported that TAE
reduced the requirement for chemotherapy in patients with
intrahepatic ruptured hepatoblastoma.
We herein report a case of intrahepatic ruptured
hepatoblastoma successfully treated with TAE, cisplatin monotherapy
and surgery.
Case report
A 1-year-old female patient was admitted to the
Guangzhou Women and Children's Medical Center with acute
hemorrhagic shock during November 2016. Blood examination revealed
a hemoglobin level of 67 g/l (normal range, 110–160 g/l). Abdominal
ultrasound revealed an intrahepatic mass and intrahepatic bleeding.
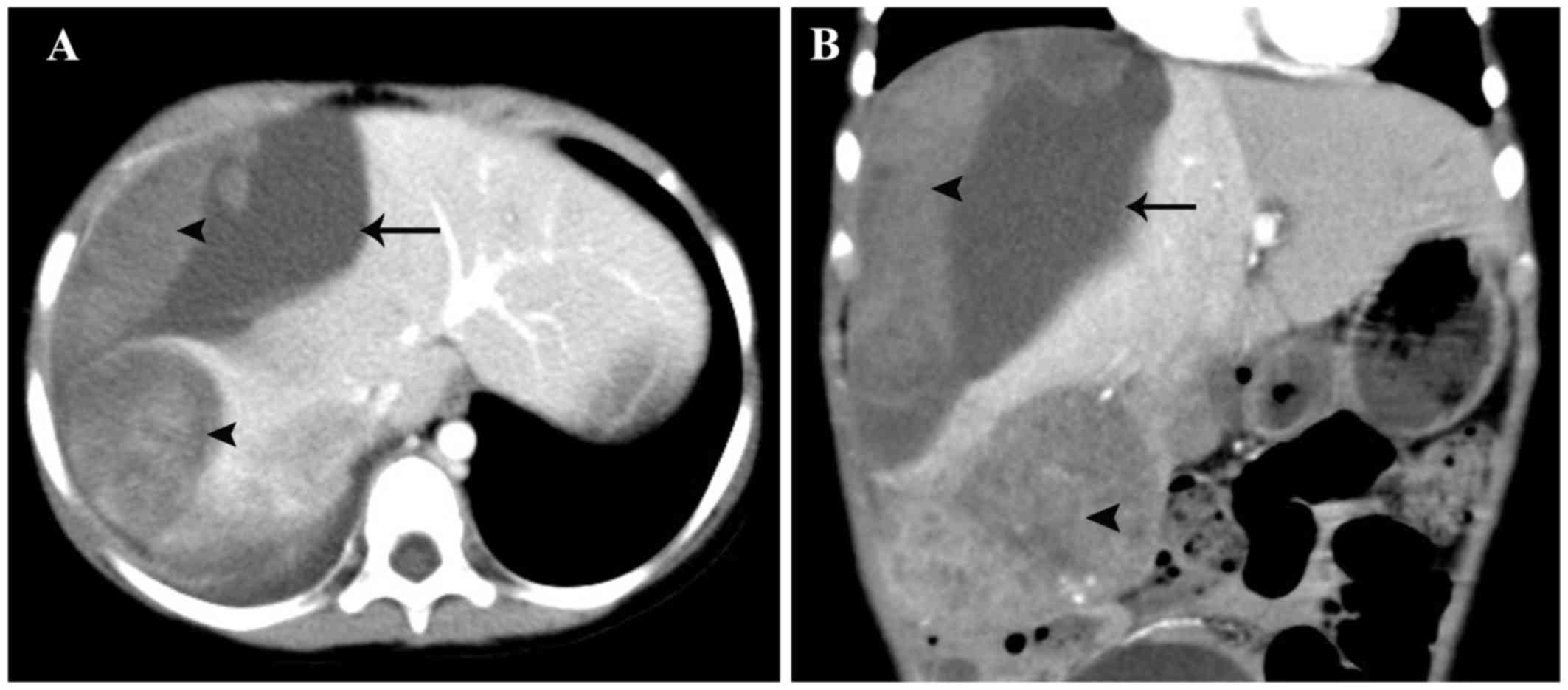
Computed tomography (CT) examination confirmed the presence of a
ruptured tumor in the right hemiliver and an intact hepatic capsule
(Fig. 1). The α-fetoprotein (AFP)
level was 59,318.26 ng/ml (normal range, <10 ng/ml). A ruptured
hepatoblastoma, PRETEXT stage II, was diagnosed. The patient
underwent fluid resuscitation and blood transfusion as an emergency
measure and, once she was hematologically stable, TAE was performed
using the Seldinger technique (4)
under general anesthesia. Hepatic arteriography showed that the
tumor was supplied by branches of the right hepatic artery. The
feeding arteries were embolized via superselective embolization
using a 100–300-µm embolic microsphere and polyvinyl alcohol. The
bleeding was successfully controlled via TAE.
The patient was scheduled to receive four courses of
neoadjuvant cisplatin monochemotherapy at doses of 100
mg/m2 of body surface area over 5 days, starting 1 week
after TAE and continuing at the same dose at 21-day intervals.
Repeated CT after four courses of chemotherapy revealed a smaller
and post-treatment extent of disease (POST-TEXT) stage II
hepatoblastoma in the right hemiliver. A right hepatectomy was
performed with a clear resection margin. Pathological examination
of the resected tumor confirmed a hepatoblastoma of mixed
epithelial and stromal subtype. The patient underwent four
additional courses of cisplatin postoperatively. The AFP level
dropped to within the normal range after the fifth cycle of
chemotherapy and remained normal thereafter. The patient remained
disease-free up until the last follow-up appointment, which took
place on April 2018.
Discussion
Traditionally, a ruptured hepatoblastoma has been
considered a high-risk occurrence, necessitating a chemotherapy
regimen usually consisting of cisplatin alternating with
carboplatin plus doxorubicin, based on SIOPEL studies (5). Although this dose-dense cisplatin-based
chemotherapy regimen is feasible and efficacious for patients with
high-risk hepatoblastoma, 29% of patients suffer serious side
effects, including death.
TAE is a practical and effective alternative
treatment option for hepatic malignancies and is performed
worldwide on adult patients with inoperable hepatocellular
carcinoma (4). TAE is less
frequently performed on childhood hepatoblastomas and is mainly
used to shrink advanced-stage hepatoblastomas following initial
systemic chemotherapy (6,7). Embolization of the selected hepatic
arteries causes ischemic tumor necrosis. Theoretically, TAE reduces
the tumor burden by truncating the feeding artery and inducing
subsequent necrosis. This may also help to reduce the doses used in
the chemotherapy regimen.
Ruptured hepatoblastomas are uncommon, comprising
~5% of all hepatoblastomas (8),
based on data obtained from the Children's Hepatic Tumors
International Collaboration. Therefore, the study of this rare
subtype of hepatoblastoma presents a considerable challenge.
Ruptured hepatoblastomas may be subdivided into those that rupture
within the liver and those that rupture into the abdominal cavity.
Ruptured hepatoblastomas with intact hepatic capsules may differ
from those that rupture into the abdominal cavity. In the case
described herein. TAE was used to successfully control bleeding
from a ruptured hepatoblastoma with an intact hepatic capsule.
However, hepatoblastomas that bleed into the abdominal cavity may
require an emergency hepatectomy. There are no specific guidelines
for this small subset of cases.
According to the current risk-stratified staging of
pediatric hepatoblastomas developed by the Children's Hepatic
Tumors International Collaboration, tumor rupture is considered an
intermediate risk, based on an analysis of 1,605 pediatric patients
treated in eight multicenter hepatoblastoma trials over a period of
25 years. This classification system indicates that cisplatin
monochemotherapy may be suitable for ruptured hepatoblastomas.
However, to the best of our knowledge, no previous study has yet
examined the efficacy of cisplatin monochemotherapy on ruptured
hepatoblastomas. The present case suggests that this subset of
patients may benefit from an abbreviated chemotherapy regimen
following bleeding control via TAE. As predicted, cisplatin alone
resulted in markedly lower hematological toxicity.
Although firm conclusions based on a single patient
cannot be drawn, it is hypothesized that certain ruptured
hepatoblastomas may benefit from cisplatin monochemotherapy after
TAE: Ruptured hepatoblastomas with intact hepatic capsules may form
a unique subgroup that can be successfully treated with TAE,
cisplatin monotherapy and surgical excision. However, given the
rarity of ruptured hepatoblastomas, further study of patients
within this subgroup is required to confirm our findings.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81602199), which had no role
in the preparation, review or approval of the manuscript.
Availability of data and materials
Not applicable.
Authors' contributions
TY conceptualized and designed the study. YZ
conceptualized and designed the study, coordinated and supervised
data collection, and critically reviewed the manuscript for
important intellectual content. JL, TT, JY, JP, CH and TX designed
the data collection instruments, collected the data and reviewed
and revised the manuscript. The first draft of this manuscript was
written by TY, TT and JY. All the authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Guangzhou Women and Children's Medical Center (Guangzhou,
China), which waived the need for informed consent for the
retrospective collection of demographic, clinical and hospital
outcome data. The patient's records and data were anonymized and
de-identified prior to analysis.
Patient consent for publication
Consent for the publication of the case details and
associated images was obtained from the patient's parents.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aronson DC and Meyers RL: Malignant tumors
of the liver in children. Semin Pediatr Surg. 25:265–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roebuck DJ, Aronson D, Clapuyt P,
Czauderna P, de Ville de Goyet J, Gauthier F, Mackinlay G, Maibach
R, McHugh K, Olsen OE, et al: International Childrhood Liver Tumor
Strategy Group: 2005 PRETEXT: A revised staging system for primary
malignant liver tumours of childhood developed by the SIOPEL group.
Pediatr Radiol. 37:123–132; quiz 249–250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers RL, Tiao G, de Ville de Goyet J,
Superina R and Aronson DC: Hepatoblastoma state of the art:
Pre-treatment extent of disease, surgical resection guidelines and
the role of liver transplantation. Curr Opin Pediatr. 26:29–36.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Barcelona Liver Cancer Group: Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: A randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zsiros J, Brugieres L, Brock P, Roebuck D,
Maibach R, Zimmermann A, Childs M, Pariente D, Laithier V, Otte JB,
et al: International Childhood Liver Tumours Strategy Group
(SIOPEL): Dose-dense cisplatin-based chemotherapy and surgery for
children with high-risk hepatoblastoma (SIOPEL-4): A prospective,
single-arm, feasibility study. Lancet Oncol. 14:834–842. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xuewu J, Jianhong L, Xianliang H and
Zhongxian C: Combined treatment of hepatoblastoma with
transcatheter arterial chemoembolization and surgery. Pediatr
Hematol Oncol. 23:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogl TJ, Scheller A, Jakob U, Zangos S,
Ahmed M and Nabil M: Transarterial chemoembolization in the
treatment of hepatoblastoma in children. Eur Radiol. 16:1393–1396.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Czauderna P, Haeberle B, Hiyama E,
Rangaswami A, Krailo M, Maibach R, Rinaldi E, Feng Y, Aronson D,
Malogolowkin M, et al: The Children's Hepatic tumors International
Collaboration (CHIC): Novel global rare tumor database yields new
prognostic factors in hepatoblastoma and becomes a research model.
Eur J Cancer. 52:92–101. 2016. View Article : Google Scholar : PubMed/NCBI
|