Introduction
In patients with ovarian cancer, at least 80% will
experience relapse and will require second-line therapy. Additional
treatment of these patients is currently complex, but the length of
the platinum-free interval (PFI) is an important clinical
consideration (1). Since the
effectiveness of platinum retreatment is dependent on the
relapse-free and treatment-free intervals, stratification of
patients based on PFI can help clinicians to decide the optimal
therapeutic strategy. At present, according to the PFI, patients
are normally classified as those with fully platinum sensitive
disease (PFI >12 months), partially platinum sensitive disease
(PFI 6–12 months), platinum resistant disease (PFI <6 months)
and refractory disease (progression during the last line of
platinum therapy or within 4 weeks of the last platinum dose)
(2). Recently, there has been some
discussion that the patients should no longer to be categorized
using the PFI (with an arbitrary cut-off of 6-months), but rather
‘according to the question: Is platinum still an option for the
patient?’ (3).
Notwithstanding, in recurrent ovarian tumors, the
PFI remains a primary prognostic factor for both progression-free
survival (PFS) and overall survival (OS) (4,5).
Nonetheless, it must be considered that PFI is in reality a
continuous variable that does not always adequately reflect disease
prognosis. Patients with a fully platinum-sensitive relapse
typically receive a second-line salvage therapy based on
retreatment with platinum-containing regimens with response rates
that vary from 30 to 75%, while patients with partially
platinum-sensitive recurrent disease typically have lower response
rates when retreated with platinum (27–33%) (6). Moreover, from 20 to 40% of all ovarian
cancer patients will have partially platinum-sensitive disease for
whom the optimal treatment sequence is still controversial
(6). Indeed, among those with
platinum sensitive disease, patients with partially platinum
sensitive disease are the most challenging to clinically manage.
Given this, there is thus intense interest in providing new
therapeutic options for these patients. Agents that may resensitize
tumors to platinum have been generating interest (7), and among these trabectedin is being
investigated in relapsed ovarian cancer.
Trabectedin is a multitarget agent that was
originally extracted from a species of marine tunicate,
Ecteinascidia turbinate, and has a complex, novel mechanism
of action. Firstly, trabectedin has been shown to bind to the minor
groove of double-stranded DNA, causing double-strand breaks
(8,9). Secondly, trabectedin appears to affect
the cell cycle by causing apoptosis of cancer cells and by
downregulating transcription factors associated with cell
proliferation (9). Due to its
cytotoxic effects on tumor-associated macrophages, trabectedin may
further inhibit the release of cytokines by monocytes and
macrophages in the tumor microenvironment (10). Importantly, trabectedin has also been
reported to interact directly with components involved in
nucleotide excision repair (NER), thereby inhibiting repair of
specific NER substrates and forming a ternary complex that appears
to be associated with cell death (7). Since the NER pathway is responsible for
repairing platinum-DNA adducts in cellular DNA, NER aberrations
will increase the sensitivity of tumors to platinum. This novel
aspect of trabectedin is thus related to the high-sensitivity seen
in NER-proficient cells upon exposure to platinum, which are
generally more resistant to platinum compounds. These findings thus
provide a clear rationale for the benefits of sequential treatment
with trabectedin and platinum compounds (7).
First approved in the EU as monotherapy for
treatment of advanced soft tissue sarcoma in 2007, trabectedin is
also indicated for the treatment of patients with relapsed,
platinum-sensitive ovarian cancer in combination with
pegylated liposomal doxorubicin (PLD) since 2009.
In ovarian tumors, trabectedin in combination with
PLD has best been studied in the OVA-301 trial (11). In fact, among the various options
investigated, early studies with trabectedin in combination with
PLD suggested that the combination was associated with a
significant advantage in survival compared to PLD alone (11). In the entire population, median PFS
was 7.3 months with trabectedin + PLD vs. 5.8 months with PLD (HR,
0.79; P=0.0190). In the partially platinum-sensitive subpopulation,
trabectedin + PLD led to a 41% decrease in the risk of death (HR,
0.59; P=0.0015), with median survival of 23.0 vs. 17.1 months for
PLD alone (12). Thus, trabectedin +
PLD appeared to be of particular benefit for those with partially
platinum sensitive disease from second to further lines (12). Another post-hoc analysis of OVA-301
reported that in patients with relapsed ovarian cancer, trabectedin
+ PLD delays third-line chemotherapy and prolongs the platinum-free
interval by about 2.5 months, and OS was also significantly
prolonged in the partially platinum-sensitive disease subgroup
(13). This adds additional support
to the possibility that the enhanced survival benefits in the
partially platinum-sensitive subset might be related to the ability
of trabectedin to resensitize tumors to subsequent platinum
rechallenge (7).
To further characterize the clinical efficacy of the
trabectedin + PLD combination, we carried out a retrospective
analysis involving 11 cases of recurrent ovarian tumor and partial
platinum sensitivity.
Materials and methods
Patients and eligible criteria
A retrospective analysis was carried out at the
Oncological Pharmacology Department at the University Hospital of
Florence involving 11 patients with a diagnosis of ovarian
epithelial tumor. All patients had previously undergone 1 cycle of
platinum-based (carboplatin-taxol) chemotherapy, without the
addition of bevacizumab, from October 2011 to July 2014. All
patients underwent chemotherapy with trabectedin and PLD, after a
PFI of 6–12 months. An ECOG PS ≤2 was required before initiating
therapy as well as the following laboratory parameters: (Hb ≥9
g/dl, neutrophils ≥1.5×109/l, platelets
≥100×109/l, creatinine <1.5 mg/dl; bilirubin ≤ upper
normal limit (ULN); ALT, AST, alkaline phosphatase, and creatinine
phosphatase ≤2.5 times ULN, albumin ≥25 g/l, and alkaline
phosphatase ≤2.5 times ULN.
Treatment and assessment
Trabectedin was administered every 3 weeks (i.v. 1.1
mg/m2 over 3 h) immediately after the administration of
PLD (30 mg/m2). All patients were premedicated with
corticosteroids: Dexamethasone 20 mg i.v. (prior to chemotherapy as
an antiemetic), oral prednisone (10 mg BID starting the day before
chemotherapy), followed by 1 day of 5 mg BID (to avoid liver and
hematologic toxicity due to trabectedin). Response to therapy was
assessed by imaging (ultrasound and CT) every 3 cycles and by serum
CA 125 levels at the beginning of each cycle. Patients with
negative disease status underwent clinical and instrumental
follow-up (ultrasound, CT, tumor markers, PET as needed) every 3
months. Recist 1.1 criteria were used for evaluation of response.
The PFI was calculated from the last administration of
chemotherapy. OS was calculated as the time from surgical
intervention to death or last follow-up visit.
Adverse events
Adverse events were evaluated by clinical and
laboratory assessment according to severity. In case of toxicity,
the dose of trabectedin was reduced in 2 steps (1st step, 0.9
mg/m2 trabectedin and 25 mg/m2 PLD; 2nd step,
0.75 mg/m2 trabectedin and 20 mg/m2 PLD).
Statistical analysis
Statistical analysis was performed to evaluate
objective response and PFS. PFS was expressed as median and
interquartile range. Descriptive statistics were reported in terms
of absolute frequencies and percentages for the qualitative data.
PFS and OS were estimated according to the Kaplan-Meier method. The
PFS and OS analyses were defined as the time interval from the last
administration of trabectedin + PLD to the earliest date of disease
progression or death for PFS, whereas OS was defined as the time
from surgical intervention to death or last follow-up visit. The
results were expressed as median values and mean values, with 95%
confidence intervals (CIs).
Results
Patient cohort
A total of 11 patients, with median age of 60 years
(range, 45–75 years), with ovarian tumors were enrolled (Table I). All patients underwent debulking
surgery (total hysterectomy, bilateral annessiectomy, omentectomy,
appendicectomy and removal of peritoneal implants, when possible).
In 9 patients, this was done at the beginning of clinical history,
in 2 patients neoadjuvant chemotherapy was necessary (in 1 case for
pelvic infiltration, in other case for pulmonary metastases) with
subsequent interval surgery. Histological examination showed the
following histotypes: 7 serous carcinomas, 1 endometrial
carcinomas, 2 undifferentiated carcinomas, and 1 mucinous
carcinoma. The degree of differentiation was low (G3) in 10
patients, while 1 patient had an intermediate degree of
differentiation (G2). Regarding pathological stage: 2 cases were
stage II, 7 cases stage III, and 2 cases stage IV. At the time of
diagnosis, CA 125 was elevated in 10 patients, while it was below
the threshold value in 1 patient. All patients underwent first-line
chemotherapy with carboplatin and taxol for a total number of
cycles ranging from 6 to 9.
 | Table I.Baseline characteristics of the
patient cohort (n=11). |
Table I.
Baseline characteristics of the
patient cohort (n=11).
| Characteristic | Total, n (%) |
|---|
| Median age, years
(range) | 60 (45–75) |
| Surgery |
|
|
Primary | 9 (82) |
|
Interval | 2 (18) |
| Stage |
|
| IIB | 1 (9) |
| IIC | 1 (9) |
| IIIB | 1 (9) |
| IIIC | 4 (37) |
| IV | 4 (37) |
| Histological
type |
|
|
Serous | 7 (64) |
|
Endometrial | 1 (9) |
|
Mucinous | 1 (9) |
|
Undifferentiated | 2 (18) |
| Grading |
|
| 1 | 0 |
| 2 | 1 (9) |
| 3 | 1 (91) |
| Residual tumor |
|
|
Absent | 2 (18) |
| <1
cm | 7 (64) |
| >1
<2 cm | 2 (18) |
| Ca 125, U/ml |
|
|
<35 | 1 (9) |
|
>35 | 1 (91) |
The 2 patients undergoing neoadjuvant chemotherapy
performed both 4 pre-intervention chemotherapy cycles and 4 cycles
of the same pattern after interval surgery. The recurrences, all
with a free interval between 6 and 12 months, were found in 4
patients at lymph node sites, (1 of these patients experienced a
thyroid metastasis), and in 7 patients at peritoneal sites
(peritoneal carcinosis). At the time of recurrence, CA 125 was
positive in 7 of 11 patients.
All 11 patients had undergone trabectedin and PLD as
second-line therapy. A median of 6 cycles of chemotherapy were
administered (range 1–12). The 7 patients who underwent a third
line were treated with carboplatin and gemcitabine. At that time, a
genetic test for the BRCA 1–2 gene was not routinely performed, and
so data are available for only 2 of the 3 patients who are still
alive at the time of writing. One patient has a BRCA1 mutation, 1
wild-type, and for the third genetic response is pending.
Efficacy
In the 11 patients, 4 showed complete response, 3
achieved stable disease, and 4 had progression of disease.
Accordingly, 63.6% of patients responded to therapy (complete or
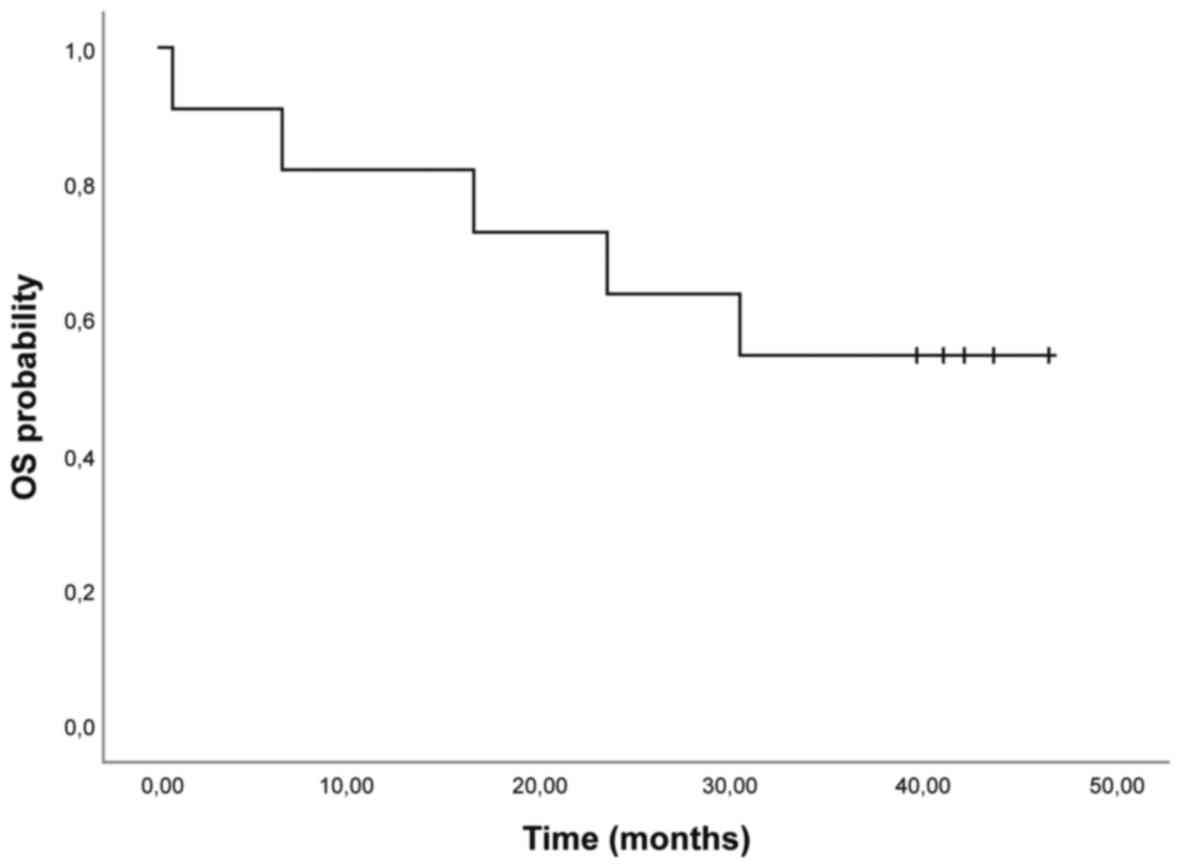
partial response or stable disease) (Table II). Mean OS was 32.42 months (95% CI
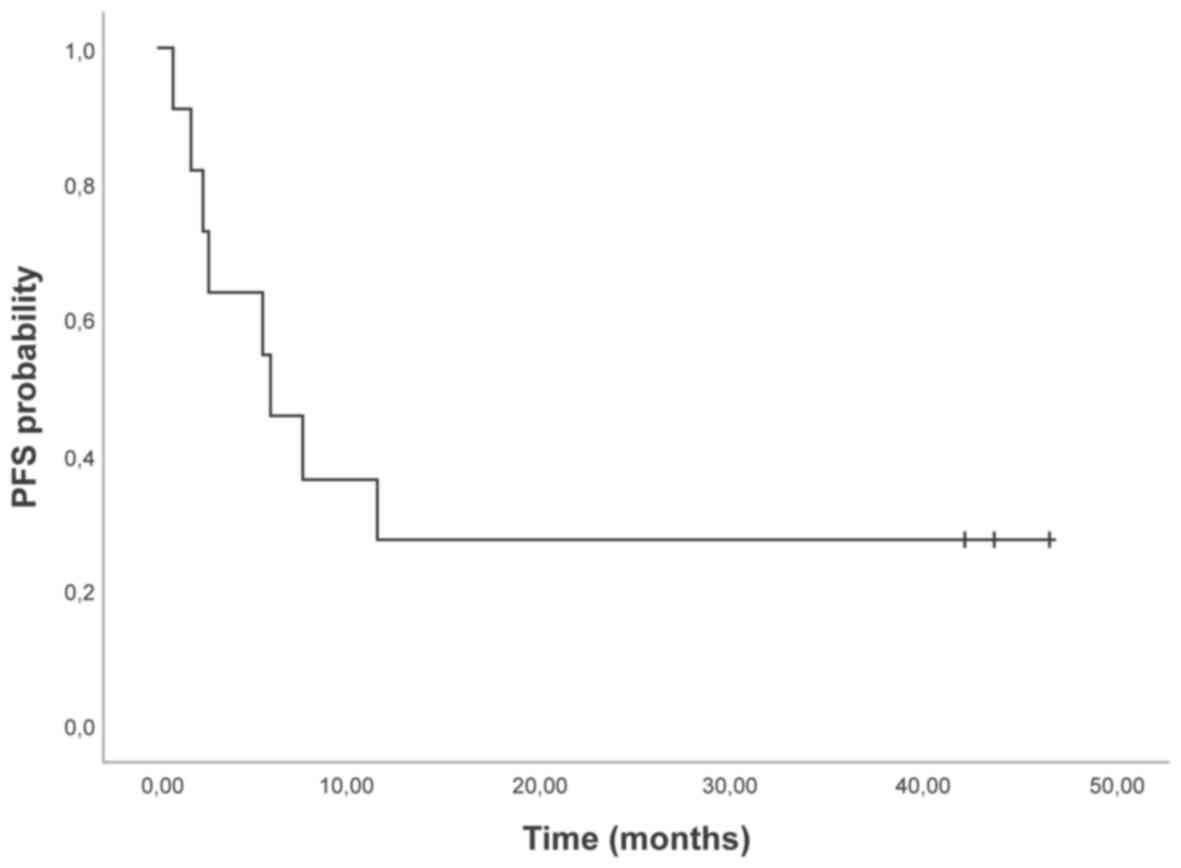
22.4; 42.6) (Fig. 1). The median PFS
was 5.9 months (95% CI 0.6; 11.2) (Table II, Fig.
2). Of the 4 patients who progressed during therapy, 2 patients
had a serous histology, 1 patient had mucinous histology (the only
patient with stage II), and 1 patient had endometrial carcinoma.
The complete responses were in patients with serous (n=2) and
undifferentiated carcinoma (n=1), and the partial response was in a
patient with serious disease. Of the 4 patients with progression, 1
died after a single cycle of therapy. The 7 patients who had
relapsed all underwent a third line with carboplatin and
gemcitabine. Of these patients, 2 had a complete response, 2 a
partial response, 2 were stationary, and 1 had progression after
the third cycle of therapy. The 2 patients who had a complete
response had a disease-free interval of 6 and 8 months,
respectively. Median OS for these patients was 45 months (range
7–75 months).
 | Table II.Response in the patient cohort
(n=11). |
Table II.
Response in the patient cohort
(n=11).
| Response | Trabectedin plus PLD,
n (%) |
|---|
| Complete
response | 3 (27.3) |
| Partial response | 1 (9.0) |
| Stable disease | 3 (27.3) |
| Progressive
disease | 4 (36.4) |
| Objective response
rate | 7 (63.6) |
| Median
progression-free survival, months (95% CI) | 5.9 (0.6; 11.2) |
| Mean overall
survivala, months (95%
CI) | 32.4 (22.4;
42.6) |
Tolerability
Regarding treatment-related toxicity, grade 3
cutaneous toxicity was seen in 18.2% of patients, despite
prophylaxis with vitamin B6 (300 mg/day orally). Trabectedin in
combination with PLD was well tolerated in terms of
gastrointestinal and hematological toxicity. Grade 3 neutropenia
was observed in 18.2% of patients, although there was no need for
treatment delay, discontinuation, or dose adjustments, but only
growth factors to treat neutropenia (Table III).
 | Table III.Adverse events (n=11). |
Table III.
Adverse events (n=11).
|
| Trabectedin plus PLD,
n (%) |
|---|
|
|
|
|---|
| Adverse event | Grade 1 | Grade 2 | Grade 3 |
|---|
| Neutropenia | 4 (36.3) | 3 (27.2) | 2 (18.2) |
| Anemia | 5 (45.4) | 3 (27.2) | 0 |
| Thrombocytopenia | 3 (27.2) | 1 (0.1) | 0 |
| Cutaneous
toxicity | 2 (18.2) | 2 (18.2) | 2 (18.2) |
|
Nausea/vomiting | 2 (18.2) | 2 (18.2) | 0 |
| Hepatotoxicity | 4 (36.3) | 3 (27.2) | 0 |
| Asthenia | 3 (27.2) | 1 (0.1) | 0 |
| Blood creatine
phosphokinase increased | 2 (18.2) | 0 | 0 |
Discussion
In the present retrospective study, median PFS was 9
months in the 11 patients with recurrent ovarian cancer and a PFI
of 6–12 months receiving the combination of trabectedin + PLD. This
is in good general agreement with data from the OVA-301 trial where
patients with a PFI of 6–12 months receiving the same combination
were found to have a median PFS of 7.4 months (13). Our data further reinforce the utility
of trabectedin + PLD in patients with relapsed ovarian cancer, and
especially in those with partial platinum sensitivity. Considering
the available data, trabectedin and PLD appears to be a valid
therapeutic option for second and further-line therapy in patients
experiencing relapse after 6–12 months following first-line therapy
with carboplatin-paclitaxel-bevacizumab.
In the final analysis of OVA-301, median OS in the
overall population for the trabectedin + PLD and PLD arms was 22.2
and 18.9 months, respectively (HR, 0.86; P=0.0835) (14). Moreover, in an exploratory analysis,
the subset of patients with a PFI of 6–12 months had the largest
difference in OS (22.4 vs. 16.4 months with HR=0.64; P=0.0027). As
noted previously, the positive trend toward a survival advantage
with the trabectedin + PLD compared to PLD alone in the PFI 6- to
12-month subgroup of patients does not appear to be related to the
effects of subsequent therapies received by the patients after
discontinuing study medication (12). In fact, about the same proportion of
patients in each group received subsequent therapy, and patients
treated with trabectedin + PLD actually had a slightly lower
proportion of additional platinum-based treatment than did patients
treated with PLD alone. In OVA-301, the administration of
additional platinum-containing lines of therapy was significantly
delayed for patients receiving trabectedin + PLD, which
significantly prolonged survival starting from the initiation of
subsequent platinum. Accordingly, the survival benefits with
trabectedin + PLD compared to PLD alone in patients with partially
platinum-sensitive disease is possibly due to extension of the PFI
through resensitization of tumors to platinum by trabectedin
(7,15). In addition to an advantage in
survival the increased interval before reinitiating therapy also
provides the patient more time to recover from the adverse effects
of previous platinum therapy.
Our results are also similar to those published in a
recent real-life study in 17 patients treated with trabectedin
alone or combined with PLD (16). In
that study, median PFS was 6.7 months, while median OS was 17.6
months. Positive experience in a smaller real-life setting was also
reported in 6 patients; the authors further documented a
heavily-treated patient with advanced recurrent ovarian cancer
treated with trabectedin + PLD at fourth-line who was later
rechallenged at seventh-line, with treatment continuing until
disease progression (17). The
findings are also in broad agreement with a small retrospective
analysis of heavily pretreated patients with platinum-sensitive
ovarian cancer in which the ORR was 32.4% with median OS of 16.3
months; most responses were in (9 of 11) patients with partially
platinum-sensitive disease (18).
Regarding tolerability, there were no unexpected
safety signals in the present cohort of patients. In the OVA-301
trial, the safety profile in the subgroup of patients with a PFI of
6–12 months was not different from that in the overall population,
even if compared with single-agent PLD, trabectedin + PLD was
associated with a higher incidence of transient neutropenia and
transaminase elevations (12).
Moreover, the addition of trabectedin to PLD did not lead to a
decrease in overall health status as assessed by patient reported
outcomes. Grade 3 neutropenia was observed in ≤20% of patients, and
treatment was neither delayed nor discontinued, and no dose
adjustments were needed. This finding is consistent with the
real-life study by Moriceau where the most frequent grade 3–4
toxicities were neutropenia (24%) and nausea/vomiting (24%)
(16). Thus, trabectedin combined
with PLD appears to be both effective and well tolerated in a
real-life setting of women with recurrent ovarian cancer, and the
toxicity of this combination is predictable and manageable. This is
an important aspect as there is a high unmet need for new and
tolerable therapies.
A good tolerability profile for trabectedin was also
reported in the large case study of Araki comparing trabectedin
with the best supportive care in patients with advanced
translocation-related sarcoma after failure of standard
chemotherapy (19) and in a recent
case report of our group where the patient continued the treatment
with trabectedin for a total of 30 months without significant
toxicity (20).
Of note, the trabectedin + PLD combination has been
recommended by ESMO in patients with relapsed partially
platinum-sensitive ovarian cancer (21). While the limitations of the present
study include its retrospective design and small number of
patients, our results nonetheless reinforce the validity of
trabectedin + PLD in patients with relapsed ovarian cancer and
partial platinum sensitivity.
Acknowledgements
Not applicable.
Funding
The present study was supported by PharmaMar, S.A.
(Madrid, Spain).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KT, LV and AV conceived and designed the study. VR
and LV collected the patient data. SN, KT, LV, VR and AV
participated in data analysis and interpretation. LV, AV, GA, TM
and EM drafted and revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained at the time of
original data collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PFI
|
platinum-free interval
|
|
NER
|
nucleotide excision repair
|
|
PLD
|
pegylated liposomal doxorubicin
|
References
|
1
|
Colombo N: Optimizing treatment of the
partially platinum-sensitive ovarian cancer patient. Future Oncol.
9 12 Suppl:S19–S23. 2013. View Article : Google Scholar
|
|
2
|
Friedlander M, Trimble E, Tinker A,
Alberts D, Avall-Lundqvist E, Brady M, Harter P, Pignata S,
Pujade-Lauraine E, Sehouli J, et al: Clinical trials in recurrent
ovarian cancer. Int J Gynecol Cancer. 21:771–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
González-Martín A: Update on relapsed
ovarian cancer treatment: From new consensus to daily clinical
practice. Future Oncol. 13(23s): 1–9. 2017. View Article : Google Scholar
|
|
4
|
Gore ME, Fryatt I, Wiltshaw E and Dawson
T: Treatment of relapsed carcinoma of the ovary with cisplatin or
carboplatin following initial treatment with these compounds.
Gynecol Oncol. 36:207–211. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
López-Guerrero JA, Romero I and Poveda A:
Trabectedin therapy as an emerging treatment strategy for recurrent
platinum-sensitive ovarian cancer. Chin J Cancer. 34:41–49. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
González-Martín A and du Bois A: Factors
to consider and questions to ask in the management of recurrent
ovarian cancer: A focus on the role of trabectedin + pegylated
liposomal doxorubicin. Expert Rev Anticancer Ther. 16 Sup1:S3–S10.
2016. View Article : Google Scholar
|
|
8
|
D'Incalci M and Galmarini CM: A review of
trabectedin (ET-743): A unique mechanism of action. Mol Cancer
Ther. 9:2157–2163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guirouilh-Barbat J, Redon C and Pommier Y:
Transcription-coupled DNA double-strand breaks are mediated via the
nucleotide excision repair and the Mre11-Rad50-Nbs1 complex. Mol
Biol Cell. 19:3969–3981. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allavena P, Signorelli M, Chieppa M, Erba
E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni
A, et al: Anti-inflammatory properties of the novel antitumor agent
yondelis (trabectedin): Inhibition of macrophage differentiation
and cytokine production. Cancer Res. 65:2964–2971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monk BJ, Herzog TJ, Kaye SB, Krasner CN,
Vermorken JB, Muggia FM, Pujade-Lauraine E, Lisyanskaya AS, Makhson
AN, Rolski J, et al: Trabectedin plus pegylated liposomal
Doxorubicin in recurrent ovarian cancer. J Clin Oncol.
28:3107–3114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poveda A, Vergote I, Tjulandin S, Kong B,
Roy M, Chan S, Filipczyk-Cisarz E, Hagberg H, Kaye SB, Colombo N,
et al: Trabectedin plus pegylated liposomal doxorubicin in relapsed
ovarian cancer: Outcomes in the partially platinum-sensitive
(platinum-free interval 6–12 months) subpopulation of OVA-301 phase
III randomized trial. Ann Oncol. 22:39–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaye SB, Colombo N, Monk BJ, Tjulandin S,
Kong B, Roy M, Chan S, Filipczyk-Cisarz E, Hagberg H, Vergote I, et
al: Trabectedin plus pegylated liposomal doxorubicin in relapsed
ovarian cancer delays third-line chemotherapy and prolongs the
platinum-free interval. Ann Oncol. 22:49–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monk BJ, Herzog TJ, Kaye SB, Krasner CN,
Vermorken JB, Muggia FM, Pujade-Lauraine E, Park YC, Parekh TV and
Poveda AM: Trabectedin plus pegylated liposomal doxorubicin (PLD)
versus PLD in recurrent ovarian cancer: Overall survival analysis.
Eur J Cancer. 48:2361–2368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colombo N: Optimising the treatment of the
partially platinum-sensitive relapsed ovarian cancer patient. EJC
Suppl. 12:7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moriceau G, Rivoirard R, Méry B, Vallard
A, Pacaut C, Trone JC, Espenel S, Bosacki C, Jacquin JP and Magné
N: Real-world outcomes of combination chemotherapy with trabectedin
plus pegylated liposomal doxorubicin in patients with recurrent
ovarian cancer: a single-center experience. Chemotherapy.
61:122–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tahir S: Real-life experience using
trabectedin plus pegylated liposomal doxorubicin combination to
treat patients with relapsed ovarian cancer. EJC Suppl. 12:17–20.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nicoletto MO, Baldoni A, Casarin A, Randon
G, Nardin M, Baretta Z, Lardelli P, Nieto A, Alfaro V, Rigamonti C
and Conte PF: Trabectedin plus pegylated liposomal doxorubicin:
Retrospective analysis in heavily pretreated platinum-sensitive
ovarian cancer. Tumori. 101:506–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Araki N, Takahashi S, Sugiura H, Ueda T,
Yonemoto T, Takahashi M, Morioka H, Hiraga H, Hiruma T, Kunisada T,
et al: Retrospective inter- and intra-patient evaluation of
trabectedin after best supportive care for patients with advanced
translocation-related sarcoma after failure of standard
chemotherapy. Eur J Cancer. 56:122–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tavella K, Villanucci A, Vannini L,
Lavacchi D, Montelatici S, Amunni G and Mazzei T: Stable disease in
a patient with metastatic leiomyosarcoma treated with trabectedin.
Anticancer Drugs. 28:465–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ledermann JA, Raja FA, Fotopoulou C,
Gonzalez-Martin A, Colombo N and Sessa C; ESMO, Guidelines Working
Group, : Newly diagnosed and relapsed epithelial ovarian carcinoma:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 Suppl 6:vi24–vi32. 2013. View Article : Google Scholar : PubMed/NCBI
|