Introduction
Lung cancer remains the leading cause of
cancer-related mortality in Japan, with non-small cell lung cancer
(NSCLC) accounting for 87% of all cases (1); death from disease recurrence occurs in
~20% of patients with stage I and II NSCLC who underwent surgery
(2). The expression levels and
prognostic value of several genes have been investigated in lung
cancer (3,4), but the results are inconclusive.
It is important to identify the most suitable
chemotherapeutic drugs for high-risk patients following complete
resection of early-stage NSCLC. Thymidine phosphorylase (TP),
dihydropyrimidine dehydrogenase (DPD), thymidylate synthase (TS),
and orotate phosphoribosyltransferase (OPRT) are all key enzymes in
the 5-fluorouracil (5-FU) metabolic pathway and are prognostic and
predictive factors in several types of cancer (5–8). The
combination of uracil and tegafur (often referred to as UFT)
administered orally has been shown to improve the overall survival
of patients with stage IB adenocarcinoma following complete
resection, and is recommended for such patients by the Japan Lung
Cancer Society (9,10).
The primary aim of the present study was to
investigate the association between the disease-specific survival
(DSS) of patients with stage I and II NSCLC and the mRNA levels of
TP, DPD, TS, and OPRT. The secondary aim was to
evaluate the association between the mRNA levels of these factors
and the pathological characteristics of this patient
population.
Materials and methods
Patients and clinical tissue
samples
Intratumoral mRNA levels were measured in 115
patients with lung cancer at Showa University Hospital (Tokyo,
Japan) between January 1998 and December 2007. After excluding
those with hilar or mediastinal lymph node metastasis, distant
metastasis, and pulmonary metastasis, 66 patients who underwent R0
resection for pathological stage I and II NSCLC (adenocarcinoma or
squamous cell carcinoma) were enrolled in the study. All patients
underwent radical lobectomy with lymph node dissection. Surgical
resection is the most effective treatment for NSCLC localized in
the lung. However, there are currently no criteria other than stage
for selecting cases that require postoperative adjuvant therapy. In
some cases, recurrence resulting in death may occur even after
complete resection of NSCLC. Therefore, NSCLC patients with stage I
and II disease, without lymph node, distant, or pulmonary
metastasis, were selected. Pathological classification was
determined according to the 7th Edition of the Union for
International Cancer Control TNM Classification (11). All specimens were formalin
(10%)-fixed and paraffin-embedded (FFPE), and were reviewed by
pathologists at our institution. None of the patients received
preoperative chemo- or radiotherapy. Blood counts, blood
biochemistry tests, serum tumor marker level assessment and chest
roentgenogram were performed every 2 or 3 months in the first 2
years after surgery and every 6 months in the subsequent 3 years.
Furthermore, a chest computed tomography (CT) scan was performed
once or twice per year. Positron emission tomography-CT, brain
magnetic resonance imaging and bone scintigraphy were performed
when tumor recurrence or a second primary malignancy was suspected.
The protocol of the present study was approved by the Showa
University Ethics Committee, and written informed consent was
obtained from all participating patients.
Danenberg tumor profile (DTP)
method
The DTP method (12)
is used to evaluate mRNA expression levels in FFPE specimens and is
superior to other methods in terms of accuracy and practicality.
Standard polymerase chain reaction (PCR), biochemical assays and
most other conventional techniques are impractical due to the
requirement for fresh samples that are often difficult to store. In
addition, other proposed methods have low accuracy and precision,
since it is difficult to distinguish between cancerous stroma and
normal tissues in fresh-frozen specimens. The DTP method overcomes
these problems by determining the mRNA expression profiles of FFPE
specimens. The procedure has been previously described in detail
(13–15). The FFPE tumor specimens included in
the present study were selected by an experienced pathologist
following examination of hematoxylin and eosin-stained slides.
Sections (10-µm) were stained with neutral fast red to visualize
histological characteristics during laser capture microdissection.
RNA was isolated from the FFPE specimens using a novel proprietary
procedure (United States Patent Number 6,248,535; Response
Genetics, Los Angeles, CA, USA). After RNA isolation, cDNA was
derived from each sample as previously described (13). Quantification of the four genes of
interest (TP, DPD, TS and OPRT) and an internal
reference gene (β-actin) was performed with a
fluorescence-based quantitative PCR system [ABI PRISM 7900 Sequence
Detection System (TaqMan); Applied Biosystems, Foster City, CA,
USA]. The PCR reaction mixture contained primers, dATP, dCTP, dGTP
and dUTP, MgCl2 and TaqMan buffer; the final volume of
the reaction mixture, cycling conditions, primers and probes have
been previously described (14). The
assay yielded quantification cycle (Cq) values that were inversely
proportional to the amount of cDNA in the reaction. The relative
mRNA levels are expressed as the ratio (difference between Cq
values) of the gene of interest and β-actin. Therefore, the
expression level of β-actin mRNA in the same tissues was
used as the control in our study.
Statistical analysis
Spearman's rank correlation coefficient was used to
assess correlations among the mRNA expression levels of TP, DPD,
TS and OPRT and other continuous parameters. The
Mann-Whitney U test or the Kruskal-Wallis test was used to compare
variables, and DSS was estimated with the Kaplan-Meier method. The
mRNA expression levels were evaluated with the DTP method, and the
patients were divided into high and low expression groups according
to the mean mRNA level of TP, DPD, TS, or OPRT.
Differences in DSS were evaluated with a stratified log-rank test.
Multivariate analyses with a Cox proportional hazards model were
used to estimate the simultaneous effects of prognostic factors on
DSS, which was defined as the time from surgery until death from
NSCLC. Interactions of prognostic factors were also examined using
the Cox proportional hazards model. JMP statistical software
package, version Pro12.0 (SAS Institute, Cary, NC, USA) was used
for all calculations. The level of significance was set at
P<0.05. All statistical tests were two-sided.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The study included 66
patients (median age, 68.5 years; range, 33–85 years), with 45
(68.2%) men and 21 (31.8%) women; 50 (75.8%) of the patients had
adenocarcinoma and 16 (24.2%) had squamous cell carcinoma.
Histologically, the extent of the disease ranged from stage IA to
IIB (IA, n=29; IB, n=26; IIA, n=5; and IIB, n=6) and pathological
N0 disease was confirmed in all patients. A total of 33 tumors were
well-differentiated, 24 were moderately differentiated and 9 were
poorly differentiated, and the median maximum diameter was 26.5 mm
(range, 7–80 mm). The median preoperative serum level of
carcinoembryonic antigen was 3.4 ng/ml (range, 0.8–308.2 ng/ml).
Pleural invasion, vascular invasion and lymphatic permeation were
confirmed in 25 (37.9%), 45 (68.2%) and 36 (54.5%) patients,
respectively. No patient received induction chemo- or radiotherapy
or molecular-targeted therapy. Postoperative adjuvant therapy
included platinum-based chemotherapy in 5 patients and UFT
administration in 19 patients. Tumor recurrence occurred in 28
patients (42.4%), who were then treated by platinum-based
chemotherapy (n=15), chemoradiotherapy (n=5) and surgery (n=5); the
remaining 3 patients received no treatment for tumor recurrence due
to their poor general condition. The median follow-up period was
76.5 months (range, 2–191 months).
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
|
Characteristics | No. |
|---|
| Age, years |
| Median
(range) | 68.5 (33–85) |
|
| Sex |
|
Male | 45 | 68.2% |
|
Female | 21 | 31.8% |
| Pathological
type |
|
Adenocarcinoma | 50 | 75.8% |
|
Squamous cell carcinoma | 16 | 24.2% |
| Pathological
stage |
| IA | 29 | 43.9% |
| IB | 26 | 39.4% |
|
IIA | 5 | 7.6% |
|
IIB | 6 | 9.1% |
| Differentiation
degree |
| Well
differentiated | 33 | 50.0% |
|
Moderately differentiated | 24 | 36.4% |
| Poorly
differentiated | 9 | 13.6% |
| Maximum tumor
diameter, mm |
| Median
(range) | 26.5 (7–80) |
|
| Preoperative serum
CEA levels, ng/ml |
| Median
(range) | 3.4
(0.8–308.2) |
|
| Pleural
invasion |
|
Negative | 41 | 62.1% |
|
Positive | 25 | 37.9% |
| Vascular
invasion |
|
Negative | 21 | 31.8% |
|
Positive | 45 | 68.2% |
| Lymphatic
permeation |
|
Negative | 30 | 45.4% |
|
Positive | 36 | 54.5% |
| UFT administration
following surgery |
|
UFT | 19 | 28.8% |
| Surgery
alone | 47 | 71.2% |
| Follow-up period,
months |
| Median
(range) | 76.5 (2–191) |
|
The correlations between the mRNA levels of TP,
DPD, TS, and OPRT genes and clinicopathological factors
are shown in Table II. The mean
expression levels of TP, DPD, TS, and OPRT in NSCLC
patients were 9.03 (range, 0.53–40.48), 1.96 (range, 0.20–6.15),
2.45 (range, 0.36–18.54) and 0.71 (range, 0.16–4.42), respectively.
TP levels were higher in patients with vascular invasion.
DPD levels were negatively correlated with maximum tumor
diameter (P<0.001, r=−0.553), and were higher in patients with
adenocarcinoma compared with those with squamous cell carcinoma
(P<0.001), whereas OPRT mRNA expression levels were
higher in patients with squamous cell carcinoma compared with those
with adenocarcinoma (P<0.001). TS levels were higher in
patients with more poorly differentiated NSCLC (P=0.033). There was
also a positive correlation between TS and OPRT
levels (P<0.001, r=0.542).
 | Table II.Association between
clinicopathological variables and mRNA expression levels of four
key enzymes in non-small-cell lung cancer. |
Table II.
Association between
clinicopathological variables and mRNA expression levels of four
key enzymes in non-small-cell lung cancer.
| Variables | Patient no. | TP levels, mean
(range) | DPD levels, mean
(range) | TS levels, mean
(range) | OPRT levels, mean
(range) |
|---|
| Sex |
| NS | NS | NS | NS |
|
Male | 45 | 10.23
(0.53–40.48) | 1.85
(0.21–5.88) | 2.26
(0.36–7.08) | 1.09
(0.16–4.42) |
|
Female | 21 | 8.11
(3.29–25.08) | 1.92
(0.20–6.15) | 2.92
(0.52–18.54) | 0.88
(0.22–2.95) |
| Age (years) |
| NS | NS | NS | NS |
|
<70 | 35 | 8.59
(1.76–36.05) | 1.85
(0.21–6.15) | 2.14
(0.55–18.54) | 0.98
(0.22–4.42) |
|
≥70 | 31 | 10.71
(0.53–40.48) | 1.89
(0.20–4.73) | 2.84
(0.36–12.82) | 1.07
(0.16–3.04) |
| Histology |
| NS | P<0.001 | NS | P<0.001 |
|
Adenocarcinoma | 50 | 9.19
(2.22–36.05) | 2.16
(0.35–6.15) | 2.15
(0.36–12.82) | 0.80
(0.16–2.90) |
|
Squamous cell carcinoma | 16 | 10.85
(0.53–40.48) | 0.95
(0.20–3.77) | 3.45
(0.74–18.54) | 1.72
(0.49–4.42) |
| Differentiation
degree |
| NS | NS | P=0.033 | NS |
|
Well | 33 | 7.91
(2.99–13.84) | 2.20
(0.37–6.15) | 1.69
(0.36–6.62) | 0.84
(0.22–4.42) |
|
Moderately | 24 | 9.48
(0.53–25.95) | 1.68
(0.20–4.64) | 2.68
(0.52–7.08) | 1.15
(0.16–3.04) |
|
Poorly | 9 | 10.13
(2.22–40.48) | 1.18
(0.42–4.73) | 4.76
(0.76–18.54) | 1.37
(0.32–2.95) |
| Preoperative CEA
serum level (ng/ml) |
| NS | NS | NS | NS |
|
<5.0 | 48 | 9.10
(0.53–40.48) | 1.98
(0.20–6.15) | 2.39
(0.36–18.54) | 0.94
(0.22–3.33) |
|
≥5.0 | 18 | 10.89
(2.99–36.05) | 1.83
(0.37–4.64) | 2.67
(1.04–7.08) | 1.06
(0.16–4.42) |
| Pleural
invasion |
| NS | NS | NS | NS |
|
Negative | 41 | 8.71
(0.53–40.48) | 2.13
(0.28–6.15) | 2.44
(0.45–6.62) | 0.95
(0.16–3.33) |
|
Positive | 25 | 10.12
(2.09–36.05) | 1.44
(0.20–4.64) | 2.46
(0.36–18.54) | 1.07
(0.22–4.42) |
| Vascular
invasion |
| P=0.042 | NS | NS | NS |
|
Negative | 21 | 6.8
(2.09–16.83) | 2.27
(0.35–4.64) | 1.80
(0.36–18.54) | 0.83
(0.25–3.33) |
|
Positive | 45 | 10.89
(0.53–40.48) | 1.68
(0.20–6.15) | 3.02
(0.45–7.08) | 1.12
(0.16–4.42) |
| Lymphatic
permeation |
| NS | NS | NS | NS |
|
Negative | 30 | 8.06
(1.76–24.43) | 2.09
(0.21–5.88) | 1.79
(0.45–7.08) | 0.94
(0.16–4.92) |
|
Positive | 36 | 10.89
(0.53–40.48) | 1.68
(0.20–6.15) | 3.02
(0.36–18.54) | 1.09
(0.25–2.95) |
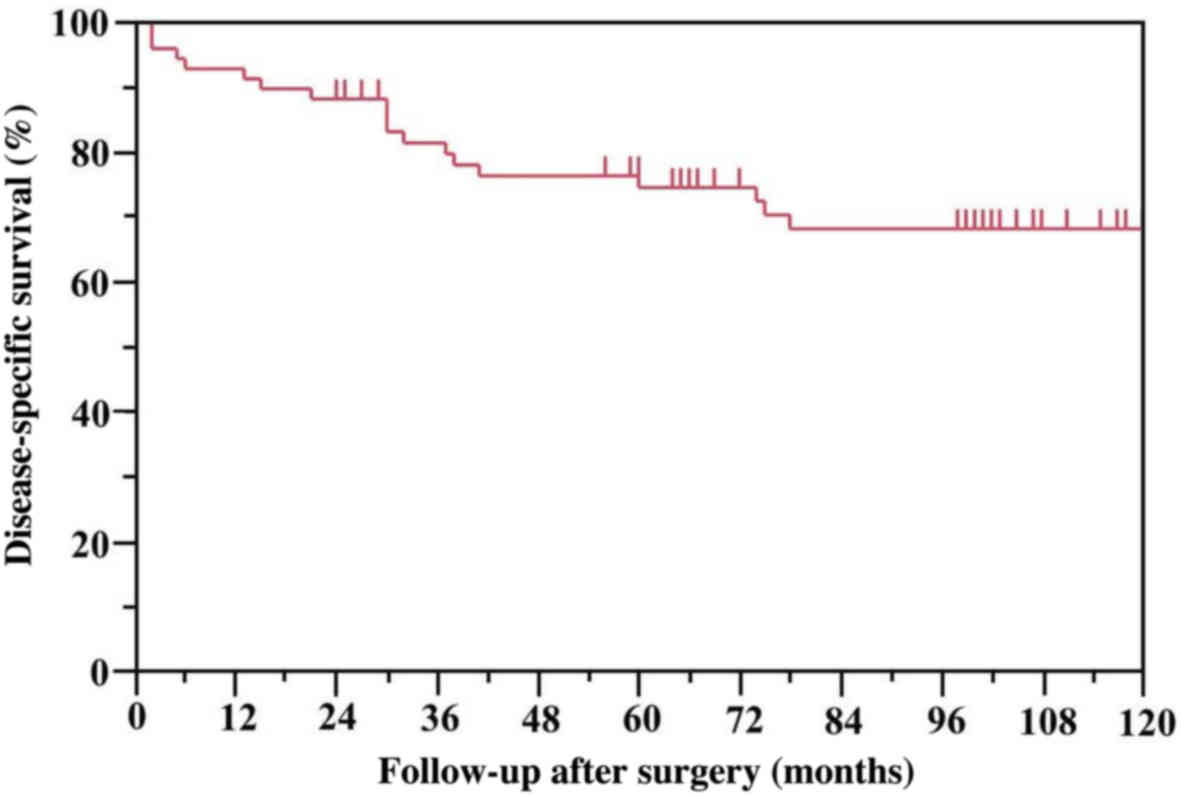
The 5-year DSS rate of all 66 patients who underwent
complete resection was 74.2% (Fig.
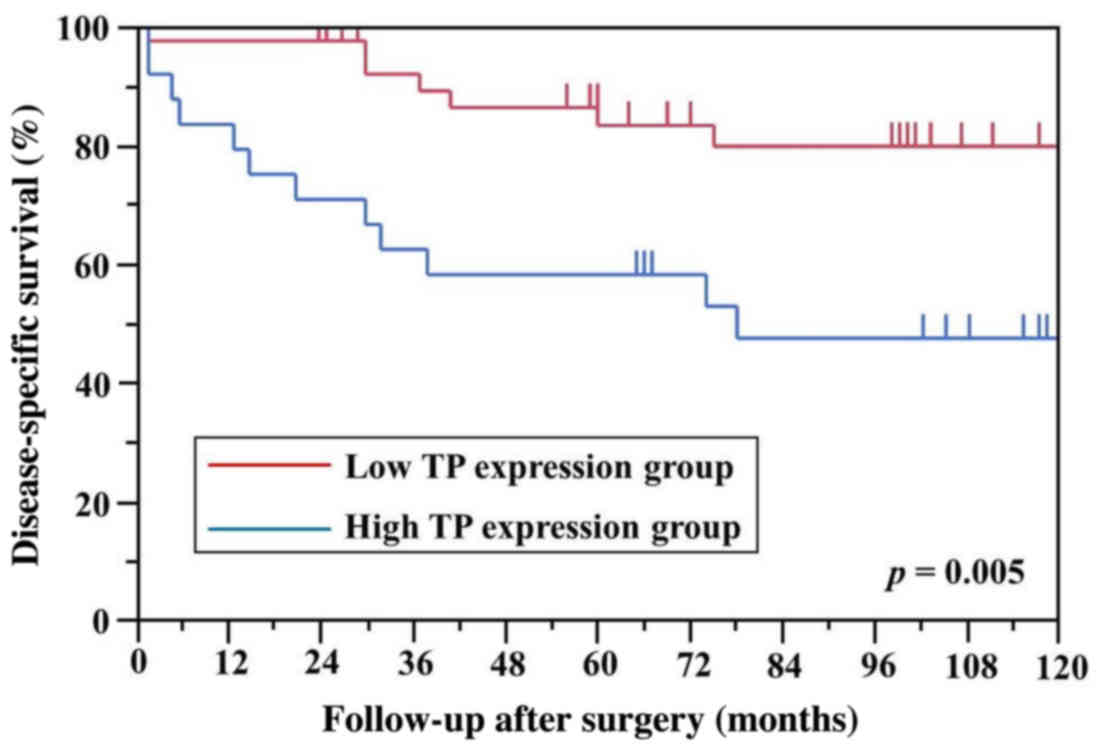
1). Among the examined genes, only TP levels differed
significantly between the high and low mRNA expression groups for
DSS (Fig. 2). There were no
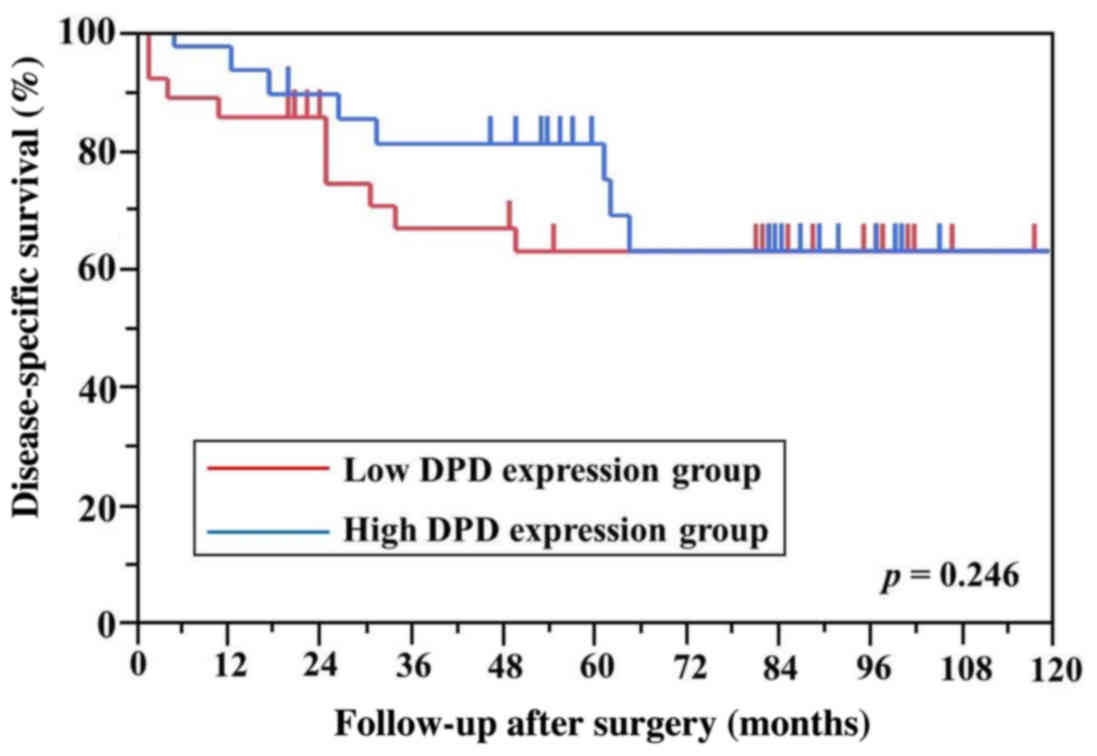
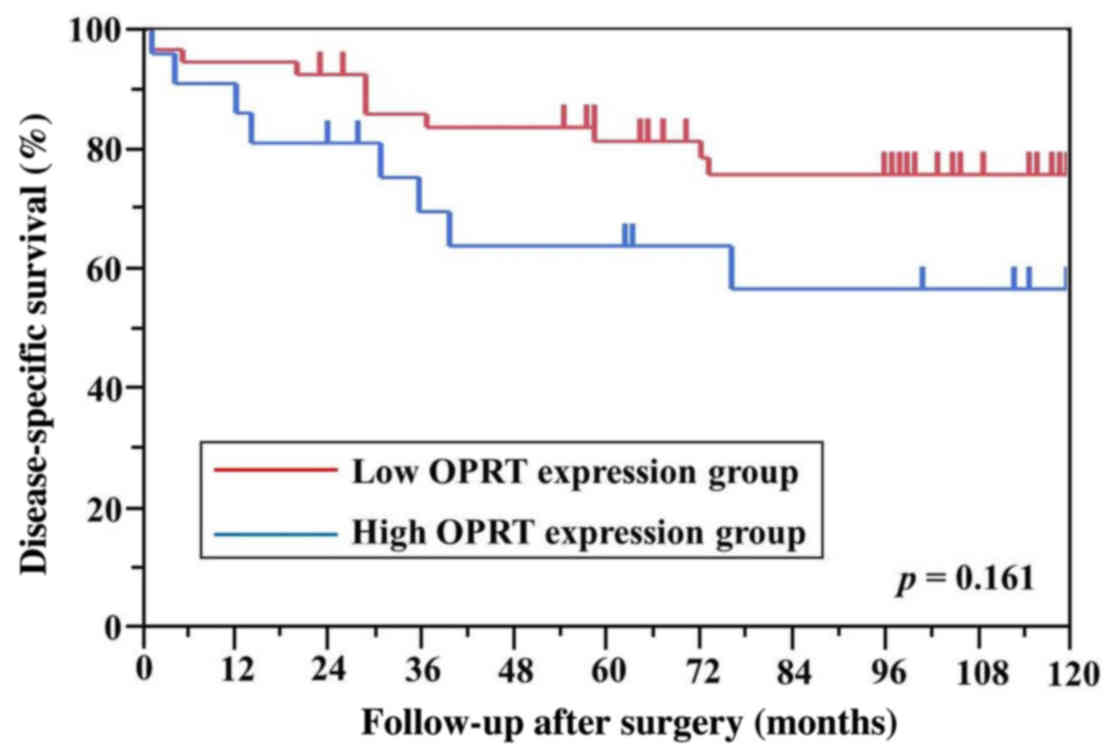
differences in DPD, TS and OPRT levels between the
high and low mRNA expression groups for DSS (Figs. 3–5). A
univariate analysis revealed that DSS was better in patients who
were female, those with pathological stage I NSCLC and those with
low TP mRNA levels, and the Cox proportional hazards model
revealed that sex, pathological stage, and TP mRNA
expression were independent prognostic factors for DSS (Table III).
 | Table III.Cox analysis of potential prognostic
factors of DSS: Univariate and multivariate analysis. |
Table III.
Cox analysis of potential prognostic
factors of DSS: Univariate and multivariate analysis.
| Variables | n | 5-year DSS (%) | P-value | HR | 95% CI | P-value |
|---|
| Age (<70 vs. ≥70
years) | 35/31 | 76.4/71.5 | 0.506 |
|
|
|
| Sex (male vs.
female) | 45/21 | 63.9/95.0 | 0.003 | 7.85 | 1.56–14.30 | 0.047 |
| Adenocarcinoma vs.
squamous cell carcinoma | 50/16 | 76.9/67.7 | 0.144 |
|
|
|
| Pathological stage
(I vs. II) | 55/11 | 80.7/40.9 | 0.003 | 0.19 | 0.06–0.64 | 0.005 |
| Well vs. moderately
differentiated | 33/24 | 80.6/73.5 | 0.218 |
|
|
|
| Moderately vs.
poorly differentiated | 24/9 | 73.5/55.6 | 0.386 |
|
|
|
| Well vs. poorly
differentiated | 33/9 | 80.6/55.6 | 0.054 |
|
|
|
| Preoperative serum
CEA levels (low vs. high) | 48/18 | 76.9/63.5 | 0.665 |
|
|
|
| Pleural invasion
(negative vs. positive) | 41/25 | 79.8/64.0 | 0.267 |
|
|
|
| Vascular invasion
(negative vs. positive) | 21/45 | 93.3/62.0 | 0.052 |
|
|
|
| Lymphatic
permeation (negative vs. positive) | 30/36 | 82.9/58.6 | 0.072 |
|
|
|
| TP (low vs. high)
mean: 9.03 | 41/25 | 83.4/58.6 | 0.005 | 0.19 | 0.06–0.56 | 0.003 |
| DPD (low vs. high)
mean: 1.96 | 35/31 | 62.3/84.6 | 0.246 |
|
|
|
| TS (low vs. high)
mean: 2.45 | 39/27 | 81.1/67.0 | 0.165 |
|
|
|
| OPRT (low vs. high)
mean: 0.71 | 34/32 | 81.4/66.5 | 0.161 |
|
|
|
Discussion
In the present study, the association between the
expression levels of key enzymes associated with 5-FU metabolism
and the pathological characteristics of patients with stage I and
II NSCLC was investigated. The TS expression level was low
in patients with well-differentiated NSCLC; DPD was
downregulated in patients with squamous cell carcinoma, which was
negatively correlated with maximum tumor diameter; TP was
highly expressed in patients with vascular invasion; and the
OPRT level was decreased in patients with adenocarcinoma.
The reason for these findings is not known; however, it was
reported that adenocarcinoma in situ, previously classified
as bronchioloalveolar carcinoma (16), has a significantly higher epidermal
growth factor receptor (EGFR) mutation frequency and DPD
mRNA levels compared with other histological types (17), which may explain the high DPD
mRNA levels observed in adenocarcinoma. On the other hand, OPRT
activity is known to be increased in rapidly growing cells,
including normal cells, such as those in the testis (18). Squamous cell carcinoma exhibited
higher OPRT mRNA levels compared with adenocarcinoma, due to
a shorter doubling time (19). TS is
an enzyme that plays an important role in DNA biosynthesis and
repair. Evidence from in vitro and in vivo studies
indicates that TS contributes to cancer development through cell
cycle regulation (20). More poorly
differentiated carcinomas exhibit higher rates of tumor cell
proliferation. Thus, an increased TS expression level in
NSCLC likely reflects poor differentiation.
The observed associations between the mRNA levels of
factors related to 5-FU metabolism and pathological characteristics
in this study are in agreement with previous reports (21–24).
TP mRNA level was found to be an independent prognostic
factor for DSS in NSCLC. TP is a nucleoside metabolic enzyme that
plays an important role in the pyrimidine salvage pathway. 5-FU is
transformed into a deoxyribose fluorouracil nucleoside
monophosphate through TP, which forms a complex with methylene
tetrahydrofolate that inhibits TS activity, thereby interfering
with DNA replication and inhibiting tumor cell growth and
proliferation. DPD is the initial and rate-limiting enzyme in 5-FU
catabolism. In vivo, >85% of 5-FU is reduced to inactive
metabolites by enzymes produced in the liver and other tissues,
which are then excreted by the kidneys (25).
TP catalyzes the reversible conversion of thymidine
to thymine and 2-deoxy-α-d-ribose-1-phosphate, and the
phosphorolysis of deoxyuridine to uracil and
2-deoxy-α-d-ribose-1-phosphate (26). A major function of TP is to control
the intracellular levels of thymidine, which is cytotoxic at high
concentrations and causes errors in DNA replication. Thus, TP
expression is crucial for the efficacy of 5-FU-based chemotherapy
(25,26).
Apart from its role as a nucleoside metabolism
enzyme, TP also functions as an angiogenic factor that is identical
to platelet-derived endothelial cell growth factor (PD-ECGF)
(27). TP enhances angiogenesis in
the tumor via two distinct mechanisms: By stimulating endothelial
cell migration, and the release of angiogenic factors from
malignant cells and stromal cells into the tumor microenvironment.
TP-expressing cells were shown to secrete angiogenic factors
(interleukin-8, basic fibroblast growth factor and tumor necrosis
factor-α) that stimulated endothelial cell migration and invasion,
but not proliferation (26). It was
also reported that a high level of TP caused more aggressive cancer
growth with a higher incidence of vascular infiltration and
metastasis in breast, colorectal and gastric cancers (28–30).
PD-ECGF/TP has been implicated in the pathogenesis of NSCLC, and
its upregulation defines a more aggressive tumor phenotype
associated with a poor prognosis, particularly in cases without
nodal involvement. However, PD-ECGF/TP expression was unrelated to
the degree of differentiation, nodal status and histology, or the
expression of Ki67, EGFR and p53 (31).
The ‘Nottingham Prognostic Index’ is an evaluation
of breast cancer based on tumor diameter, lymph node metastasis,
and differentiation level (32).
Therefore, we have not used this score in our study, because cases
with lymph node metastasis were excluded, and the tumor diameter
was reflected almost exactly by pathological stage. Moreover, it is
not suitable for evaluating angiogenesis.
In this study, the low TP expression group
had better DSS compared with the high expression group in stage I
and II NSCLC. The TP expression level was also higher in
patients with vascular invasion. These results indicate that
TP expression is a potential marker for tumor malignancy,
including vascular invasion or micrometastasis. However, our study
had certain limitations: adjuvant chemotherapy was administered
according to the physician's preference. Moreover, we were unable
to retrieve information on certain patient characteristics, such as
smoking habit, other angiogenic factors, and mutational status. In
addition, we did not measure the enzyme expression levels in the
normal lung tissue or in the healthy tissue surrounding the lung
cancer tissue. Finally, considering that an optimal cut-off value
for mRNA expression level was not defined in previous studies, we
considered the mean value as the cut-off value based on the
following thoughts (33–36). Enzyme expression levels are presented
in a quantitative manner and there are no excessive differences
between the expression levels of distinct enzymes.
There have been few investigations on the utility of
TP as a prognostic factor for patients with completely resectable
NSCLC, whereas no studies to date have quantitatively analyzed TP
expression in this patient population (31,37). Our
results demonstrated that intratumoral TP expression is an
important prognostic factor in patients with completely resected
NSCLC without metastasis. Thus, additional and more powerful
adjuvant therapies should be considered for early-stage NSCLC with
high TP mRNA levels in order to improve patient outcome.
Acknowledgements
The authors would like to thank Taiho Pharmaceutical
Co., Ltd. (Tokyo, Japan) for performing the molecular analyses in
this study.
Funding
No funding was received.
Availability of data and materials
The data generated and analyzed in the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to the design of the study
and the writing of the manuscript. NH, DK, SY and MK undertook the
research and performed the analyses. All authors reviewed and
approved the final version of the manuscript for publication.
Ethics approval and consent to
participate
The study was conducted with the approval of the
Institutional Ethics Committee at Showa University Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
CT
|
computed tomography
|
|
DPD
|
dihydropyrimidine dehydrogenase
|
|
DSS
|
disease-specific survival
|
|
DTP
|
Danenberg tumor profile
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
NSCLC
|
non-small-cell lung cancer
|
|
OPRT
|
orotate phosphoribosyltransferase
|
|
PCR
|
polymerase chain reaction
|
|
TP
|
thymidine phosphorylase
|
|
TS
|
thymidylate synthase
|
|
UFT
|
uracil and tegafur
|
References
|
1
|
Committee for Scientific Affairs, The
Japanese Association for Thoracic Surgery, . Masuda M, Kuwano H,
Okumura M, Arai H, Endo S, Doki Y, Kobayashi J, Motomura N, et al:
Thoracic and cardiovascular surgery in Japan during 2013: Annual
report by The Japanese Association for Thoracic Surgery. Gen Thorac
Cardiovasc Surg. 63:670–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sawabata N, Miyaoka E, Asamura H,
Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M and
Yokoi K; Japanese Joint Committee for Lung Cancer Registration, :
Japanese lung cancer registry study of 11,663 surgical cases in
2004: Demographic and prognosis changes over decade. J Thorac
Oncol. 6:1229–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brabender J, Park J, Metzger R, Schneider
PM, Lord RV, Hölscher AH, Danenberg KD and Danenberg PV: Prognostic
significance of cyclooxygenase 2 mRNA expression in non-small cell
lung cancer. Ann Surg. 235:440–443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grimminger P, Ling FC, Neiss S, Vallböhmer
D, Lurje G, Schneider PM, Hölscher AH, Metzger R and Brabender J:
The role of the homeobox genes BFT and CDX2 in the pathogenesis of
non-small cell lung cancer. Anticancer Res. 29:1281–1286.
2009.PubMed/NCBI
|
|
5
|
Farrugia DC, Ford HE, Cunningham D,
Danenberg KD, Danenberg PV, Brabender J, McVicar AD, Aherne GW,
Hardcastle A, McCarthy K and Jackman AL: Thymidylate synthase
expression in advanced colorectal cancer predicts for response to
raltitrexed. Clin Cancer Res. 9:792–801. 2003.PubMed/NCBI
|
|
6
|
Lenz HJ, Leichman CG, Danenberg KD,
Danenberg PV, Groshen S, Cohen H, Laine L, Crookes P, Silberman H,
Baranda J, et al: Thymidylate synthase mRNA level in adenocarcinoma
of the stomach: A predictor for primary tumor response and overall
survival. J Clin Oncol. 14:176–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyoshi Y, Uemura H, Ishiguro H, Kitamura
H, Nomura N, Danenberg PV and Kubota Y: Expression of thymidylate
synthase, dihydropyrimidine dehydrogenase, thymidine phosphorylase,
and orotate phosphoribosyl transferase in prostate cancer. Prostate
Cancer Prostatic Dis. 8:260–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uchida K, Danenberg PV, Danenberg KD and
Grem JL: Thymidylate synthase, dihydropyrimidine dehydrogenase,
ERCC1, and thymidine phosphorylase gene expression in primary and
metastatic gastrointestinal adenocarcinoma tissue in patients
treated on a phase I trial of oxaliplatin and capecitabine. BMC
Cancer. 8:3862008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wada H, Hitomi S and Teramatsu T: Adjuvant
chemotherapy after complete resection in non-small-cell lung
cancer. West Japan Study Group for Lung Cancer Surgery. J Clin
Oncol. 14:1048–1054. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato H, Ichinose Y, Ohta M, Hata E,
Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, et al:
A randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH, Gospodarowicz MK and Wittekind
Ch: International Union Against Cancer (UICC) TNM Classification of
Malignant Tumours. 7th. Oxford, UK: Wiley-Blackwell; 2009
|
|
12
|
Horikoshi T, Danenberg K, Volkenandt M,
Stadlbauer T and Danenberg PV: Quantitative measurement of relative
gene expression in human tumors. Methods Mol Biol. 15:177–188.
1993.PubMed/NCBI
|
|
13
|
Lord RV, Salonga D, Danenberg KD, Peters
JH, DeMeester TR, Park JM, Johansson J, Skinner KA, Chandrasoma P,
DeMeester SR, et al: Telomerase reverse transcriptase expression is
increased early in the Barrett's metaplasia, dysplasia, carcinoma
sequence. Gastrointest Surg. 4:135–142. 2000. View Article : Google Scholar
|
|
14
|
Matsubara J, Nishina T, Yamada Y, Moriwaki
T, Shimoda T, Kajiwara T, Nakajima TE, Kato K, Hamaguchi T, Shimada
Y, et al: Impacts of excision repair cross-complementing gene 1
(ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth
factor receptor on the outcomes of patients with advanced gastric
cancer. Br J Cancer. 98:832–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eguchi K, Oyama T, Tajima A, Abiko T,
Sawafuji M, Horio H, Hashizume T, Matsutani N, Kato R, Nakayama M,
et al: Intratumoral gene expression of 5-fluorouracil
pharmacokinetics-related enzymes in stage I and II non-small cell
lung cancer patients treated with uracil-tegafur after surgery: A
prospective multi-institutional study in Japan. Lung Cancer.
87:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mochinaga K, Tsuchiya T, Nagasaki T, Arai
J, Tominaga T, Yamasaki N, Matsumoto K, Miyazaki T, Nanashima A,
Hayashi T, et al: High expression of dihydropyrimidine
dehydrogenase in lung adenocarcinoma is associated with mutations
in epidermal growth factor receptor: Implications for the treatment
of NSCLC using 5-fluorouracil. Clin Lung Cancer. 15:136–140.e4.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tominaga T, Tsuchiya T, Mochinaga K, Arai
J, Yamasaki N, Matsumoto K, Miyazaki T, Nagasaki T, Nanashima A,
Tsukamoto K and Nagayasu T: Epidermal growth factor signals
regulate dihydropyrimidine dehydrogenase expression in EGFR-mutated
non-small-cell lung cancer. BMC Cancer. 16:3542016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizutani Y, Wada H, Fukushima M, Yoshida
O, Nakanishi H, Li YN and Miki T: Prognostic significance of
orotate phosphoribosyltransferase activity in bladder carcinoma.
Cancer. 100:723–731. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mackintosh JA, Marshall HM, Yang IA,
Bowman RV and Fong KM: A retrospective study of volume doubling
time in surgically resected non-small cell lung cancer.
Respirology. 19:755–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SW, Chen TJ, Lin LC, Li CF, Chen LT,
Hsing CH, Hsu HP, Tsai CJ, Huang HY and Shiue YL: Overexpression of
thymidylate synthetase confers an independent prognostic indicator
in nasopharyngeal carcinoma. Exp Mol Pathol. 95:83–90. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shintani Y, Ohta M, Hirabayashi H, Tanaka
H, Iuchi K, Nakagawa K, Maeda H, Kido T, Miyoshi S and Matsuda H:
Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA
levels in tumor tissues and the efficacy of 5-fluorouracil in
patients with non-small-cell lung cancer. Lung Cancer. 45:189–196.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakano J, Huang C, Liu D, Masuya D,
Nakashima T, Yokomise H, Ueno M, Wada H and Fukushima M:
Evaluations of biomarkers associated with 5-FU sensitivity for
non-small-cell lung cancer patients postoperatively treated with
UFT. Br J Cancer. 95:607–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basaki Y, Chikahisa L, Aoyagi K, Miyadera
K, Yonekura K, Hashimoto A, Okabe S, Wierzba K and Yamada Y:
Gamma-hydroxybutyric acid and 5-fluorouracil, metabolites of UFT,
inhibit the angiogenesis induced by vascular endothelial growth
factor. Angiogenesis. 4:163–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishihama H, Chida M, Araki O, Karube Y,
Seki N, Tamura M, Umezu H, Honma K, Masawa N and Miyoshi S:
Comparison of 5-fluorouracil-related gene expression levels between
adenocarcinomas and squamous cell carcinomas of the lung. Jpn J
Clin Oncol. 39:33–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Che J, Pan L, Yang X, Liu Z, Huang L, Wen
C, Lin A and Liu H: Thymidine phosphorylase expression and
prognosis in colorectal cancer treated with 5-fluorouracil-based
chemotherapy: A meta-analysis. Mol Clin Oncol. 7:943–952.
2017.PubMed/NCBI
|
|
26
|
Elamin YY, Rafee S, Osman N, Byrne O KJ
and Gately K: Thymidine phosphorylase in cancer; enemy or friend?
Cancer Microenviron. 1:33–43. 2016. View Article : Google Scholar
|
|
27
|
Metzger R, Danenberg K, Leichman CG,
Salonga D, Schwartz EL, Wadler S, Lenz HJ, Groshen S, Leichman L
and Danenberg PV: High basal level gene expression of thymidine
phosphorylase (platelet-derived endothelial cell growth factor) in
colorectal tumors is associated with nonresponse to 5-fluorouracil.
Clin Cancer Res. 4:2371–2376. 1998.PubMed/NCBI
|
|
28
|
Toi M, Hoshina S, Taniguchi T, Yamamoto Y,
Ishitsuka H and Tominaga T: Expression of platelet-derived
endothelial cell growth factor/thymidine phosphorylase in human
breast cancer. Int J Cancer. 64:79–82. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amaya H, Tanigawa N, Lu C, Matsumura M,
Shimomatsuya T, Horiuchi T and Muraoka R: Association of vascular
endothelial growth factor expression with tumor angiogenesis,
survival and thymidine phosphorylase/platelet-derived endothelial
cell growth factor expression in human colorectal cancer. Cancer
Lett. 119:227–235. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maeda K, Kang SM, Ogawa M, Onoda N, Sawada
T, Nakata B, Kato Y, Chung YS and Sowa M: Combined analysis of
vascular endothelial growth factor and platelet-derived endothelial
cell growth factor expression in gastric carcinoma. Int J Cancer.
74:545–550. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koukourakis MI, Giatromanolaki A, O'Byrne
KJ, Comley M, Whitehouse RM, Talbot DC, Gatter KC and Harris AL:
Platelet-derived endothelial cell growth factor expression
correlates with tumour angiogenesis and prognosis in non-small-cell
lung cancer. Br J Cancer. 75:477–481. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haybittle JL, Blamey RW, Elston CW,
Johnson J, Doyle PJ, Campbell FC, Nicholson RI and Griffiths K: A
prognostic index in primary breast cancer. Br J Cancer. 45:361–366.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsuji T, Sawai T, Yamashita H, Takeshita
H, Nakagoe T, Shindou H, Fukuoka H, Yoshinaga M, Hidaka S, Yasutake
T, et al: Platelet-derived endothelial cell growth factor
expression is an independent prognostic factor in colorectal cancer
patients after curative surgery. Eur J Surg Oncol. 30:296–302.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsunoda Y, Suzuki K, Tsunoda A, Takimoto M
and Kusano M: Evaluation of 5-fluorouracil related genes in breast
cancer to predict the effect of adjuvant therapy with oral
fluorouracil derivatives. Oncol Rep. 23:771–777. 2010.PubMed/NCBI
|
|
35
|
Nishina T, Hyodo I, Miyaike J, Inaba T,
Suzuki S and Shiratori Y: The ratio of thymidine phosphorylase to
dihydropyrimidine dehydrogenase in tumour tissues of patients with
metastatic gastric cancer is predictive of the clinical response to
5′-deoxy-5-fluorouridine. Eur J Cancer. 40:1556–1571. 2004.
View Article : Google Scholar
|
|
36
|
Ahlin C, Aaltonen K, Amini RM, Nevanlinna
H, Fjällskog ML and Blomqvist C: Ki67 and cyclin A as prognostic
factors in early breast cancer. What are the optimal cut-off
values? Histopathology. 51:491–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kojima H, Shijubo N and Abe S: Thymidine
phosphorylase and vascular endothelial growth factor in patients
with Stage I lung adenocarcinoma. Cancer. 94:1083–1093. 2002.
View Article : Google Scholar : PubMed/NCBI
|