Introduction
In recent years, several immune cells endowed with
potent regulatory properties have been identified. The best-known
are the regulatory T (Treg) cells, which serve a critical role in
modulating immune responses in a variety of conditions (1). By analogy to Treg cells, a small subset
of B-cells, termed regulatory B (Breg) cells, was identified and
found to exert its immunosuppressive effects via the production of
interleukin-10 (IL-10), an inhibitory cytokine also utilized by
Treg cells (2). In addition to their
interaction with Treg cells, Breg cells promote activation of
invariant natural killer (iNKT) cells that also display
immunoregulatory properties and share several functional hallmarks
with Treg cells (3–5). This cross-talk among Treg, Breg and
iNKT cell subsets indicates a link between innate and adaptive
immunity in order to elicit strong immune responses to protect the
body from diseases.
While their immunogenic role is well-established,
some reports revealed that Breg cells may also promote
tumorigenesis and may limit the therapeutic efficacy of B-cell
depletion therapies with rituximab, a monoclonal antibody directed
against the CD20 antigen that is commonly used in the treatment of
patients with B-cell malignancies (6–8).
Interestingly, even small number of residual murine Breg cells was
able to inhibit rituximab activity, and only their total removal
was associated with optimal clearance of malignant B-cells
(8). Collectively, these findings
support the hypothesis that preferential depletion of Breg cells
may enhance tumor-specific immune responses and control tumor
growth. Thus, it is necessary to elucidate the various
immunological barriers in order to optimize and increase the
efficacy of antitumor responses.
Despite the fact that an increasing number of
patients with B-cell malignancies achieve complete remission (CR)
following rituximab-based therapies, a significant number relapse
or are refractory to these treatments. There is a need for novel
markers that have the potential to identify those patients who are
more prone to relapse and modify their therapy to improve outcomes.
In this context, Breg cells may serve as a potential novel
candidate.
To date, limited data on the levels of Treg, iNKT
and Breg cell subsets and their interrelationships have been
reported in patients who receive rituximab-based regimens and
achieve CR. In addition, little is known on the levels of these
regulatory cell subsets in the bone marrow (BM), which constitutes
a key site providing important information for the diagnosis and
follow-up of patients with B-cell malignancies. Therefore, there
remains the question of whether Treg, iNKT and Breg cell subsets
change following therapy in rituximab-treated patients who achieve
CR. The aim of the present study was to investigate the levels of
Treg, iNKT and Breg cell subsets and their interrelationships in
the peripheral blood (PB) and BM of patients who received
rituximab-based regimens for the treatment of B-cell non-Hodgkin
lymphoma (B-NHL) at diagnosis and after achieving CR.
Patients and methods
Patients
Patients with B-NHL were recruited at the Sultan
Qaboos University (SQU) Hospital, Sultanate of Oman. All patients
were previously treated with rituximab in combination with
cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP),
or other chemotherapeutic agents as part of standard protocols.
Each case was reviewed and the final diagnosis was made according
to the 2016 World Health Organization classification of tumors of
the hematopoietic and lymphoid tissues (9). All patients had sustained CR for at
least 2 months prior to sampling. Age and sex-matched healthy
controls were also recruited for comparison. This study was
performed according to the principles outlined in the Declaration
of Helsinki and approved by the Institutional Research Ethics
Committee of the SQU (Sultanate of Oman). All the study
participants signed an informed consent form prior to
enrollment.
Cell isolation
PB samples were drawn into vacutainer tubes
containing ethylenediaminetetraacetic acid as anticoagulant (BD
Biosciences, San Jose, CA, USA) and processed within 2 h of
collection. PB mononuclear cells (PBMC) were separated by
Ficoll-Hypaque density centrifugation, washed and re-suspended in
phosphate-buffered saline (PBS) containing heat-inactivated fetal
calf serum (FCS). The freshly processed PBMC samples were either
stained with appropriate monoclonal antibodies or stored in liquid
nitrogen until used. Similarly, mononuclear cells from BM samples
were prepared by Ficoll-Hypaque density centrifugation and stored
until used.
Monoclonal antibodies
Specific human monoclonal antibodies conjugated to
fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-CF594,
PECy-7, allophycocyanin (APC), APC-H7 and
peridin-chlorophyll-protein (PerCP) were used to identify cellular
antigens. 5 µl of anti-CD3-APC/H7 (cat. no. 560176), 7.5 µl of
anti-CD4-APC (cat. no. 555349) 7.5 µl of anti-CD8-PerCp (cat. no.
347314), 9 µl of anti-CD19-APC (cat. no. 555415), 7.5 µl of
anti-CD24-FITC (cat. no. 560992), 5 µl of anti-CD25-PE-CF594 (cat.
no. 562403), 5 µl of anti-CD38-PE-CF594 (cat. no. 562288), 5 µl of
anti-CD127-PECy-7 (cat. no. 560822), 12.5 µl of anti-FoxP3-PE (cat.
no. 560046), 12.5 µl of anti-IL-10-PE (cat. no. 562035), and 10 µl
of anti-iNKT-PE (cat. no. 562051) antibodies were obtained from BD
Biosciences (Franklin Lakes, NJ, USA).
Surface and intracellular
staining
Immunostaining was performed as recently reported
(10). Briefly, in a 96-well
polystyrene plate, appropriate amounts of each surface monoclonal
antibody and 2×106 of PBMC were incubated in the dark
for 30 min at 4°C. The cells were then washed twice with PBS
containing 1% FCS and fixed with Cytofix™ buffer (BD Biosciences).
Subsequently, the Cytoperm™ Plus buffer was used for
permeabilization (BD Biosciences). Cells were washed and stained
intracellularly with anti-FoxP3 antibody, then fixed for cell
acquisition by flow cytometry. For Breg cells, the PBMC were first
stimulated and cultured for 48 h. They were then stained
extracellularly, fixed with Cytofix™ buffer and permeabilized with
Cytoperm™ Plus buffer (BD Biosciences). The cells were then stained
intracellularly with anti-IL-10 antibody and fixed for analysis by
flow cytometry.
Immunophenotypic analysis
The LSR Fortessa and FACSDIVA™ Software version
8.0.1 (BD Biosciences) were used for cell acquisition and analysis,
respectively, as previously reported (11). In brief, the sensitivity of
fluorescence detectors was set and monitored using calibrated beads
according to the manufacturer's recommendations (BD Biosciences).
Fluorescence voltages and compensation values were determined using
a combination of CS&T beads and CompBeads (BD Biosciences).
Lymphocytes were first identified according to their
light-scattering properties and then analyzed for expression of
specific markers. Treg cells were defined as
CD3+CD4+CD25hiCD127loFoxP3+, iNKT cells as CD3+CD4+6B11+ and Breg
cells as CD19+CD24hiCD38hiIL-10+. For each sample, a minimum of
50×106 events in live cell gate were accumulated. To
discriminate live from dead cells, the LIVE/DEAD Stain kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used in all experiments.
Statistical analysis
Quantitative data were expressed as means ± standard
deviations. Differences in the levels of Treg, Breg, and iNKT cell
subsets in PB and BM were evaluated using Wilcoxon's test for
paired samples and Mann-Whitney U test for unpaired samples, as
data were not normally distributed. Correlations among regulatory
cell subsets, study parameters and clinical data were evaluated by
Spearman's correlation test. All tests were two-tailed with an α
level of 0.05, which was considered as significant. Analyses were
performed using the GraphPad PRISM 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
Patient characteristics
A total of 20 patients with B-NHL in CR following
treatment with rituximab and chemotherapy and 20 healthy age- and
sex-matched healthy controls were enrolled. The baseline
characteristics of the participants at study enrollment are
presented in Table I. Overall, the
sociodemographic data were similar between patients and controls.
The majority of the enrolled patients were diagnosed with diffuse
large B-cell lymphoma (DLBCL). In order to study as homogeneous a
group of patients as possible, all DLBCL patients received R-CHOP,
while patients with follicular lymphoma received rituximab with
cyclophosphamide, vincristine and prednisone (R-CVR). Full doses of
cytostatic agents were administered to all patients. Of note, none
of the patients received bendamustine-based chemotherapy, given its
potential impact on immune reconstitution. As expected, hemoglobin
levels were slightly lower in patients compared with those in
healthy controls. Both patients and healthy controls were negative
for infections with human immunodeficiency virus, hepatitis C virus
(anti-HCV antibody) and hepatitis B virus (HBVsAg). All patients
were disease-free for at least 8 weeks prior to sampling, with a
mean length of time of 3±1 months (range, 2–4 months). The patients
received between 6 and 8 cycles of rituximab-based treatment, which
was well-tolerated.
 | Table I.Baseline characteristics of study
participants. |
Table I.
Baseline characteristics of study
participants.
| Characteristics | Patients (n=20) | Healthy controls
(n=20) |
|---|
| Age (years) | 53±5 (18–69) | 54±3 (18–68) |
| Male sex, n (%) | 14 (70) | 14 (70) |
| Haemoglobin
(g/dl) | 11±3 (9–14) | 14±2 (13–15) |
| Lactate dehydrogenase
(U/l) | 268±47 (128–239) | ND |
| Lymphocyte/monocyte
ratio | 4±1.9 (0.92–8.1) | 5±1.4 (1.6–8.1) |
| Diagnosis, n (%) |
| NA |
| Diffuse
large B-cell lymphoma | 16 (80) |
|
|
Follicular lymphoma | 4 (20) |
|
| B symptoms, n
(%) |
| NA |
|
Present | 6 (30) |
|
| Extranodal lesions, n
(%) |
| NA |
|
Present | 4 (20) |
|
| Bone marrow
involvement, n (%) |
| NA |
|
Present | 5 (25) |
|
| International
prognostic index, n (%) |
| NA |
| 0–1 | 6 (30) |
|
| 2 | 4 (20) |
|
| 3 | 3 (15) |
|
| 4–5 | 7 (35) |
|
Levels of circulating regulatory cell subsets prior
to therapy. To determine whether the levels of regulatory cell
subsets differ between patients and healthy controls, we first
compared their levels prior to rituximab-based regimen
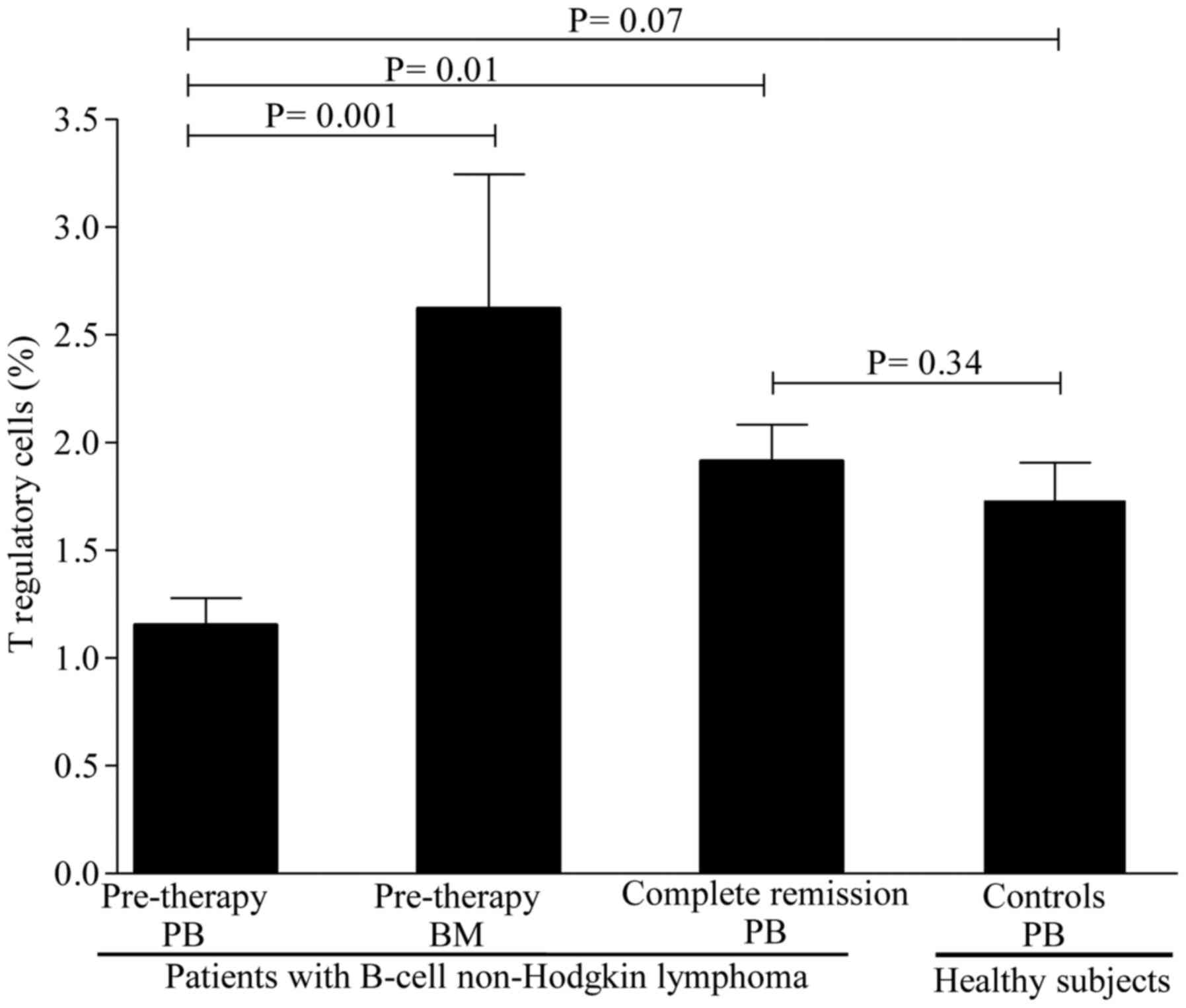
administration. As shown in Fig. 1,
the levels of Treg cells were lower in patients compared with those
in healthy controls, but the difference did not reach statistical
significance. A mean value of Treg cells of 1.15±0.12% (range,
0.39–2.10%) was observed in patients compared with 1.73±0.17%
(range, 0.88–3.30%) in healthy controls (P=0.07). In contrast to
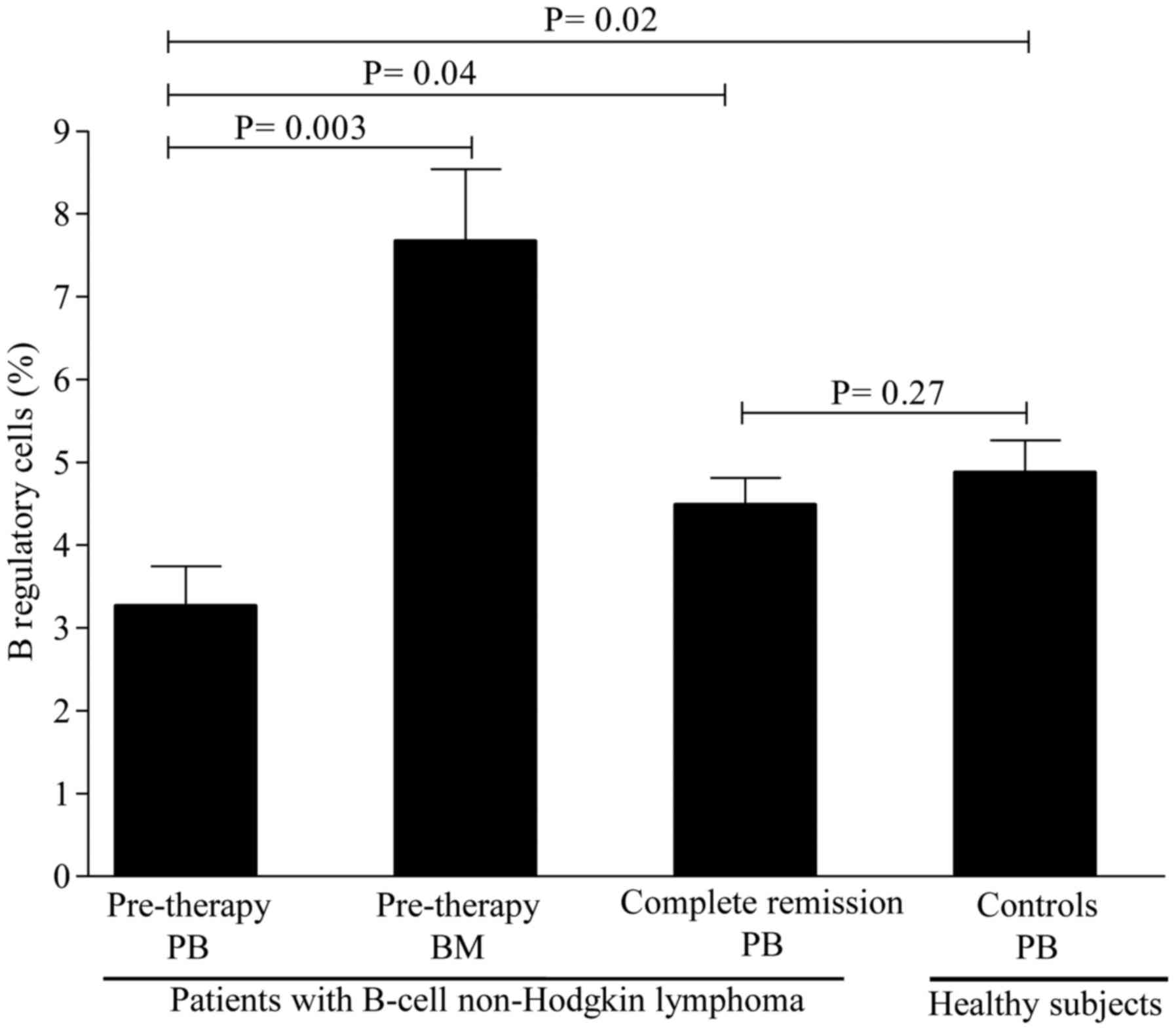
Treg cells, the levels of Breg cells were substantially lower in
patients [3.26±0.48% (range, 0.11–6.7%)] compared with healthy
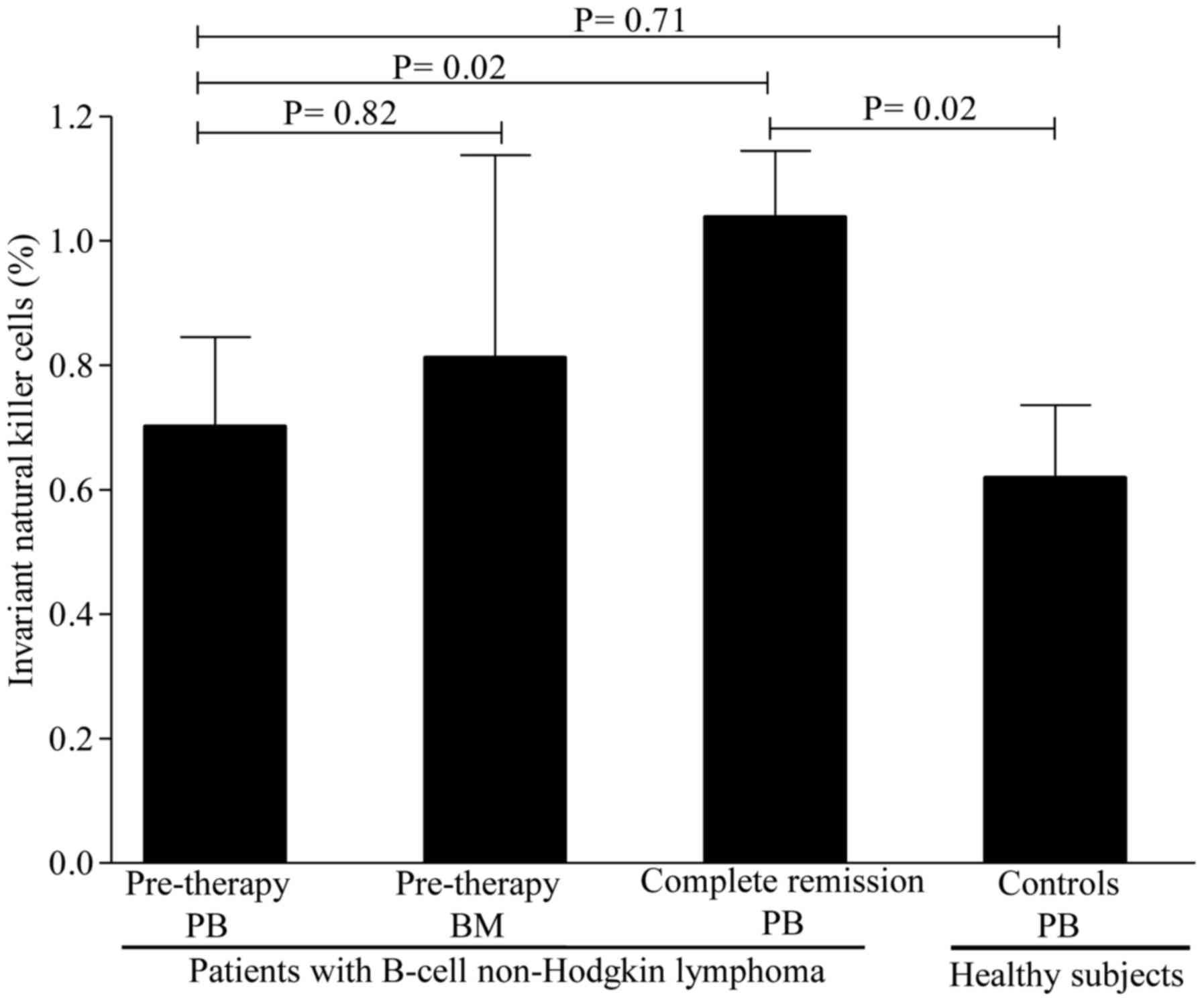
controls [4.97±0.39% (range, 2.10–7.3%)] (P=0.02; Fig. 2). Interestingly, the levels of iNKT
cells were similar between patients [0.71±0.14% (range, 0.11–1.9%)]
and healthy controls [0.61±0.11% (range, 0.11–1.90%)] (P=0.71;
Fig. 3). Collectively, these data
suggest that patients with B-cell NHL displayed a pronounced
reduction in Breg cells and, to a lesser degree, Treg cells, as
compared to controls, but with comparable levels of iNKT cells.
Levels of regulatory cell subsets in
the BM
As the BM acts as a reservoir for regulatory cells,
whether the levels of Treg, Breg and iNKT cells differ between BM
and PB was next investigated. The levels of Treg cells were higher
in the BM [2.62±0.62% (range, 1.21–4.80%)] compared with those in
the PB [1.15±0.12% (range, 0.39–2.10%)] (P=0.001) as evidenced by
the 1-fold increase in BM levels (Fig.
1). Similarly, the levels of Breg cells were markedly higher in
the BM [7.67±0.86% (range, 5.60–10.50%)] compared with those in the
PB [3.26±0.48% (range, 0.11–6.7%)], with a nearly 2-fold increase
in BM levels (Fig. 2). The levels of
iNKT cells, however, were similar between BM and PB (Fig. 3). Of note, no differences were
observed in the levels of regulatory cells in patients with or
without BM involvement, B symptoms, or displaying >1 extranodal
sites (P=0.51). Similarly, no statistically significant differences
in regulatory cells were identified between patients with favorable
and poor prognostic markers (P=0.77). Taken together, these
findings suggest that the Treg and Breg cell subsets, but not the
iNKT cell subset, are retained in the BM compared with the PB.
Levels of circulating regulatory cell
subsets after CR
To study the impact of rituximab-based regimens on
regulatory cell subsets, their levels were assessed in patients
achieving CR. A significant increase was observed in the levels of
Treg cells [from 1.15±0.12% (range, 0.39–2.10%) to 1.81±0.16%
(range, 0.68–3.10%); P=0.01] after CR (Fig. 1). However, only a slight increase was
observed in the levels of Breg cells [from 3.26±0.48% (range,
0.11–6.7%) to 4.63±0.45% (range, 1.50–9.30); P=0.04] after CR
(Fig. 2). In contrast to Breg cells,
a marked increase in iNKT cells was noticed in almost all patients
[from a pre-therapy level of 0.71±0.14% (range, 0.11–1.9%) to
1.10±0.11% (0.41–2.30); P=0.02] after CR (Fig. 3). Of note, regulatory cell subsets at
CR reached the levels of normal or nearly normal values in the
majority of the patients. In addition, there were no significant
differences in the levels of regulatory cells between patients who
were in CR for <3 months and those in CR for >3 months
(P=0.37). Overall, these results suggest that Breg cells only
slowly repopulated the PB, while the Treg and iNKT cell subsets
recovered rapidly after CR.
Associations among regulatory cell
subsets
We next assessed the associations among the levels
of regulatory cell subsets and study parameters. Interestingly, the
levels of Breg cells were negatively associated with both Treg
cells (Rho = −0.29; P=0.13) and iNKT cells (Rho = −0.31; P=0.10),
but the differences did not reach statistical significance. The
majority of the other correlations among study variables were not
statistically significant (data not shown). Of note, the levels of
Breg cells were positively correlated with the length of time after
CR (Rho = 0.37; P=0.08), but this did not reach statistical
significance.
Discussion
The pathophysiology of B-cell NHL involves impaired
mechanisms of cellular regulation that control the maintenance of
normal homeostasis. Hence, probing the regulatory activities at a
single-cell level may improve our understanding of the disease and
eventually result in more specific and effective therapeutic
measures. The interactions among regulatory cells in B-cell NHL
have not yet been fully elucidated. This prospective study aimed to
investigate the levels of Treg, Breg and iNKT cell subsets and
their interrelationships in the PB and BM of patients with B-cell
NHL who were treated with rituximab-based regimens and achieved a
CR.
Thus far, published data on the levels of
circulating Treg cells in newly diagnosed patients with NHL have
yielded conflicting results. While some studies reported that the
pre-therapy levels of Treg cells in patients with B-cell NHL are
higher in PB compared with those of healthy individuals, other
studies reported the opposite, or no association (12–17). In
the present study, the levels of circulating Treg cells tended to
be lower in patients compared with healthy controls prior to the
initiation of rituximab-based treatment, but their levels were
markedly increased after CR. These findings appear to differ from
those studies reporting increased pre-therapy levels of circulating
Treg cells in patients with NHL compared with healthy controls.
Several possible explanations may account for these differences.
First, they may be due to an increase in the migration of Treg
cells from the PB to the BM. This hypothesis is supported by our
findings showing that the levels of Treg cells are higher in the BM
compared with the PB. It is also supported by earlier studies
showing increased homing of Treg cells into the tumor sites
compared with other compartments (14,15).
Second, these findings may be explained by the fact that Treg cell
measurements are subject to marked immunophenotyping variability
across diverse studies where different markers are being used. In
the present study, five markers were employed simultaneously to
accurately define Treg cell phenotype. In fact, the combination of
these markers was shown to better reflect Treg cell functions,
which are enriched within the CD25hiCD127loFoxP3+ gated
population (18). Finally, it is
possible that the studied populations are somehow heterogeneous, as
they differ in several factors, including sociodemographic and
histological subtypes of NHL. It has been demonstrated that
patients with unhealthy habits, such as smoking and alcohol abuse,
or those suffering from extreme stress, exhibited high levels of
Treg cells in the PB (19).
The findings of the present study also extended to
investigation of the levels of Breg cells in patients with B-cell
NHL. In a recent study, Qiu et al (20) reported a long-lasting
overrepresentation of circulating CD19+CD20-CD27hiIL-s10-producing
B cells in R-CHOP-treated patients with DLBCL who were in
remission. Although Qiu et al used different cellular makers
to phenotypically define Breg cells, our results confirmed their
findings, showing increased levels of Breg cells after achieving
CR, but with a slow repopulation of the PB. This may be due in part
to the fact that rituximab treatment induced depletion of most
CD20-expressing cells, despite the fact that Breg cells express low
levels of the CD20 antigen and may escape depletion therapies, as
described in murine models (6–8).
Indirectly, our data are in line with this observation, as a
positive association between the levels of Breg cells and length of
time after CR was observed, although this did not reach statistical
significance. Additionally, increased levels of Breg cells were
found in the BM compared with the PB prior to starting
rituximab-based treatment. The observed compartmental differences
in the levels of Breg cells may suggest mobilization and homing of
these cells from the PB to the BM to exert their regulatory
effects. This hypothesis is supported by studies reporting that BM
acts as a reservoir for immune cells, including Breg and Treg cells
(21,22).
Previous studies in animal models have demonstrated
that iNKT cells are endowed with potent antitumor activity in
different types of cancer, including B-cell NHL (23). However, only a limited number of
studies have focused on their levels in patients with B-cell NHL
who achieved CR following rituximab-based therapy. In an earlier
study, Yoneda et al (24)
reported that patients with malignant lymphoma who achieved a CR
exhibited comparable absolute numbers of circulating
Vα24+ NKT cells, which are equivalent to iNKT cells, to
those of healthy individuals. In another study, Hus et al
(25) found lower levels of
circulating iNKT cells in patients with B-cell NHL prior to
chemotherapy compared with healthy controls, and these levels
markedly increased after the completion of R-CHOP. Our results are
quite similar, except that the pre-therapy levels of iNKT cells
were not different from those in healthy controls in the present
study. In addition, it was demonstrated that the levels of iNKT
cells were comparable between PB and BM, suggesting a balance
between the two compartments.
One of the objectives of the present study was to
evaluate the interrelationships among the regulatory cell subsets,
as these cells are known to interact with each other to establish a
potent immunoregulatory environment to control tumor growth.
Associations among circulating regulatory cell subsets and standard
disease markers in patients with B-cell NHL has been evaluated by
few studies with conflicting results (12–17,20). The
findings of the present study revealed that the levels of
circulating Treg cells were not significantly correlated with Breg
or iNKT cells, either pre- or post-rituximab-based therapy.
Similarly, weak and insignificant associations were observed
between the levels of regulatory cell subsets and clinical
parameters, including lymphocyte-to-monocyte ratio, which was
recently identified as an independent prognostic factor in patients
with B-cell NHL (26). The lack of
significant correlations may be explained in part by the fact that
the levels of regulatory cell subsets in the PB do not accurately
reflect their intratumoral counterparts. Whether the associations
among circulating regulatory cell subsets are truly insignificants
requires further investigation, along with co-culture assays using
sorted cells to better mimic their interactions in ex vivo
studies.
There were certain limitations to the present study.
The sample size was relatively small, but it reflects the routine
care in our clinical settings. This may have affected the
statistical power to discriminate the effects of tested
correlations between the analyzed groups. However, the low level of
statistical dispersion of the results suggests that increasing the
number of patients would not have had a major effect on the
significance of the obtained results. Another limitation is that
all enrolled patients were in CR; therefore, it was not possible to
evaluate the effects of regulatory cell subsets as factors
predictive of response. In addition, due to the limited sample
availability, functional cellular assays were not performed. It
would be useful to perform such studies to investigate whether the
Treg, Breg and iNKT cell subsets are also functionally impaired in
future studies.
In conclusion, the present study demonstrated that
patients with B-cell NHL who receive rituximab-based regimens and
achieve CR display lower pre-therapy levels of Breg cells and, to a
lesser degree, Treg cells, but not iNKT cells in the PB when
compared with healthy individuals. Compartmental differences in the
levels of Treg and Breg cells, but not iNKT cells, exist between PB
and BM, suggesting increased trafficking through the blood of these
regulatory cell subsets to the marrow. Despite the fact that the
levels of circulating Breg, Treg and iNKT cells increased after CR
in almost all patients, no significant associations were
identified. Overall, these results provide new insights into the
role of regulatory cell subsets in patients with B-cell NHL.
Further investigation into the interrelationships among these
regulatory cells may help to elucidate the mechanisms underlying
antitumor control.
Acknowledgements
The authors would like to thank all participants and
the staff from the Department of Microbiology and Immunology for
the technical assistance.
Funding
The present study was supported by a grant from the
Sultan Qaboos University (grant no. IG/MED/HAEM/14/01).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are not publicly available due to SQU regulations,
but are available from the corresponding author on reasonable
request.
Authors' contributions
MB designed, performed and analyzed data and wrote
the manuscript. ZQ, NA, HH and AB performed the experiments and
analyzed the data. IB, HK, RQ, VP and KF recruited participants,
collected clinical data and critically reviewed the manuscript.
Ethics approval and consent to
participate
This study was performed according to the principles
outlined in the Declaration of Helsinki and approved by the
Institutional Research Ethic Committee of the Sultan Qaboos
University (SQU), Sultanate of Oman. Further approval was obtained
from the hospital management before accessing the patient medical
records. All study participants signed an informed consent form
prior to enrollment.
Patient consent for publication
Consents, verbal and written, were obtained from all
the participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune system.
Nat Rev Immunol. 10:490–500. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mauri C and Menon M: Human regulatory B
cells in health and disease: Therapeutic potential. J Clin Invest.
127:772–779. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flores-Borja F, Bosma A, Ng D, Reddy V,
Ehrenstein MR, Isenberg DA and Mauri C: CD19+CD24hiCD38hi B cells
maintain regulatory T cells while limiting TH1 and TH17
differentiation. Sci Transl Med. 5:173ra232013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oleinika K, Rosser EC, Matei DE, Nistala
K, Bosma A, Drozdov I and Mauri C: CD1d-dependent immune
suppression mediated by regulatory B cells through modulations of
iNKT cells. Nat Commun. 9:6842018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vomhof-DeKrey EE, Yates J, Hägglöf T,
Lanthier P, Amiel E, Veerapen N, Besra GS, Karlsson MC and
Leadbetter EA: Cognate interaction with iNKT cells expands
IL-10-producing B regulatory cells. Proc Natl Acad Sci USA.
112:12474–12479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olkhanud PB, Damdinsuren B, Bodogai M,
Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP and Biragyn A:
Tumor-evoked regulatory B cells promote breast cancer metastasis by
converting resting CD4+ T cells to T-regulatory cells.
Cancer Res. 71:3505–3515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bodogai M, Lee Chang C, Wejksza K, Lai J,
Merino M, Wersto RP, Gress RE, Chan AC, Hesdorffer C and Biragyn A:
Anti-CD20 antibody promotes cancer escape via enrichment of
tumor-evoked regulatory B cells expressing low levels of CD20 and
CD137L. Cancer Res. 73:2127–2138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horikawa M, Minard-Colin V, Matsushita T
and Tedder TF: Regulatory B cell production of IL-10 inhibits
lymphoma depletion during CD20 immunotherapy in mice. J Clin
Invest. 121:4268–4280. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, et al:
The 2016 revision of the World Health Organization classification
of lymphoid neoplasms. Blood. 127:2375–2390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boulassel MR, Al-Ghonimi M, Al-Balushi B,
Al-Naamani A, Al-Qarni Z, Wali Y, Elshinawy M, Al-Shezawi M, Khan
H, Nazir H, et al: Regulatory B Cells Are Functionally Impaired in
Patients Having Hemophilia A With Inhibitors. Clin Appl Thromb
Hemost. 24:618–624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boulassel MR, Mercier F, Gilmore N and
Routy JP: Immunophenotypic patterns of CD8+ T cell subsets
expressing CD8α and IL-7Rα in viremic, aviremic and slow progressor
HIV-1-infected subjects. Clin Immunol. 124:149–157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gunduz E, Sermet S and Musmul A:
Peripheral blood regulatory T cell levels are correlated with some
poor prognostic markers in newly diagnosed lymphoma patients.
Cytometry B Clin Cytom. 90:449–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin H, Sun XF, Zhen ZJ, Xia Y, Ling JY,
Huang HQ, Xia ZJ and Lin TY: Correlation between peripheral blood
CD4+CD25high CD127low regulatory T cell and clinical
characteristics of patients with non-Hodgkin's lymphoma. Chin J
Cancer. 28:1186–1192. 2009. View Article : Google Scholar
|
|
14
|
Han Y, Wu J, Bi L, Xiong S, Gao S, Yin L,
Jiang L, Chen C, Yu K and Zhang S: Malignant B cells induce the
conversion of CD4+CD25- T cells to regulatory T cells in
B-cell non-Hodgkin lymphoma. PLoS One. 6:e286492011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mittal S, Marshall NA, Duncan L, Culligan
DJ, Barker RN and Vickers MA: Local and systemic induction of
CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma.
Blood. 111:5359–5370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao J, Guo P, Xiao S and Fu X:
Relationship of regulatory T cell level in peripheral blood with
curative efficacy and prognosis of patients with non-Hodgkin's
lymphoma. Int J Clin Exp Pathol. 9:11876–11882. 2016.
|
|
17
|
Chang C, Wu SY, Kang YW, Lin KP, Chen TY,
Medeiros LJ and Chang KC: High levels of regulatory T cells in
blood are a poor prognostic factor in patients with diffuse large
B-cell lymphoma. Am J Clin Pathol. 144:935–944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohr A, Malhotra R, Mayer G, Gorochov G
and Miyara M: Human FOXP3+ T regulatory cell heterogeneity. Clin
Transl Immunology. 7:e10052018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J and Ke XY: The four types of Tregs
in malignant lymphomas. J Hematol Oncol. 4:502011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu H, Li J, Feng Z, Yuan J, Lu J, Hu X,
Gao L, Lv S, Yang J and Chen L: CD19(+) CD20(−) CD27(hi)
IL-s10-producing B cells are overrepresented in R-CHOP-treated
DLBCL patients in complete remission. Clin Exp Pharmacol Physiol.
43:795–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pierini A, Nishikii H, Baker J, Kimura T,
Kwon HS, Pan Y, Chen Y, Alvarez M, Strober W, Velardi A, et al:
Foxp3+ regulatory T cells maintain the bone marrow microenvironment
for B cell lymphopoiesis. Nat Commun. 8:150682017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu
Y, Wang G and Zou W: Bone marrow and the control of immunity. Cell
Mol Immunol. 9:11–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Metelitsa LS: Anti-tumor potential of
type-I NKT cells against CD1d-positive and CD1d-negative tumors in
humans. Clin Immunol. 140:119–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoneda K, Morii T, Nieda M, Tsukaguchi N,
Amano I, Tanaka H, Yagi H, Narita N and Kimura H: The peripheral
blood Vα24+ NKT cell numbers decrease in patients with
haematopoietic malignancy. Leuk Res. 29:147–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hus I, Bojarska-Junak A, Kamińska M,
Dobrzyńska-Rutkowska A, Szatan K, Szymczyk A, Kukiełka-Budny B,
Szczepanek D and Roliński J: Imbalance in circulatory iNKT, Th17
and T regulatory cell frequencies in patients with B-cell
non-Hodgkin's lymphoma. Oncol Lett. 14:7957–7964. 2017.PubMed/NCBI
|
|
26
|
Yan-Li L, Kang-Sheng G, Yue-Yin P, Yang J
and Zhi-Min Z: The lower peripheral blood lymphocyte/monocyte ratio
assessed during routine follow-up after standard first-line
chemotherapy is a risk factor for predicting relapse in patients
with diffuse large B-cell lymphoma. Leuk Res. 38:323–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|