Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer (1).
Liver cancer is the seventh most common type of cancer and the
third leading cause of cancer-related mortality worldwide.
According to GLOBOCAN 2020 (The International Agency for Research
on Cancer), 905,677 new cases of liver cancer and 830,180 liver
cancer-related deaths were recorded (2). Although the survival rates of
patients with HCC have increased due to recent medical advances,
the overall survival (OS) of patients with HCC remains low, with
reported 1-, 2-, 3- and 5-year survival rates of 49.3, 35.3, 26.6
and 19.5%, respectively (3). The
5-year survival rate remains poor, even in patients who undergo
curative surgical resection (56.2% for OS and 35.2% for
recurrence-free survival) (4). A
previous study reported that the 1-year survival rates of
Indonesian patients with HCC were 24.1% in 1998-1999 and 29.4% in
2013-2014. This finding may be due to the fact that the majority of
patients are diagnosed with HCC at a later stage of the disease
(5).
Identifying biomarkers associated with a poor
prognosis, that could improve the risk stratification of patients
with HCC at the time of diagnosis, is of utmost significance.
Molecular pathological diagnosis involves various techniques, such
as in situ hybridization, reverse transcription polymerase
chain reaction (RT-PCR) and DNA sequencing. However,
immunohistochemistry is the most frequently used technique due to
its broad application, ease of performance and evaluation, and
reasonable costs. The results of immunohistochemistry occasionally
reflect specific genetic mutations (6). Furthermore, the ability to obtain
imaging studies, combined with the ability to test patients' blood
for elevated levels of alpha-fetoprotein (AFP), render it possible
to detect HCC at an earlier stage. However, the lack of that an
elevated AFP level is not always associated with HCC. Thus, AFP is
only associated with issues with sensitivity, but also with
specificity (7).
p53 is a protein encoded by the TP53 gene
located on human chromosome 17(8).
Initially identified as an anti-oncogene, p53 functions as a tumor
suppressor gene, which is activated when cells experience stress,
thus promoting the uncontrolled division and proliferation of cells
(9). p53 suppresses tumor
progression and growth by inducing cell cycle arrest through p21
expression, and inducing apoptosis by activating pro-apoptotic
proteins (10,11). When p53 is mutated, it loses its
function, thus promoting uncontrolled cell growth. Mutated p53 has
been detected in several types of cancer, including breast, colon,
lung, liver, prostate, bladder and skin cancer, while it has been
reported that mutant p53 is accumulated in the cell nuclei of
transformed cells (8,12). Τhe development of anti-p53
monoclonal antibodies, has enabled researchers to examine the
expression of p53 in human tissues (12).
The relevance of p53 as a prognostic factor for the
survival of patients with HCC has been extensively investigated in
two studies [Zhan and Ji (13) and
Ji et al (14)]. Therefore,
emerging evidence has suggested that patients with HCC with a
positive p53 expression have a poorer prognosis (13,14).
However, to date, at least to the best of our knowledge, such a
study has not been performed in an Indonesian population.
Therefore, the present study aimed to analyze the expression of p53
in Indonesian patients with HCC, and to evaluate its association
with different prognostic factors, such as HCC stage, grade and
subtype.
Patients and methods
Sample collection
In the present cross-sectional study, a total of 41
patients with HCC who underwent surgical resection between January,
2013 and December, 2020 at Dr. Cipto Mangunkusumo Hospital in
Jakarta, Indonesia were enrolled. Patients whose paraffin blocks
were not adequate for evaluation were excluded from the study. The
inclusion criteria consisted of cases with histopathologically
diagnosed hepatocellular carcinoma at the Department of Anatomical
Pathology FKUI-RSCM (Jakarta, Indonesia) from 2013-2020.
Hepatocellular carcinoma cases were taken from liver resection
tissue with adequate quality for immunohistochemical staining. The
exclusion criteria were patients with primary double tumors. The
clinical data were obtained from the medical records each patient.
To verify the diagnosis and obtain histopathological data, the
slides and paraffin blocks were collected and reassessed
independently by two pathologists specializing in liver pathology.
The present study (protocol no. 21-05-0466) was approved by the
Medical Ethics Committee of the Faculty of Medicine, Universitas
Indonesia Dr. Cipto Mangunkusumo Hospital (approval no.
KET-427/UN2.F1/ETIK/PPM.00.02/2021). Patient consent was waived by
the committee as the study included already existing data (waiver
statement no. ND-784/UN2.F1/ETIK/PPM.00.02/2022).
Immunohistochemical (IHC)
staining
IHC staining was performed on 3-4-µm thick
paraffin-embedded tissue sections. The sections were mounted on
poly-l-lysine coated slides and heated on a slide warmer for 60 min
at 60˚C. Subsequently, the slides were deparaffinized thrice in a
graded xylol (Smart Lab Indonesia) eries for 5 min each, rehydrated
with 96 and 70% alcohol for 4 min each, followed by rinsing with
running water for 3 min. For antigen retrieval, the sections were
boiled for 20 min at 96˚C in tris EDTA (pH 9) in a decloaking
chamber (BIOGEAR, Biozatix), followed by cooling for 25 min.
Subsequently, the tissue sections were washed with PBS (pH 7.4) for
3 min and the unspecific binding sites were then blocked. A PAP pen
(Scytek, Medipath Biosains) was used to label the tissue sections.
To block endogenous peroxidase, the tissues were incubated with
peroxidase block (Novolink Polymer®; Novocastra) for 30
min, before being washed with PBS (pH 7.4) for 3 min. The slides
were then incubated [at room temperature (20-25˚C)] for 30 min in
protein block (Novolink Polymer®; Novocastra), washed
with PBS (BIOGEAR, Biozatix) (pH 7.4) for 3 min to remove
non-specific proteins, followed by an overnight incubation with a
primary (20-25˚C) antibody against p53 (dilution 1:500; clone DO-7;
Cell Marque, cat. no. 453M-94). Following washing with PBS (pH 7.4)
for 3 min, the tissue sections were incubated with the
corresponding ready to use secondary antibody for 30 min in 20-25˚C
(Novolink Polymer®; Novocastra, cat. no. RE7140-K),
followed by washing with PBS for 3 min. The slides were covered
with 3,3'-diaminobenzidine tetrahydrochloride solution (Novolink
Polymer®; Novocastra) supplemented with 5% lithium
carbonate and were then counterstained with Mayer's hematoxylin
(Thermo Fisher Scientific, Inc.) at 20-25˚C for 10 sec. Finally,
the sections were dehydrated with an ascending concentration of
alcohol for 5 min, soaked in xylol (clearing) for 5 min and mounted
under a cover slip.
Assessment of p53 expression
Using IHC staining, two independent pathologists,
blinded to the patients' clinicopathological data, evaluated the
expression levels of p53 in the tissue sections under a light
microscope (Leica ICC 50 HD; Leica Microsystems GmbH;
magnification, x40). A positive p53 expression was indicated by the
presence of brown-stained nuclei. Therefore, cells with
brown-stained nuclei were captured and analyzed using ImageJ
software 20.0 version (National Institutes of Health). The
expression levels of p53 are expressed as a percentage, based on
the number of positively-stained cancer cells/500 cancer cells
ratio. Nuclear staining of <10, 10-30, 30-50 and >50% was
considered to indicate negative, weakly positive, moderately
positive and strongly positive for p53 expression,
respectively.
Statistical analysis
All statistical analyses were performed using the
Statistical Package for Social Sciences software (version 25.0; IBM
Corp.). Categorical variables were compared using the Chi-squared
test or Fisher's exact test. In addition, continuous variables were
compared using the Kruskal-Wallis test, followed by the
Dunn-Bonferroni post hoc test. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Study population
The demographic characteristics of the study
population are presented in Table
I. The average age of patients was 53.32±12.455 years. The
majority of the patients were males (85.4%), <60 years old
(63.4%) and with a history of hepatitis (68.3%). Hepatitis B was
more common compared with hepatitis C (56.1 and 12.2%,
respectively). The median tumor size was 7.9 cm. A tumor diameter
>5 cm (75.6%) and vascular invasion (73.2%) were recorded in the
majority of patients with HCC. Approximately 41.5, 36.6 and 22.0%
of the patients suffered from stage IIIA, II and IB HCC,
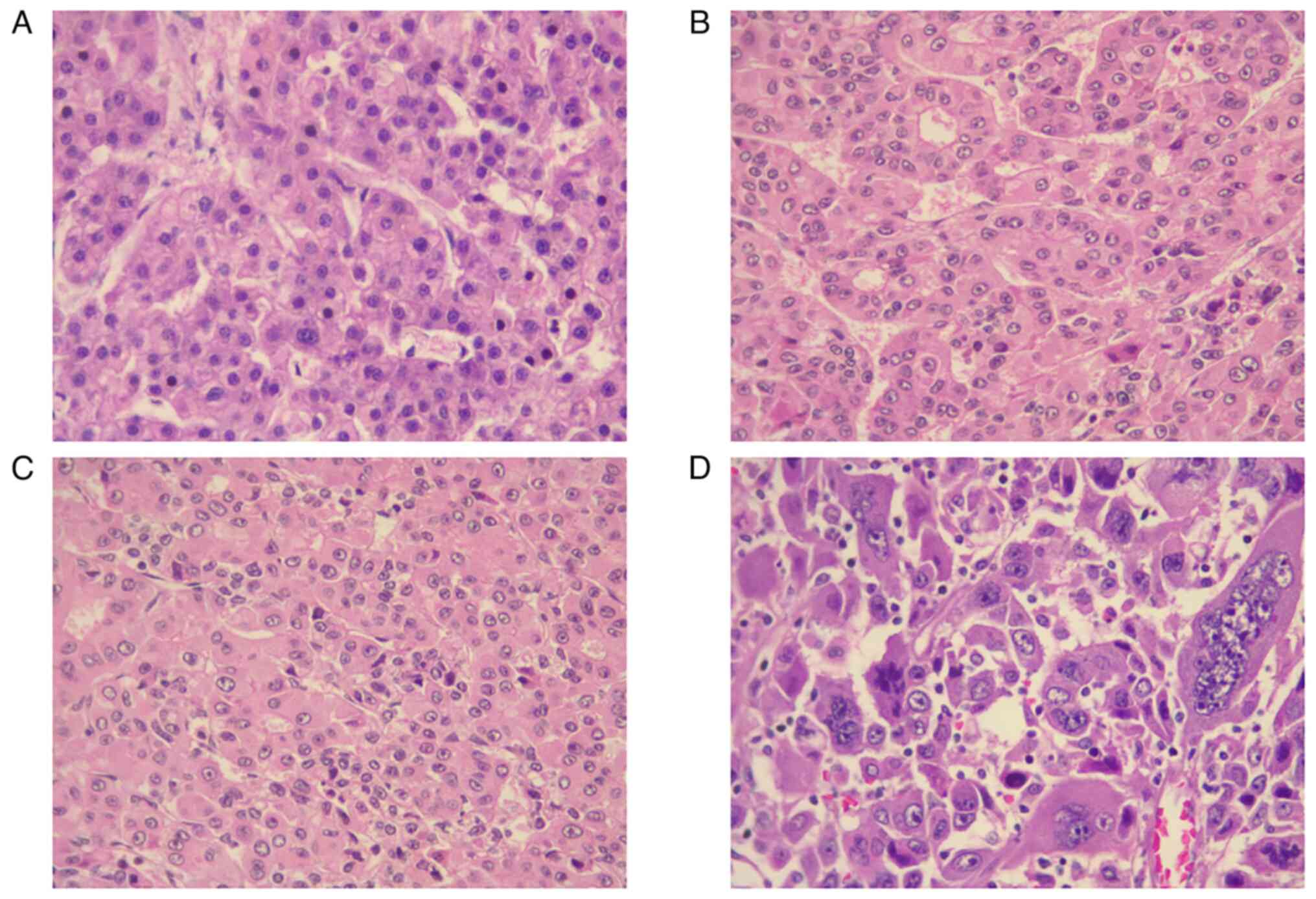
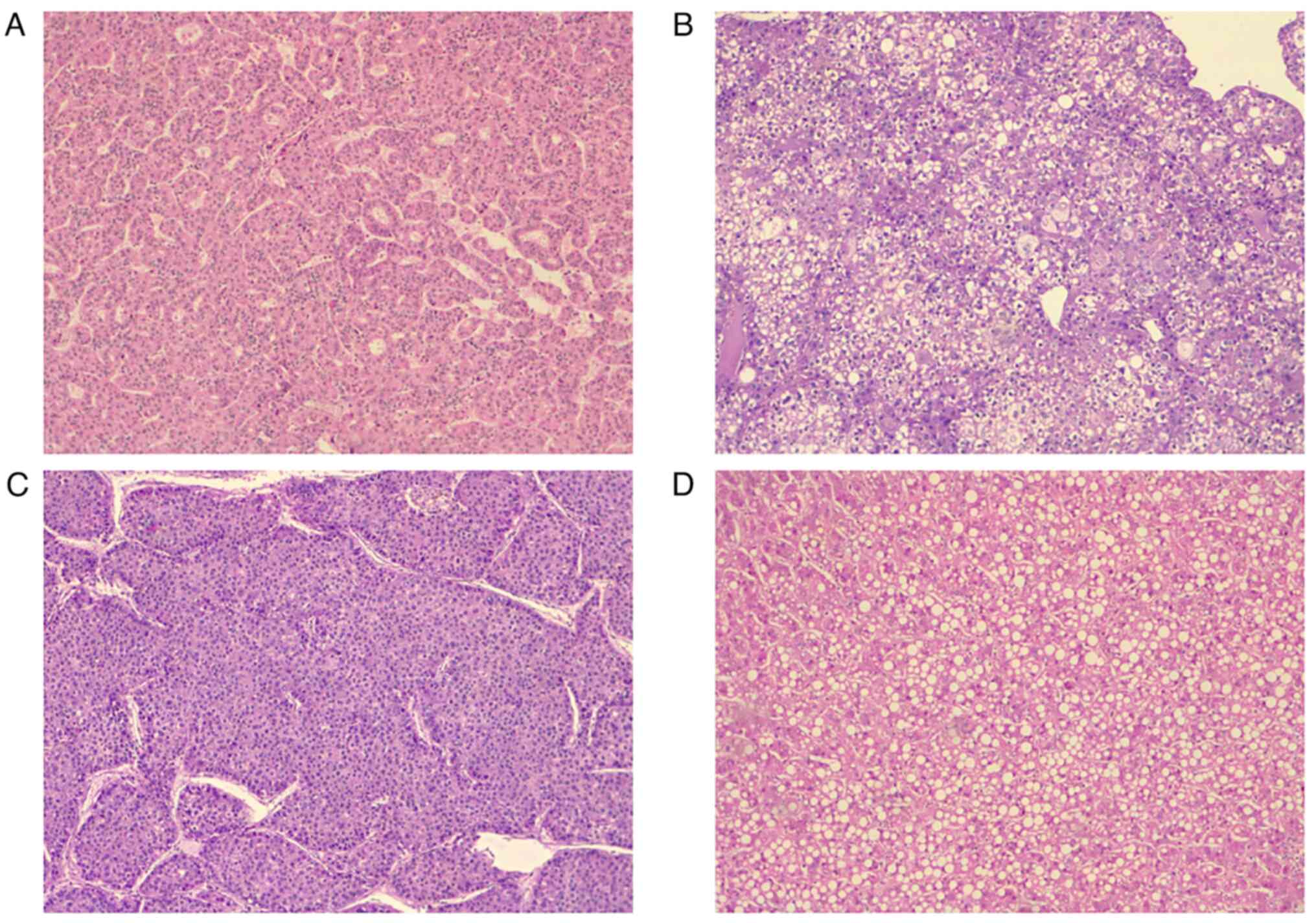
respectively. Finally, moderately differentiated classic HCC was
the most common pathological finding in the aforementioned patients
(Figs. 1 and 2).
 | Table IClinicopathological characteristics
of the study population. |
Table I
Clinicopathological characteristics
of the study population.
| Characteristic | Category | n (%) |
|---|
| Sex | Male | 35 (85.4) |
| | Female | 6 (14.6) |
| Age (mean/standard
deviation SD) | 53.32/12.455 | |
| Age | <60 years
old | 26 (63.4) |
| | ≥60 years old | 15 (36.6) |
| History of
hepatitis | Hepatitis B | 23 (56.1) |
| | Hepatitis C | 5 (12.2) |
| | No history of
hepatitis | 3 (7.3) |
| | Unknown | 10 (24.4) |
| Tumor size
(median/min-max) | 7.9/2.5-20.0 | |
| Tumor size | ≤5 cm | 10 (24.4) |
| | >5 cm | 31 (75.6) |
| Number of
nodules | Solitary | 23 (56.1) |
| | Multiple | 18 (43.9) |
| Vascular
invasion | Absent | 11 (26.8) |
| | Present | 30 (73.2) |
| Liver
cirrhosis | Absent | 19 (46.3) |
| | Present | 22 (53.7) |
| AJCC staging | Stage IB | 9 (22.0) |
| | Stage II | 15 (36.6) |
| | Stage IIIA | 17 (41.5) |
| Tumor grade |
Well-differentiated | 3 (7.3) |
| | Moderately
differentiated | 23 (56.1) |
| | Poorly
differentiated | 15 (36.6) |
| Histological
subtypes | Classic HCC | 18 (43.9) |
| | Clear cell HCC | 16 (39.0) |
| |
Macrotrabecular-massive HCC | 4 (9.8) |
| | Steatohepatitic
HCC | 3 (7.3) |
| p53 expression
(mean/SD) | 40.40/25.697 | |
| p53 expression | Negative | 6 (14.6) |
| | Positive | (85.4) |
| |
Weakly
positive | (19.5) |
| |
Moderately
positive | (26.8) |
| |
Strongly
positive | 16 (39.0) |
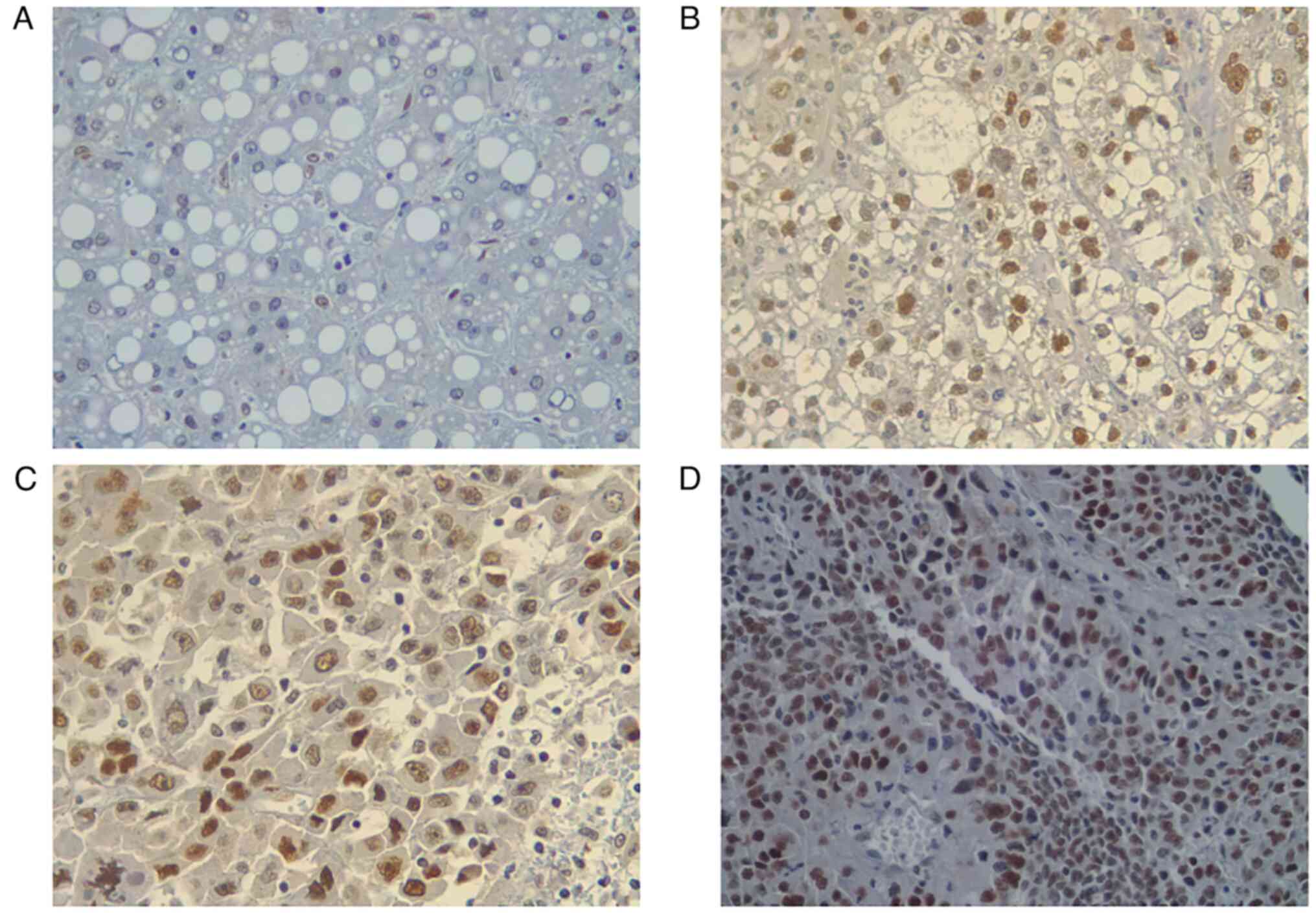
p53 expression is upregulated in HCC
tissues
The IHC staining of p53 expression revealed that the
mean expression levels of p53 in patients with HCC were
40.40±25.697%. The majority of patients (85.4%) were positive for
p53 expression. More specifically, 8 patients exhibited a weak
(19.5%), 11 patients a moderate (26.8%) and 16 patients a strong
(39.0%) p53 expression (Fig. 3).
The characteristics of the study population based on p53 expression
are displayed in Table II. The
patients with HCC with a positive p53 expression were mostly males,
>60 years old, with solitary nodules >5 cm in diameter and
vascular invasion. Statistical analysis revealed that a positive
p53 expression was associated with well- and poorly differentiated
HCC (P=0.002). With the exception of tumor grade, no statistically
significant differences were obtained for the clinicopathological
features between p53-negative and p53-positive patients with
HCC.
 | Table IIClinicopathological characteristics
of the study population according to p53 expression. |
Table II
Clinicopathological characteristics
of the study population according to p53 expression.
| Characteristic | p53- negative
(n=6) | p53- positive
(n=35) | P-value |
|---|
| Sex | | | |
|
Male | 5 (83.3) | 30 (85.7) | 0.999a |
|
Female | 1 (16.7) | 5 (14.3) | |
| Age | | | |
|
<60 years
old | 4 (66.7) | 22 (62.9) | 0.999a |
|
≥60 years
old | 2 (33.3) | 13 (37.1) | |
| Tumor size | | | |
|
≤5 cm | 3 (50.0) | 7 (20.0) | 0.143a |
|
>5
cm | 3 (50.0) | 28 (80.0) | |
| Number of
nodules | | | |
|
Solitary | 1 (16.7) | 22 (62.9) | 0.070a |
|
Multiple | 5 (83.3) | 13 (37.1) | |
| Vascular
invasion | | | |
|
Absent | 3 (50.0) | 8 (22.9) | 0.361a |
|
Present | 3 (50.0) | 27 (77.1) | |
| Liver
cirrhosis | | | |
|
Absent | 1 (16.7) | 18 (51.4) | 0.191a |
|
Present | 5 (83.3) | 17 (48.6) | |
| AJCC staging | | | |
|
Stage
IB-II | 2 (33.3) | 22 (62.9) | 0.212a |
|
Stage
IIIA | 4 (66.7) | 13 (37.1) | |
| Tumor grade | | | |
|
Well-differentiated | 3 (50.0) | 0 (0) |
0.002a |
|
Moderately-poorly
differentiated | 3 (50.0) | 35 (100.0) | |
| Histologic
subtypes | | | |
|
Non-macrotrabecular-massive
HCC | 6 (100.0) | 31 (88.6) | 0.999a |
|
Macrotrabecular-massive
HCC | 0 (0) | 4 (11.4) | |
p53 expression is associated with the
differentiation status of HCC
Further analysis was performed to evaluate the
association between the expression levels of p53 and tumor stage,
tumor grade and histological subtype (Table III). No statistically significant
differences were observed in the expression levels of p53 between
patients with stage IB, stage II and stage IIIA HCC (P=0.893).
Additionally, compared with well-differentiated HCC, the expression
levels of p53 were notably increased in poorly differentiated HCC
(P=0.018). More specifically, the p53 expression levels were higher
in poorly differentiated HCC compared with moderately
differentiated HCC. However, no statistically significant
difference was observed between well-moderate differentiated and
moderate-poorly differentiated, respectively (P=0.056 and P=0.999.
Additionally, no statistically significant differences were
observed in the expression levels of p53 among the four
histological subtypes of HCC (P=0.076).
 | Table IIIp53 expression in relation to tumor
stage, grade and subtype. |
Table III
p53 expression in relation to tumor
stage, grade and subtype.
| Characteristic | p53 expression
(%) | P-value | Post hoc analysis
P-value |
|---|
| AJCC staging | | | |
|
Stage
IB | 37.00 (0-74) |
0.8931 | Not performed |
|
Stage
II | 42.00 (2-88) | | |
|
Stage
IIIA | 33.00 (2-81) | | |
| Tumor grade | | | |
|
Well-differentiated | 2.30 (0-6) |
0.023a | Well vs. moderate:
0.0562 |
|
Moderately
differentiated | 37.00 (2-88) | | Well vs. poor:
0.018b |
|
Poorly
differentiated | 55.00 (2-84) | | Moderate vs. poor:
0.9992 |
| Histologic
subtypes | | | |
|
Classic
HCC | 40.50 (0-88) |
0.0761 | Not performed |
|
Clear cell
HCC | 46.00 (2-81) | | |
|
Macrotrabecular-massive
HCC | 24.00 (14-68) | | |
|
Steatohepatitic
HCC | 5.00 (2-6) | | |
Discussion
An impaired p53 activity has been shown to be
associated with liver carcinogenesis (15,16).
However, HCC pathogenesis remains unclear. Previous whole-genome
sequencing results have revealed that TP53 was the most
commonly mutated tumor suppressor gene in HCC samples (15). Furthermore, exome sequencing
results revealed that mutations in TP53 were associated with
hepatitis B virus infection, a risk factor for HCC (16). Alterations in the activation of the
p53/p21 and retinoblastoma protein 1 signaling pathways have also
been shown to be associated with the development of HCC by
regulating genomic stability and cell cycle distribution (17). In addition, HCC-inducing extrinsic
factors, such as aflatoxin B1, non-alcoholic liver disease, iron
overload and hepatitis virus infection, have also been found to be
associated with p53(18). Gene
sequencing is considered the gold standard for identifying genetic
alterations in p53. However, due to its high cost and limited
availability, gene sequencing was not performed in the present
study. A previous meta-analysis, including 36 studies, demonstrated
that an increased p53 expression, detected using IHC staining,
could predict mutations in TP53 in patients with HCC
(19). The p53 family plays a
central role in the tumorigenesis, treatment response and prognosis
of HCC. In recent years it has been revealed that all members of
the p53 family are expressed as a diverse variety of isoforms.
Depending on the isoform expressed, the role of a gene can change
from a tumor suppressor to an oncogene. Consequently, emphasis
needs to be placed on the DN isoforms of p63 and p73, which have
been shown to be critical for carcinogenesis and chemoresistance.
Thus, targeting specific p53 family isoforms may be the key to the
development of novel therapeutic strategies for HCC and other human
cancers (19). There have been
several proposed purely molecular HCC classifications and HCC
classification systems using combined histology and molecular
findings; however, none of these have been incorporated into
clinical care, as they do not provide additional relevant clinical
information beyond that obtained by imaging and histology.
Integrated morphological-molecular classifications of HCC are the
most likely to be robust and clinically useful, although this has
not yet been fully achieved (20).
Therefore, in the present study, IHC staining was performed to
determine the protein expression levels of mutant p53, which can
accumulate in the nuclei of cancer cells.
The results of the current study revealed a p53
immuno-positive rate of 85.4% in patients with HCC. However, the
positive p53 expression rate in patients with HCC was higher
compared with that of previous studies from Malaysia (21), the USA (22), Egypt (23) and Brazil (24), which demonstrated positive p53
expression rates of 38, 22.8, 41 and 42%, respectively. Consistent
with the results of the present study, a previous study from
Romania and another one from China, also found that p53 was
expressed in more than the half of the patients with HCC examined.
More specifically, 91 and 72.9% of patients with HCC in the
Romanian (25) and Chinese
(26) study, respectively, were
positive for p53 expression. Although all the aforementioned
studies interpreted nuclear staining as positive, not all of them
used a cut-off value of 10% to define a positive p53
expression.
In the present study, further analysis revealed no
association between p53 expression and sex, age, tumor size, number
of nodules, vascular invasion, liver cirrhosis, American Joint
Committee on Cancer (AJCC) staging and histological subtype.
However, a higher rate of p53-positive HCC cells was observed in
tumors >5 cm in diameter, single nodule HCC and HCC with
vascular invasion compared with their counterparts (80 vs. 20%,
62.9 vs. 37.1% and 77.1 vs. 22.9%, respectively). This finding may
be due the common occurrence of p53 mutations in large HCC with
microvascular invasion (27). A
previous study from Indonesia also demonstrated that p53 expression
was upregulated in HCC with tumor diameter of >10 cm (28). However, p53 mutations are more
frequently found in multinodular HCC (27) compared with a previous study, which
demonstrated that positive p53 expression in HCC was significantly
associated with single tumor and single lobe involvement (22). The prognosis of patients with HCC
associated with recurrence following hepatic resection remains a
major obstacle. Therefore, a previous study reported that the
5-year recurrence rate in HCC was ~70%, even in patients with a
single nodule of ≤2 cm in diameter (29). Nevertheless, the association
between p53 expression and the number of nodules in HCC warrants
further investigation.
Herein, the AJCC 8th edition staging system
(30,31) was used to stratify patient
prognosis. In the present study, patients of stage IB, II and IIIA
HCC were enrolled, while p53 expression was highest in those with
stage II HCC, followed by stage IB and stage IIIA. By contrast, a
previous study demonstrated that p53 expression was positively
associated with tumor staging, with stage III-IV HCC patients
showing a 90.9% positive p53 expression rate (26). Several previous studies included
HCC samples from patients up to stage IV (26,27).
In the aforementioned studies, patients with a higher HCC stage
exhibited increased p53 expression levels, whereas these levels
were significantly higher in the advanced stages compared with
those in the early stages of the disease (26). Herein, only patients with stage IA,
II and III HCC were included. However, the expression levels of p53
did not differ significantly among the three HCC stages.
Several distinct histological subtypes of HCC have
been recognized. Apart from the classic HCC, herein, patients
suffering from clear cell HCC, macrotrabecular-massive HCC and
steatohepatitic HCC were enrolled. Clear cell HCC, a subtype of
HCC, is characterized by clear and/or acidophilic ground glass
hepatocytes with cytoplasmic clearing due to glycogen accumulation
and fat storage in the cytoplasm (32). A previous study demonstrated that
clear cell HCC exhibits a more favorable prognosis compared with
classic HCC, while it is associated with the presence of
IDH1 mutations (33).
Macrotrabecular-massive HCC is characterized by the presence of
neoplastic cells arranged in thick trabeculae, coated by
endothelial cells and surrounded by dilated vascular spaces. This
HCC subtype is associated with a poor survival, vascular invasion
and TP53 mutations (34).
Macrovesicular steatosis, ballooning and inflammation are
histological features of steatohepatitic HCC. Steatohepatitic HCC
is associated with non-alcoholic fatty liver disease, the
activation of IL-6/JAK/STAT signaling, and less frequently, with
CTNNB1 and TP53 mutations (33,34).
In the present study, the expression levels of p53 were highest in
classic HCC followed by clear cell HCC, macrotrabecular-massive HCC
and steatohepatitic HCC. However, no statistically significant
differences were obtained in the expression levels of p53 among
these four HCC subtypes.
In the present study, only four samples from
patients with the massive macrotrabecular HCC subtype were
collected and all of them were positive for p53 expression. This
finding was consistent with the findings of previous research
demonstrating that TP53 inactivation was also notably
associated with the macrotrabecular massive HCC subtype (34). However, the molecular complexity of
HCC and other biological conditions, such as hepatitis B virus
infection, can enhance p53 expression and its effects on the
development of HCC (35).
Hepatitis B virus infection in patients with different HCC
subtypes, others than macrotrabecular HCC, may be associated with
the upregulation of p53 expression. Therefore, further studies are
required to clarify the reasons for the fact that p53 expression
was not increased in macrotrabecular-massive HCC, which is
associated with TP53 mutations. TP53-mutated HCCs are
associated with an unfavorable prognosis, viral infection, high
serum AFP levels, vascular invasion and proliferation, extensive
mitotic activity resulting in chromosomal instability and stem
cell-like properties. Moreover, a previous study stated that the
presence of telomerase reverse transcriptase promoter mutations,
alone or in combination with TP53 alteration, was associated
with the morphological progression of HCC, in terms of a higher
tumor grade and an architecture more related to aggressive behavior
(solid, macrotrabecular) (36).
In the present study, the World Health Organization
(WHO) grading system (20) was
used to classify patients with HCC into well differentiated,
moderately differentiated and poorly differentiated. The
differentiation status of HCC is considered as a prediction factor
of OS and disease-free survival following resection and/or
transplantation. The tumor cells of well-differentiated HCC
resemble the morphology of a mature benign hepatocyte with minimal
to mild nuclear atypia, whereas the tumor cells of poorly
differentiated HCC are clearly malignant and lack resemblance to
mature hepatocytes (35,37). Herein, a positive p53 expression
was associated with well- and poorly differentiated HCC. Compared
with well-differentiated HCC, higher p53 expression levels were
detected in moderately and poorly differentiated HCC. The results
of the present study were consistent with those of previous studies
demonstrating that p53 expression was associated with poor
differentiation in HCC (22,38).
In addition, the results were also consistent with those of a
previous study on the phenotypic and molecular associations in HCC,
which demonstrated that TP53-tumors were poorly
differentiated, with multinucleated and pleomorphic cells (34).
In addition, a previous study demonstrated that p53
mutations exhibited discordant effects on the survival of patients
with HCC of different racial backgrounds (39). Therefore, p53 mutations were
associated with a worse prognosis in Asian patients compared with
Caucasian ones and this effect was associated with their immune
cells. Indonesian patients were included in terms of race in the
majority of studies. The research revealed similar results on the
association of p53 expression with tumor grading, although p53
expression was not associated with HCC stage and subtype, since the
results did not reach statistical significance (39). A rigorously defined, easy to use,
reproducible and broadly adopted HCC grading system remains to be
developed. However, even with the current heterogeneous approaches,
tumor grade can predict patient survival and disease-free survival
following the curative resection of cirrhotic and non-cirrhotic
livers. The prognosis of patients with HCC is generally poor,
particularly for patients with advanced-stage HCC. It has been
reported that the 5-year survival rate of patients with symptomatic
and unresectable HCC is <5%. Long-term survival is likely only
achievable for patients with small and asymptomatic HCC, that can
be treated by complete resection, liver transplantation or adequate
locoregional treatment, including percutaneous radiofrequency
ablation or transarterial chemoembolization (20). According to the WHO, several
factors, including clinical, morphological and molecular factors,
are used to predict patient outcomes (20). Clinical features that are used to
predict the prognosis of patients with HCC include serum AFP
levels, tumor size and number, vascular invasion on imaging,
comorbidity and health condition. Additionally, in terms of
morphological features, tumor grade, vascular invasion and
intrahepatic metastasis, tumor stage, tumor subtype, the presence
or absence of liver cirrhosis and the IHC expression of cytokeratin
19 (CK19) are included. Other molecular features, such as
FGF19 amplification and gene expression profiling between
proliferative vs. non-proliferative subclasses have been considered
as prognostic factors of HCC. For example, patients with HCC with a
higher tumor size and grade, and substantial CK19 immunostaining
exhibit a worse prognosis (20).
The results of the present study suggest that p53
may play a crucial role in liver carcinogenesis in Indonesian
patients. Its expression was associated with well- and poorly
differentiated HCC, thus indicating a poorer prognosis. To the best
of our knowledge, this was the first study to determine the
expression levels of p53 in Indonesian patients with HCC and
evaluate their association with HCC-related prognostic factors.
However, the present study has some limitations, including its
single-centered nature, the small sample size, the lack of gene
sequencing results to verify the presence of p53 mutations and the
lack of overall survival assessment due to limited time and access
to obtain the complete clinical and therapeutic information.
Further studies are warranted to examine other predictive or
prognostic factors, such as assessing the association between p53
expression with treatment response or overall survival.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Ministry of
Research and Technology/National Agency for Research and Innovation
through the Research and Community Service Information System
(SIMLITABMAS/BRIN) grant and the Top Basic Research in University
(PDUPT) scheme (grant no. NKB-121, year 2021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was designed by NR and MS. The data were
curated by NR, MS, DRH and EK. AGP completed the formal analysis of
the data. NR secured funds and supervised the study. NR, MS and AGP
designed and performed the experiments. NR, MS, DRH and EK also
provided resources, supervised the study and validated the results.
AGP performed the statistical analyses and the visualization of the
results. AGP and MS confirm the authenticity of all the raw data.
NR, MS and AGP prepared the original draft. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study (protocol no. 21-05-0466) was
approved by the Medical Ethics Committee of the Faculty of
Medicine, Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital
(approval no. KET-427/UN2.F1/ETIK/PPM.00.02/2021). Patient consent
was waived by the committee as the study included already existing
data (waiver statement no. ND-784/UN2.F1/ETIK/PPM.00.02/2022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balogh J, Victor D III, Asham EH,
Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial M and Monsour H:
Hepatocellular carcinoma: A review. J Hepatocell Carcinoma.
3:41–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang CY and Li S: Clinical characteristics
and prognosis of 2887 patients with hepatocellular carcinoma: A
single center 14 years experience from China. Medicine (Baltimore).
98(e14070)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reveron-Thornton RF, Teng MLP, Lee EY,
Tran A, Vajanaphanich S, Tan EX, Nerurkar SN, Ng RX, Teh R,
Tripathy DP, et al: Global and regional long-term survival
following resection for HCC in the recent decade: A meta-analysis
of 110 studies. Hepatol Commun. 6:1813–1826. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Loho IM, Hasan I, Lesmana CR, Dewiasty E
and Gani RA: Hepatocellular carcinoma in a tertiary referral
hospital in Indonesia: Lack of improvement of one-year survival
rates between 1998-1999 and 2013-2014. Asian Pac J Cancer Prev.
17:2165–2170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takahashi Y, Dungubat E, Kusano H, Ganbat
D, Tomita Y, Odgerel S and Fukusato T: Application of
immunohistochemistry in the pathological diagnosis of liver tumors.
Int J Mol Sci. 22(5780)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Roberts LR: Biomarkers for hepatocellular
carcinoma. Gastroenterol Hepatol. 12:252–255. 2016.PubMed/NCBI
|
|
8
|
Marei HE, Althani A, Afifi N, Hasan A,
Caceci T, Pozzoli G, Morrione A, Giordano A and Cenciarelli C: p53
signaling in cancer progression and therapy. Cancer Cell Int.
21(703)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kanapathipillai M: Treating p53 mutant
aggregation-associated cancer. Cancers (Basel).
10(154)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Georgakilas AG, Martin OA and Bonner WM:
p21: A two-faced genome guardian. Trends Mol Med. 23:310–319.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aubrey BJ, Kelly GL, Janic A, Herold MJ
and Strasser A: How does p53 induce apoptosis and how does this
relate to p53-mediated tumour suppression? Cell Death Differ.
25:104–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sabapathy K and Lane DP: Understanding p53
functions through p53 antibodies. J Mol Cell Biol. 11:317–329.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhan P and Ji YN: Prognostic significance
of TP53 expression for patients with hepatocellular carcinoma: A
meta-analysis. Hepatobiliary Surg Nutr. 3:11–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ji YN, Wang Q and Xue J: TP53
immunohistochemical expression is associated with the poor outcome
for hepatocellular carcinoma: Evidence from a meta-analysis. Tumour
Biol. 35:1653–1659. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kan Z, Zheng H, Liu X, Li S, Barber TD,
Gong Z, Gao H, Hao K, Willard MD, Xu J, et al: Whole-genome
sequencing identifies recurrent mutations in hepatocellular
carcinoma. Genome Res. 23:1422–1433. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schulze K, Imbeaud S, Letouzé E,
Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C,
Shinde J, Soysouvanh F, et al: Exome sequencing of hepatocellular
carcinomas identifies new mutational signatures and potential
therapeutic targets. Nat Genet. 47:505–511. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Ho DWH, Lo RCL, Chan LK and Ng IOL:
Molecular pathogenesis of hepatocellular carcinoma. Liver Cancer.
5:290–302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Link T and Iwakuma T: Roles of p53 in
extrinsic factor-induced liver carcinogenesis. Hepatoma Res.
3:95–104. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu J, Li W, Deng M, Liu D, Ma Q and Feng
X: Immunohistochemical determination of p53 protein overexpression
for predicting p53 gene mutations in hepatocellular carcinoma: A
meta-analysis. PLoS One. 11(e0159636)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Torbenson M, Ng I, Park Y, Roncalli M and
Sakamato M: Hepatocellular Carcinoma. In: WHO Classification of
Tumours: Digestive System Tumours. Vol 1. 5th edition.
International Agency for Research on Cancer, Lyon, pp229-239,
2019.
|
|
21
|
Azlin AH, Looi LM and Cheah PL: Tissue
microarray immunohistochemical profiles of p53 and pRB in
hepatocellular carcinoma and hepatoblastoma. Asian Pac J Cancer
Prev. 15:3959–3963. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
You J, Yang H, Lai Y, Simon L, Au J and
Burkart AL: AT-rich interactive domain 2, p110α, p53, and β-catenin
protein expression in hepatocellular carcinoma and
clinicopathologic implications. Hum Pathol. 46:583–592.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abdou AG, Abd-Elwahed M, Badr M, Helmy M,
Soliman EA and Maher D: The differential immunohistochemical
expression of p53, c-Jun, c-Myc, and p21 between HCV-related
hepatocellular carcinoma with and without cirrhosis. Appl
Immunohistochem Mol Morphol. 24:75–87. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Felipe-Silva A, Wakamatsu A, dos Santos
Cirqueira C and Alves VAF: Immunohistochemistry panel segregates
molecular types of hepatocellular carcinoma in Brazilian autopsy
cases. World J Gastroenterol. 22:6246–6256. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Graur F, Furcea L, Mois E, Biliuta A, Rozs
AT, Negrean V and Al Hajjar N: Analysis of p53 protein expression
in hepatocellular carcinoma. J Gastrointestin Liver Dis.
25:345–349. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Z, Han C and Feng J: Relationship of
the expression levels of XIAP and p53 genes in hepatocellular
carcinoma and the prognosis of patients. Oncol Lett. 14:4037–4042.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park NH, Chung YH, Youn KH, Song BC, Yang
SH, Kim JA, Lee HC, Yu E, Lee YS, Lee SG, et al: Close correlation
of p53 mutation to microvascular invasion in hepatocellular
carcinoma. J Clin Gastroenterol. 33:397–401. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lalisang TJM, Moenadjat Y, Siregar NC and
Stephanie M: Overexpression of p53 in extra large (more than 10 cm)
hepatocellular carcinoma. Med J Indones. 27:71–75. 2018.
|
|
29
|
Roayaie S, Obeidat K, Sposito C, Mariani
L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M and
Mazzaferro V: Resection of hepatocellular cancer ≤2 cm: Results
from two Western centers. Hepatology. 57:1426–1435. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kamarajah SK, Frankel TL, Sonnenday C, Cho
CS and Nathan H: Critical evaluation of the American joint
commission on cancer (AJCC) 8th edition staging system for patients
with hepatocellular carcinoma (HCC): A surveillance, epidemiology,
end results (SEER) analysis. J Surg Oncol. 117:644–650.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bannasch P, Ribback S, Su Q and Mayer D:
Clear cell hepatocellular carcinoma: Origin, metabolic traits and
fate of glycogenotic clear and ground glass cells. Hepatobiliary
Pancreat Dis Int. 16:570–594. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
El Jabbour T, Lagana SM and Lee H: Update
on hepatocellular carcinoma: Pathologists' review. World J
Gastroenterol. 25:1653–1665. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Calderaro J, Couchy G, Imbeaud S, Amaddeo
G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage
P, et al: Histological subtypes of hepatocellular carcinoma are
related to gene mutations and molecular tumour classification. J
Hepatol. 67:727–738. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Qu JH, Zhu MH, Lin J, Ni CR, Li FM, Zhu Z
and Yu GZ: Effects of hepatitis B virus on p53 expression in
hepatoma cell line SMMU-7721. World J Gastroenterol. 11:6212–6215.
2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Maloberti T, De Leo A, Sanza V, Gruppioni
E, Altimari A, Riefolo M, Visani M, Malvi D, D'Errico A, Tallini G,
et al: Correlation of molecular alterations with pathological
features in hepatocellular carcinoma: Literature review and
experience of an Italian center. World J Gastroenterol.
28:2854–2866. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Marthins Filho SN, Paiva C, Azevedo RS and
Alves VAF: Histological grading of hepatocellular carcinoma-A
systematic review of literature. Front Med (Lausanne).
4(193)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Z, Gou W, Liu M, Sang W, Chu H and
Zhang W: Expression of P53 and HSP70 in chronic hepatitis, liver
cirrhosis, and early and advanced hepatocellular carcinoma tissues
and their diagnostic value in hepatocellular carcinoma: An
immunohistochemical study. Med Sci Monit. 21:3209–3215.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Duan X, Cai Y, He T, Shi X, Zhao J, Zhang
H, Shen Y, Zhang H, Zhang H, Duan W, et al: The effect of the TP53
and RB1 mutations on the survival of hepatocellular carcinoma
patients with different racial backgrounds. J Gastrointest Oncol.
12:1786–1796. 2021.PubMed/NCBI View Article : Google Scholar
|