Introduction
Porocarcinoma is a rare skin appendage carcinoma
related to the sweat gland duct and is known to be detected in
0.004% of skin biopsy specimens (1). Its prognosis is not favorable due to
the fact that it carries a significant risk of local recurrence, as
well as lymph node and distant metastases (2,3). At
present, the recommended treatment of localized porocarcinoma is
wide surgical resection and in the cases where lymph node
metastasis is present, radical lymph node dissection is added.
Although anthracylin-based chemotherapy or combination of
5-fluorouracil (5-FU), taxanes and cisplatin are considered to be
the first-line treatment for metastatic or locally-advanced
porocarcinoma, this type of tumor is recognized as relatively
chemoresistant, and no standard systemic treatment exists (4).
Mammalian target of rapamycin (mTOR) is a key
protein involved in carcinogenesis and is activated via the phospha
tidylinositol-3 kinase (PI3K)/AKT pathway. mTOR phosphorylates the
eukaryotic translation initiation factor 4E-binding protein 1
(4E-BP1), and then the phosphorylated 4E-BP1 (p4E-BP1) triggers
cell cycle progression, cell proliferation and angiogenesis
(5). Therefore, mTOR is believed to
be one of the most promising therapeutic targets in various types
of carcinomas. Recently, mTOR inhibitors have been demonstrated to
prolong the progression-free survival of patients with pancreatic
neuroendocrine tumor (6). Moreover,
the expression levels of mTOR pathway proteins have been suggested
to be predictive markers to gauge response to treatment with mTOR
inhibitors (7). However, the
expression profiles of mTOR pathway proteins in porocarcinoma have
yet to be elucidated. In this preliminary study, we investigated
the expression profiles of mTOR, 4E-BP1 and p4E-BP1 in
porocarcinoma cases, and discussed whether or not mTOR inhibitors
are suitable candidates for the treatment of porocarcinoma.
Materials and methods
Expression of mTOR, 4E-BP1 and p4E-BP1 was analyzed
in five cases of porocarcinoma (four invasive and one in
situ case), using immunohistochemical methods.
Immunohistochemical analyses were carried out using an autostainer
(XT system Benchmark; Ventana Medical Systems, Tucson, AZ, USA),
according to the manufacturer’s instructions. The following primary
antibodies were used: a rabbit monoclonal antibody against mTOR
(7C10; Cell Signaling Technology, Inc., Danvers, MA, USA), a rabbit
monoclonal antibody against 4E-BP1 (53H11; Cell Signaling
Technology, Inc.) and a rabbit monoclonal antibody against p4E-BP1
(Thr 37/46) (236B4; Cell Signaling Technology, Inc.).
The immunostaining procedures of these markers were
scored using semi-quantitative scoring, as described in a previous
study (8).
Results
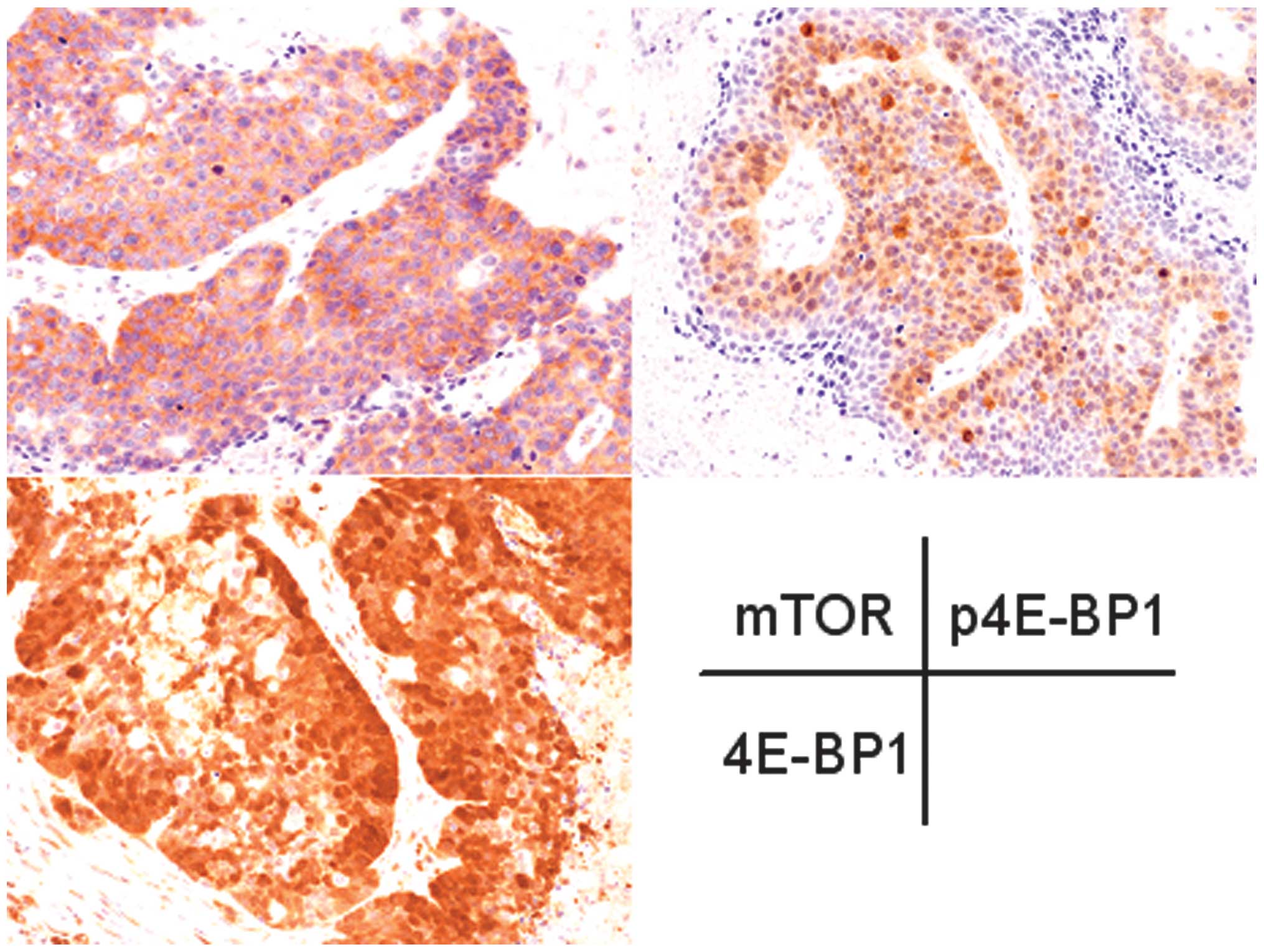
Table I shows the
immunohistochemical staining results of the mTOR pathway proteins.
The expression of mTOR was observed in the invasive porocarcinoma
cases, but not in the in situ porocarcinoma case. 4E-BP1 was
expressed in the invasive and in situ porocarconima cases.
Cytoplasmic p4E-BP1 expression was observed in 3/4 invasive
porocarcinoma cases, but not in the in situ porocarcinoma
case (Fig. 1).
 | Table I.Expression patterns of mTOR pathway
proteins in porocarcinoma cases (n=5). |
Table I.
Expression patterns of mTOR pathway
proteins in porocarcinoma cases (n=5).
| mTOR pathway proteins
|
|---|
| Porocarcinoma
cases | mTOR | 4E-BP1 | p4E-BP1 |
|---|
| Invasive (n=4) | 4/4 | 4/4 | 3/4 |
| In situ
(n=1) | 0/1 | 1/1 | 0/1 |
Discussion
To the best of our knowledge, this is the first
study to investigate the expression profiles of the mTOR pathway
proteins in porocarcinoma, and the overexpression of mTOR and its
downstream proteins in most of the included invasive porocarcinoma
cases was clearly demonstrated. Activation of the mTOR pathway has
been suggested to be highly involved in the pathogenesis of
extramammary Paget’s disease (9),
as well as in the carcinogenesis of porocarcinoma, especially
during the invasive stage.
Moreover, findings of a previous study demonstrated
that treatment with mTOR inhibitors led to growth inhibition and
cell cycle arrest in the G1 phase of carcinoma cells (10). The present study clearly
demonstrated that the mTOR pathway was activated in most of the
included invasive porocarcinoma cases. This finding, together with
the findings of previous studies, (6,10)
demonstrate that mTOR inhibitors are potential therapeutic
modalities for the treatment of metastatic or locally-advanced
porocarcinoma. Additional clinicopathological studies as well as
clinical trials concerning treatment with mTOR inhibitors in
patients with porocarcinoma are required to provide more definite
evidence.
References
|
1.
|
Mehregan AH, Hashimoto K and Rahbari H:
Eccrine adenocarcinoma. A clinicopathologic study of 35 cases. Arch
Dermatol. 119:104–114. 1992. View Article : Google Scholar
|
|
2.
|
Snow SN and Reizner GT: Eccrine
porocarcinoma of the face. J Am Acad Dermatol. 27:306–311. 1992.
View Article : Google Scholar
|
|
3.
|
Ishida M, Hotta M, Kushima R and Okabe H:
A case of porocarcinoma arising in pigmented hidroacanthoma simplex
with multiple lymph node, liver and bone metastases. J Cutan
Pathol. 38:227–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Perez-Garcia J, Morales R, Valverde CM, et
al: Eccrine porocarcinoma presenting with scrotal lymphedema: a
case report and review of systemic treatment. Ann Oncol.
21:9072010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Faivre S, Kroemer G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yao JC, Shah MH, Ito T, et al: Everolimus
for advanced pancreatic neuroendocrine tumors. N Engl J Med.
364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Duran I, Kortmansky J, Singh D, et al: A
phase II clinical and pharmacodynamic study of temsirolimus in
advanced neuroendocrine carcinomas. Br J Cancer. 96:1148–1154.
2006. View Article : Google Scholar
|
|
8.
|
Darb-Esfahani S, Faggad A, Noske A, et al:
Phospho-mTOR and phospho-4EBP1 in endometrial adenocarcinoma:
association with stage and grade in vivo and link with response to
rapamycin treatment in vitro. J Cancer Res Clin Oncol. 135:933–941.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chen S, Nakahara T, Uchi H, et al:
Immunohistochemical analysis of the mammalian target of rapamycin
signaling pathway in extramammary Paget’s disease. Br J Dermatol.
161:357–363. 2009.
|
|
10.
|
Gao N, Flynn DC, Zhang Z, et al: G1 cell
cycle progression and the expression of G1 cyclins are regulated by
PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J
Physiol Cell Physiol. 287:C281–C291. 2004. View Article : Google Scholar : PubMed/NCBI
|