Introduction
Vietnam is one of the countries in the Western part
of the Pacific-Ocean, with a high rate of hepatitis B virus (HBV)
infection and hepatocellular carcinoma (HCC). At present, the
prevalence of HBV infection in Vietnam is more than 8%, and in some
regions it may be up to 25% (1–4).
Vietnamese HBV strains share characteristics of mutations in the
core promoter and enhancement II region of the X gene in its
genome, similar to HBV strains from China, Hong Kong and Japan.
These mutations are risk factors for the development of severe
liver diseases and HCC (5–8). HBV is the leading cause of HCC in
Vietnam, with an incidence only less than that of lung and stomach
cancer in males (9,10). Recently, the Vietnamese government
has made serious efforts to persuade individuals, particularly
parents of neonates, to join the HBV vaccination program
nationwide. However, partly due to the living standards and the
differences in medical services available in the cities and
countryside, and partly due to lack of awareness, not all newborn
babies have been vaccinated at present. Serum α-fetoprotein (AFP)
is used as a routine test, although magnetic resonance imaging
(MRI) and computed tomography (CT) scans are only available in
large cities or in major provincial hospitals. Additionally, MRI
and CT scans are expensive for ordinary Vietnamese individuals.
Ultrasound (US) examination was introduced in Vietnam over 20 years
ago, however, good operators are working only in big hospitals at
present.
The above reasons render HCC a major health problem
in Vietnam, with an incidence >15/100,000 population (10,11).
Several HCC patients present in hospitals when the conditions are
irreversible, while a number of patients have liver tumor of a
diameter >5 cm. Multiple tumors within a liver parenchyma or
liver cirrhosis (LC) are extremely severe and may result in
multi-organ metastasis. Liver tumor resection, transcatheter
arterial chemoembolization (TACE), percutaneous ethanol injection
(PEIT) and radiofrequency ablation (RFA) are the key methods for
the treatment of HCC in Vietnam. However, early diagnosis of a
liver tumor remains a challenge. Similar to other countries in the
world, the serum AFP test, ultrasound examination or the
combination thereof are common methods for screening HCC at
present. However, ultrasound depends on the skill of the operator,
the body mass index of patients and is limited in differentiating
HCC from non-neoplastic nodules. In addition, varying sensitivity
and specificity of AFP has been observed in different population
studies, thus several patients are likely to be lost (12–18).
At present, newer and better markers are required for the
detection, surveillance and post-therapy follow-up of HCC. AFP is
currently the only tumor marker used in the clinical diagnosis of
HCC in Vietnam. However, few studies are available regarding the
development of new clinically applied markers for the detection of
HCC in Vietnamese patients (19).
Moreover, none of those studies have focused on protein induced by
vitamin K absence or antagonist-II (PIVKA-II). Since Liebman et
al first reported a high level of PIVKA-II in HCC patients
(20), several clinical studies
have been conducted to evaluate the role of PIVKA-II in different
patient populations worldwide (21–31).
Although several studies have reported heterogeneous results in
assessing the role of PIVKA-II in HCC patients, a number of these
studies have proven PIVKA-II to be a better tumor marker when
compared to AFP, in diagnosis as well as regarding tumor size,
progression of disease, vascular invasion and differentiating
cancer lesions from non-malignant diseases (31–36).
The present study is the first to evaluate the role of PIVKA-II in
the diagnosis of HCC in HBV-infected Vietnamese patients.
Materials and methods
Patients
A total of 166 consecutive chronic HBV-infected
patients (139 male and 27 female, mean age 39.8±16.4 years) from 15
different provinces in Northern Vietnam were enrolled in the study
[41 HCC, 43 LC, 26 chronic hepatitis B (CH) and 56 asymptomatic
carriers (ASC)]. The patients with a previous medical history of
antiviral treatment and HCC therapy or co-infection with hepatitis
C virus (HCV), human immuno-deficiency virus (HIV) were excluded.
The HCC lesions developed in patients with LC. This study was
performed in accordance with the principles of the Declaration of
Helsinki and was approved by the Ethnics Committee of Bach Mai
Hospital, Hanoi, Vietnam.
Clinical diagnostic criteria
Chronic HBV infection was confirmed based on the
medical history of the HBV status or positive HBV surface antigen
(HBsAg) test was carried out twice in the serum for at least 6
months. HCC confirmation was based on the pathophysiologic
examination or two-imaging modality with typical findings of liver
tumor that included the characteristics of a high density mass in
the arterial phase and a low density mass in the portal phase on
dynamic CT scan or MRI. The final diagnosis was evaluated by two
different histologists or radiologists unaware of additional
clinicobiochemical information on patients. LC and CH was diagnosed
based on clinical examination and biochemical test in combination
with ultrasound, CT scan and MRI, with or without upper
gastrointestinal endoscopy. ASC was evaluated by chronic HBV
infection, without any clinical signs/symptoms or abnormality of a
biochemical test and US examination.
AFP, PIVKA-II, HBV-DNA and HBV genotype
tests
The sera of collected patients were stored at −40°C
until use for further examinations at the Kobe University Graduate
School of Medicine. The AFP levels were evaluated using the
Microwell ELISA. The AFP test (Hope Laboratories, Pacoima, CA, USA)
and PIVKA-II levels were examined using Eitest PIVKA-II (Eisai Co.,
Ltd. Tokyo, Japan), according to the manufacturer’s instructions.
HBV-DNA was extracted from 200 μl of sera, using a QIAamp DNA blood
mini kit (QIAGEN GmbH, Hilden, Germany), following the
manufacturer’s instructions. The HBV-DNA levels were confirmed by
real-time PCR with a set of primers and TaqMan probe located in the
S gene, as previously described (32) and HBV genotype was classified by
PCR-RFLP, as previously reported (33).
Statistical analysis
The Chi-square, Fisher’s exact and Mann-Whitney U
tests (Wilcoxon rank-sum test), Kruskal-Wallis one-way analysis of
variance, receiver operating characteristic (ROC), univariate and
multivariate analyses were carried out to analyze the data using
the Stata software 8.0 (StataCorp LP, College Station, TX, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics of the patient
population
Table I shows the
characteristics of the study population. The mean age of the
patients was 39.8±16.4 years (range, 17–75), with the mean age in
the ASC group being the youngest (22.8±7.3 years) and it was
significantly younger compared to the other groups (P<0.0001).
The mean age in the LC and the HCC groups (50.8±12.4 and 49.8±12.4
years, respectively) was also higher (42.6±13.5 years) compared to
the CH group (P<0.05). The male to female ratio showed no
difference in the LC, CH and ASC groups (38/5, 20/6 and 42/14,
respectively), although it was significantly higher (39/2) in the
HCC, compared to the CH and ASC groups (P<0.05 and P<0.01).
The positive prevalence for HBV e antigen (HBeAg) in CH (3.8%) was
significantly lower compared to the other groups (P<0.05,
P<0.01 and P<0.001). However, the positive prevalence of
anti-HBe antibody in CH was higher compared to the ASC group (69.2
vs. 35.7%, P<0.01).
 | Table I.Characteristics of population
study. |
Table I.
Characteristics of population
study.
|
Characteristics | Total (n=166) | Clinical diagnosis
|
|---|
| HCC (n=41) | LC (n=43) | CH (n=26) | ASC (n=56) |
|---|
| Age (years) | 39.8±16.4
(17–75) |
49.8±11.0a,d(28–74) |
50.8±12.4a,d(18–75) |
42.6±13.5a,d(17–70) | 22.8±7.3d (19–51) |
| Gender (m/f) | 139/27 | 39/2a,b | 38/5 | 20/6a | 42/14b |
| HBeAg (+) | 114 (68.7%) | 9a,b (22%) | 14b (32.6%) | 1a–c (3.8%) | 28b,c (50%) |
| Anti-HBe (+) | 86 (51.8%) | 20 (48.8%) | 27b (62.8%) | 18b (69.2%) | 20b (35.7%) |
| Genotype | | | | | |
| B | 117 (70.5%) | 23a,b (56.1%) | 26a (60.5%) | 23a,b (88.5%) | 45a (80.4%) |
| C | 49 (29.5%) | 18a,b (43.9%) | 17a (39.5%) | 3a,b (11.5%) | 11a (19.6%) |
| HBV-DNA (log
copies/ml) | 5.5±2.1
(2.6–9.7) | 5.8±1.9b (2.6–9.5) | 6.2±1.8c (2.6–8.8) |
4.4±1.7a–c
(2.6–8.9) | 5.4±2.3a (2.6–9.7) |
| AFP (ng/ml) | 150±279
(0–901) | 344±356d (0–894) | 152±285d (0–863) | 143±261d (0.1–901) | 10.4±31.6d (0–179) |
| PIVKA-II
(mAU/ml) | 6,780±19,332
(4–75,000) |
16,201±25,386c,d (15–75,000) |
10,576±25,794a,c (9–75,000) |
184±431a,d(4–1,965) |
31.5±8.5c,d(17–59) |
The prevalence of the HBV genotype B in the CH and
ASC was significantly higher compared to the LC and HCC groups
(88.5, 80.4, 60.5 and 56.1%, respectively) (P<0.05 and
P<0.01), whereas the prevalence of HBV genotype C in the LC
(39.5%) and HCC (43.9%) groups was higher compared to the CH
(11.5%) and ASC (19.6%) groups. Together with the status of HBeAg
and anti-HBe, the HBV-DNA level in CH (4.4±1.7 log copies/ml) was
significantly lower compared to the other groups (P<0.05,
P<0.01 and P<0.001). The HBV-DNA level was the highest in the
LC (6.2±1.8 log copies/ml), although not significantly, compared to
the HCC (5.8±1.9 log copies/ml) and ASC (5.4±2.3 log copies ml)
groups. The AFP level was the highest in the HCC group, showing no
significant difference compared to the LC and CH groups. The
PIVKA-II level was significantly higher in the HCC compared to the
other groups, while the PIVKA-II level in the LC was higher
compared to the CH and ASC groups (P<0.05 and P<0.001).
Characteristics of AFP and PIVKA-II in
HCC
The comparison of sensitivity and specificity in AFP
and PIVKA-II is shown in Table II.
The levels of AFP and PIVKA-II showed a gradual increase from the
cut-off level (AFP 20 ng/ml and PIVKA-II 40 mAU/ml) to 2.5, 5, 10
and 20 times over the cut-off level of each marker. At each
compared level, the sensitivity of PIVKA-II was significantly
higher compared to the AFP (P=0.01, P=0.018, P=0.011 and P=0.007),
while the specificity of AFP was higher compared to PIVKA-II,
although this difference was not significant (P>0.05). The
significance of the combination of these two markers was also
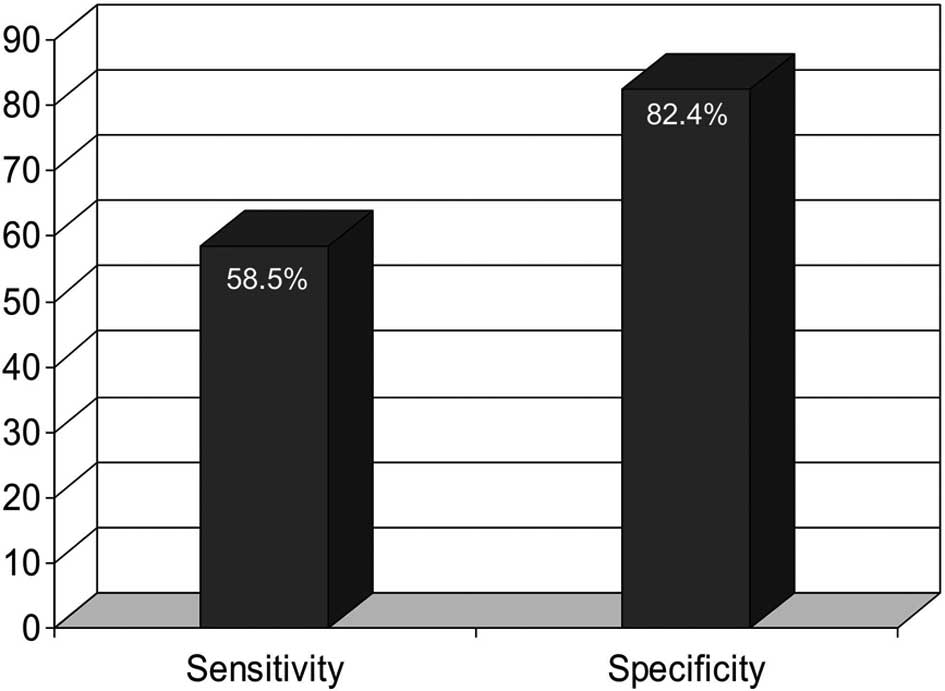
examined and compared. Fig. 1 shows
that the combination of the two markers had a significantly higher
specificity than either AFP (P=0.018) or PIVKA-II (P<0.001), and
a significantly lower sensitivity compared to PIVKA-II alone
(P=0.003), although not significantly lower compared to AFP
(P>0.05).
 | Table II.Sensitivity and specificity for the
detection of HCC using different cut-off levels of AFP and
PIVKA-II. |
Table II.
Sensitivity and specificity for the
detection of HCC using different cut-off levels of AFP and
PIVKA-II.
| AFP (ng/ml) | Sensitivity
(%) | Specificity
(%) | PIVKA-II
(mAU/ml) | Sensitivity
(%) | Specificity
(%) |
|---|
| ≥20 | 63.4a | 69.6 | ≥40 | 87.8a | 62.4 |
| ≥50 | 56.1b | 80.0 | ≥100 | 80.5b | 79.2 |
| ≥100 | 51.2c | 86.4 | ≥200 | 78.0c | 82.4 |
| ≥200 | 46.3d | 88.8 | ≥400 | 75.6d | 87.2 |
| ≥400 | 46.3 | 92.0 | ≥800 | 63.4 | 89.6 |
The characteristics of liver tumor in association
with AFP and PIVKA-II in 41 HCC patients are shown in Table III. Portal vein thrombosis was
present in 36.6% (15/41) of patients, with several liver tumors
located in the right (53.6%) or in both the left and right lobes
(34.2%), while few tumors were presented in the left lobe (12.2%).
In the present study, the liver tumors had a diameter <3 cm,
while the number of patients with a diameter of liver tumor <5
cm was ∼70.7%. AFP showed no significant association with any tumor
characteristics, including portal thrombosis, tumor localization,
number or size. By contrast, the PIVKA-II level in HCC patients
with portal vein thrombosis was significantly higher compared to
patients without portal vein thrombosis (32,860±29,954 vs.
6,590±16,312 mAU/ml, P=0.0025). In addition, PIVKA-II was
correlated with multiple tumors as compared to single tumors
(12,496±24,021 vs. 19,729±26,7207 mAU/ml, P=0.087). To further
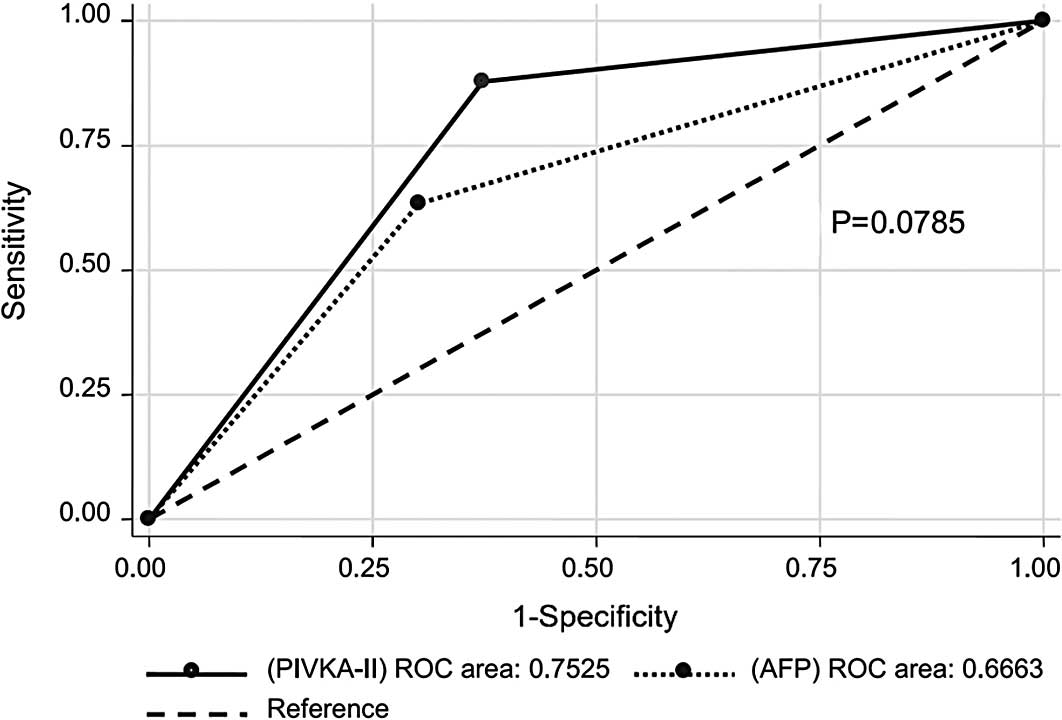
compare AFP and PIVKA-II, ROC was applied to analyze the data
(Fig. 2). The analysis demonstrated
that PIVKA-II was the better marker compared to AFP in HBV-infected
Vietnamese patients. The area under ROC (AUC) of PIVKA-II was
higher compared to AFP (0.7525 vs. 0.6663, P=0.0785).
 | Table III.Characteristics of HCC in association
with AFP and PIVKA-II. |
Table III.
Characteristics of HCC in association
with AFP and PIVKA-II.
|
Characteristics | No. (%) | AFP (ng/ml) | PIVKA-II
(mAU/ml) |
|---|
| Portal vein
thrombosis | | | |
| Present | 15 (36.6) | 427±342 |
32,860±29,954a |
| Absent | 26 (63.4) | 297±362 |
6,590±16,312a |
| Localization | | | |
| Left lobe | 5 (12.2) | 472±431 | 13,532±29,633 |
| Right lobe | 22 (53.6) | 347±368 | 15,628±25,990 |
| Both | 14 (34.2) | 295±324 | 18,055±24,791 |
| Tumor no. | | | |
| Single | 20 (48.8) | 351±384 |
12,496±24,021b |
| Multiple
(≥2) | 21 (51.2) | 338±337 |
19,729±26,720b |
| Tumor size
(cm) | | | |
| >3–5 | 12 (29.3) | 290±410 | 12,631±22,967 |
| >5 | 29 (70.7) | 367±367 | 17,678±26,565 |
Univariate and multivariate analyses of
risk factors for LC and HCC
The risk factors of LC and HCC are shown in Tables IV and V using univariate and multivariate
analyses. In Table IV, age (≥50
years), gender (male), HBV genotype C, HBV-DNA (≥5.0 log
copies/ml), AFP (≥20 ng/ml) and PIVKA-II (≥40 mAU/ml) were the risk
factors of LC by univariate analysis. By multivariate analysis,
however, only age (≥50 years) and PIVKA-II (≥40 mAU/ml) were the
independent risk factors of LC, although not for AFP (≥20 ng/ml).
Similar findings are shown in the data of Table V, i.e., the variables age (≥50
years), gender (male), HBV genotype C, AFP (≥20 ng/ml) and PIVKA-II
(≥40 mAU/ml) were the risk factors of HCC by univariate analysis.
However, by multivariate analysis, only PIVKA-II (≥40 mAU/ml) was
the independent risk factor of HCC. Nevertheless, the odds ratio of
AFP was relatively high, 2.1 (0.92–4.9) and P=0.076.
 | Table IV.Univariate and multivariate analysis
of the LC risk factors. |
Table IV.
Univariate and multivariate analysis
of the LC risk factors.
| Factors | Univariate (n=166)
| Multivariate
(n=166)
|
|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age (years) | | | | |
| ≥50 | 9.6 (4.4–21.2) | <0.0001 | 5.7 (2.2–15.0) | <0.001 |
| <50 | 1 | | 1 | |
| Gender | | | | |
| Male | 3.5 (1.4–8.9) | 0.007 | 2.2 (0.68–7.2) | 0.182 |
| Female | 1 | | 1 | |
| HBeAg | | | | |
| Positive | 0.69 (0.4–1.3) | 0.268 | 0.52 (0.2–1.5) | 0.227 |
| Negative | 1 | | 1 | |
| Genotype | | | | |
| C | 3.5 (1.7–7.1) | 0.001 | 2.6 (0.95–7.0) | 0.064 |
| B | 1 | | 1 | |
| HBV-DNA (log
copies/ml) | | | | |
| ≥5 | 2.3 (1.2–4.3) | 0.012 | 2.1 (0.79–5.7) | 0.133 |
| <5 | 1 | | 1 | |
| AFP (ng/ml) | | | | |
| ≥20 | 3.3 (1.7–6.3) | <0.0001 | 1.5 (0.6–3.7) | 0.396 |
| <20 | 1 | | 1 | |
| PIVKA-II
(mAU/ml) | | | | |
| ≥40 | 12.2
(5.9–25.3) | <0.0001 | 8.8 (3.7–20.9) | <0.0001 |
| <40 | 1 | | 1 | |
 | Table V.Univariate and multivariate analysis
of the HCC risk factors. |
Table V.
Univariate and multivariate analysis
of the HCC risk factors.
| Factors | Univariate (n=166)
| Multivariate
(n=166)
|
|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age (years) | | | | |
| ≥50 | 3.3 (1.6–6.8) | 0.001 | 1.6 (0.7–3.8) | 0.301 |
| <50 | 1 | | 1 | |
| Gender | | | | |
| Male | 4.9 (1.1–21.5) | 0.037 | 3.1 (0.6–15.9) | 0.179 |
| Female | 1 | | 1 | |
| HBeAg | | | | |
| Positive | 0.54
(0.23–1.3) | 0.140 | 0.47 (0.2–1.4) | 0.176 |
| Negative | 1 | | 1 | |
| Genotype | | | | |
| C | 2.4 (1.1–4.9) | 0.022 | 2.0 (0.76–5.3) | 0.159 |
| B | 1 | | 1 | |
| HBV-DNA (log
copies/ml) | | | | |
| ≥5 | 1.2 (0.6–2.6) | 0.563 | 1.02 (0.4–2.7) | 0.955 |
| <5 | 1 | | 1 | |
| AFP (ng/ml) | | | | |
| ≥20 | 4.1 (1.9–8.7) | <0.0001 | 2.1 (0.92–4.9) | 0.076 |
| <20 | 1 | | 1 | |
| PIVKA-II
(mAU/ml) | | | | |
| ≥40 | 11.9
(4.4–32.6) | <0.0001 | 7.4 (2.6–21.3) | <0.0001 |
| <40 | 1 | | 1 | |
Discussion
Screening is crucial to the early diagnosis of
cancer. At present, there are different methods and medications to
cure cancer patients, however, the earlier the diagnosis the better
the clinical outcomes. The combination of ultrasound and serum AFP
test is the most common method to screen HCC due to its being
cost-effective, non-invasive and available globally. However, these
tests are limited regarding clinical practice (12–18,34,36),
thus a number of patients are lost. Moreover, differentiating
cancer lesions from nodules in cirrhotic patients is challenging.
At present, a new HCC surveillance method is urgently required to
identify cost-effective and easy to use novel biomarkers suitable
for clinical activity that may replace the serum AFP test,
particularly in the diagnosis of HCC in cirrhotic patients at an
early stage of liver tumor (11,17,18,35).
PIVKA-II is one of the novel candidate markers for the detection of
HCC. In a physiological process, glutamic acids located in the
N-terminal of the prothrombin precursor are converted to γ-carboxyl
glutamic acids by vitamin K-dependent carboxylase. PIVKA-II is
produced when this pathway is disturbed in the absence of vitamin
K, or when a vitamin K antagonist is used. Liebman et al
first reported PIVKA-II in 1984 (20). PIVKA-II has since been considered a
specific marker for the detection of liver cancer in HCC patients,
and is studied worldwide. Most recently, PIVKA-II has also been
demonstrated to be highly present in advanced gastric cancer
patients (37,38).
Wang et al reported that the sensitivity of
PIVKA-II in detecting HCC was superior to AFP in patients with
liver tumor of a diameter >3 cm, as well as in patients with
liver tumor size 2–3 or <2 cm (28). However, a study comprising Japanese
patients showed that the sensitivity and utility of PIVKA-II for
the diagnosis of HCC was lower compared to AFP in the case of a
small liver tumor (<3 cm), but higher compared to AFP in case of
a large tumor (>5 cm) (23).
Durazo et al conducted a study in the American population
focusing on HBV-infected ethnic Asians, and found that PIVKA-II had
the highest sensitivity and positive predicting value compared to
AFP and AFP-L3 (39. Thus, the authors recommended that PIVKA-II be
used as the main serum test for HCC detection (39). Another American study comprising
mainly non-hispanic white, HCV-infected patients, also reported
PIVKA-II to be more sensitive and specific compared to AFP when
differentiating HCC from non-malignant chronic liver diseases
(29). By contrast, results
obtained from the HALT-C trial group in different American
ethnicities demonstrated AFP to be more sensitive compared to
PIVKA-II or APF-L3 in the detection of early or very early stages
of HCC with a new cut-off level of 10.9 ng/ml (24,25),
while another study found neither AFP nor PIVKA-II to be optimal
for the detection of HCC but served a complementary role to
ultrasound in the detection of early HCC (25). In addition, the combination of AFP
and PIVKA-II may increase the sensitivity up to 91% (25). In the present study on HBV-infected
Vietnamese patients and HCC tumors >3 cm, the data have shown
that PIVKA-II has a significantly higher sensitivity compared to
AFP at different analyzed serum levels (Table II), although the combination of
these tumor markers only enhances the specificity (Fig. 1) as previously reported in a study
on American subjects (25). The
decreased sensitivity when combining PIVKA-II to AFP in the present
study was consistent with the results obtained from a recent study
on Indian patients (31). The
results of the present study have again shown that the liver tumor
markers, AFP and PIVKA-II, are likely to be affected by etiologies
of liver diseases, patient demographics, stages of liver disease
and the characteristics of the HCC tumor. Thus, in the future,
large-scale population studies are required worldwide to examine
the role of liver tumor markers.
The number of tumors, liver tumor size and vascular
invasion indicate a poor prognosis of HCC patients (40–45).
When compared to AFP and AFP-L3, PIVKA-II is a good predictor for
portal vein thrombosis and microvascular invasion (26,27,30).
The present study reports that the level of AFP and PIVKA-II in HCC
patients with portal vein thrombosis is higher compared to HCC
patients without portal vein thrombosis. However, a statistically
significant difference was only found for PIVKA-II (32,860±29,954
vs. 6,590±16,312 mAU/ml, P=0.0025), as opposed to AFP (427±342 vs.
297±362 ng/ml, P>0.05). Demonstrating a certain percentage of
sensitivity (Table II), PIVKA-II
was found to be a better marker compared to AFP as it is a strong
independent factor for predicting LC and HCC in HBV-infected
Vietnamese patients (Tables IV and
V), while ROC analysis demonstrates
that the area under the ROC of PIVKA-II in the present study is
also higher compared to AFP (0.7525 vs. 0.6663, P=0.0785). In
contrast to a previous study (28),
findings of the present study have shown that PIVKA-II is not more
strongly correlated to liver tumor size than AFP, partly due to the
HCC patients in the study having a tumor size of more than 3 cm,
with approximately 70.7% of them with a liver tumor size of more
than 5 cm. In addition, PIVKA-II and AFP levels in the present
study were also relatively high in patients with LC and CH
(Table I).
In conclusion, the data in this first
cross-sectional study on examining the role of PIVKA-II in
HBV-infected Vietnamese patients indicate that PIVKA-II is likely
to be a good marker in the detection of HCC, the prediction of
portal vein thrombosis, as well as complementing the ultrasound in
clinical utility for screening HCC. However, further studies are
required to determine whether PIVKA-II is a better marker for the
diagnosis of HCC in chronic HBV-infected Vietnamese patients.
References
|
1.
|
Truong BX and Bang NV: The prevalence of
HBV infection, HCV infection, co-infection of HBV/HCV and HBV
genotype in the border region between Vietnam and China in Batxat
district, Laocai province. Vietnam J Med Res. 64:52–59. 2009.
|
|
2.
|
Duong TH, Nguyen PH, Henley K and Peters
M: Risk factors for hepatitis B infection in rural Vietnam. Asian
Pac J Cancer Prev. 10:97–102. 2009.PubMed/NCBI
|
|
3.
|
Nguyen VT, McLaws ML and Dore GJ: Highly
endemic hepatitis B infection in rural Vietnam. J Gastroenterol
Hepatol. 22:2093–2100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hipgrave DB, Nguyen TV, Vu MH, Hoang TL,
Do TD, Tran NT, Jolley D, Maynard JE and Biggs BA: Hepatitis B
infection in rural Vietnam and the implications for a national
program of infant immunization. Am J Trop Med Hyg. 69:288–294.
2003.PubMed/NCBI
|
|
5.
|
Truong BX, Yano Y, Seo Y, et al:
Variations in the core promoter/pre-core region in HBV genotype C
in Japanese and Northern Vietnamese patients. J Med Virol.
79:1293–1304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Truong BX, Seo Y, Yano Y, et al: Genotype
and variations in core promoter and pre-core regions are related to
progression of disease in HBV-infected patients from Northern
Vietnam. Int J Mol Med. 19:293–299. 2007.PubMed/NCBI
|
|
7.
|
Huy TT, Ushijima H, Quang VX, Ngoc TT,
Hayashi S, Sata T and Abe K: Characteristics of core promoter and
precore stop codon mutants of hepatitis B virus in Vietnam. J Med
Virol. 74:228–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Truong BX, Yano Y, Seo Y, et al: T1653
mutation (C to T1653) in the box-alpha of X gene in relation to
genotype, HBV-DNA level and HBeAg status in HBV-infected Vietnamese
patiens. Vietnam J Med Res. 45:26–31. 2006.
|
|
9.
|
Vuong DA, Velasco-Garrido M, Lai TD and
Busse R: Temporal trends of cancer incidence in Vietnam, 1993–2007.
Asian Pac J Cancer Prev. 11:739–745. 2010.PubMed/NCBI
|
|
10.
|
Anh PT and Duc NB: The situation with
cancer control in Vietnam. Jpn J Clin Oncol. 32(Suppl): S92–S97.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of world burden cancer in 2008:
GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sheu JC, Sung JL, Chen DS, et al: Early
detection of hepatocellular carcinoma by real-time ultrasonography.
A prospective study. Cancer. 56:660–666. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Oka H, Tamori A, Kuroki T, Kobayashi K and
Yamamoto S: Prospective study of alpha-fetoprotein in cirrhotic
patients monitored for development of hepatocellular carcinoma.
Hepatology. 19:61–66. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Di Bisceglie AM and Hoofnagle JH:
Elevation in serum alphafetoprotein levels in patients with chronic
hepatitis B. Cancer. 64:2117–2120. 1989.
|
|
15.
|
Bruix J, Sherman M, Llovet JM, et al:
Clinical management of hepatocellular carcinoma. Conclusion of the
Barcelona-2000 EASL conference. European Association for the Study
of the liver. J Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sherman M: Surveillance for hepatocellular
carcinoma. Semin Oncol. 28:450–459. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gupta S, Bent S and Kohlwes J: Test
characteristics of alpha-fetoprotein for detecting hepatocellular
carcinoma in patients with hepatitis C. A systemic review and
critical analysis. Ann Intern Med. 139:46–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Marrero JA and Lok AS: Newer markers for
hepatocellular carcinoma. Gastroenterology. 127(5 Suppl 1):
S113–S119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Khien VV, Mao HV, Chinh TT, Ha PT, Bang
MH, Lac BV, Hop TV, Tuan NA, Don LV, Taketa K and Satomura S:
Clinical evaluation of lectin-reactive alpha-fetoprotein-L3 in
histology-proven hepatocellular carcinoma. Int J Biol Markers.
16:105–111. 2001.
|
|
20.
|
Liebman HA, Furie BC, Tong MJ, Blanchard
RA, Lo KJ, Lee SD, Coleman MS and Furie B: Des-gamma-carboxy
(abnormal) prothrombin as a serum marker of primary hepatocellular
carcinoma. N Eng J Med. 310:1427–1431. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Cui R, He J, Zhang F, Wang B, Ding H, Shen
H, Li Y and Chen X: Diagnostic value of protein induced by vitamin
K absence (PIVKA-II) and hepatoma-specific band of serum
gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma
marker complementary to alpha-fetoprotein. Br J Cancer.
88:1878–1882. 2003. View Article : Google Scholar
|
|
22.
|
Nagaoka S, Yatsuhashi H, Hamada H, Yano K,
Matsumoto T, Daikoku M, Arisawa K, Ishibashi H, Koga M, Sata M and
Yano M: The des-gamma carboxy prothrombin index is a new prognostic
indicator for hepatocellular carcinoma. Cancer. 98:2671–2677. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nakamura S, Nouso K, Sakaguchi K, Ito YM,
Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E
and Shiratori Y: Sensitivity and specificity of des-gamma-carboxy
prothrombin for diagnosis of patients with hepatocellular
carcinomas varies according to tumor size. Am J Gastroenterol.
101:2038–2043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Marrero JA, Feng Z, Wang Y, et al:
Alpha-fetoprotein, des-gamma-carboxy prothrombin, and lectin-bound
alpha-fetoprotein in early hepatocellular carcinoma.
Gastroenterology. 137:110–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lok AS, Sterling RK, Everhart JE, Wright
EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky
HL and Dienstag JL; HALT-C Trial Group: Des-gamma-carboxy
prothrombin and alpha-fetoprotein as biomarkers for the early
detection of hepatocellular carcinoma. Gastroenterology.
138:439–502. 2010.PubMed/NCBI
|
|
26.
|
Miyaaki H, Nakashima O, Kurogi M, Eguchi K
and Kojiro M: Lens culinaris agglutinin-reactive alpha-fetoprotein
and protein induced by vitamin K absence II are potential
indicators of a poor prognosis: a histopathological study of
surgically resected hepatocellular carcinoma. J Gastroenterol.
42:962–968. 2007. View Article : Google Scholar
|
|
27.
|
Shirabe K, Itoh S, Yoshizumi T, Soejima Y,
Taketomi A, Aishima S and Maehara Y: The predictors of
microvascular invasion in candidates for liver transplantation with
hepatocellular carcinoma-with special reference of the serum levels
of des-gamma-carboxy prohrombin. J Surg Oncol. 95:235–240. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wang CS, Lin CL, Lee HC, Chen KY, Chiang
MF, Chen HS, Lin TJ and Liao LY: Usefulness of serum
des-gamma-carboxy prothrombin in detection of hepatocellular
carcinoma. World J Gastroenterol. 11:6115–6119. 2005.PubMed/NCBI
|
|
29.
|
Marrero JA, Su GL, Wei W, Emick D,
Conjeevaram HS, Fontana RJ and Lok AS: Des-gamma carboxyprothrombin
can differentiate hepatocellular carcinoma from nonmalignant
chronic liver disease in American patients. Hepatology.
37:1114–1121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Koike Y, Shiratori Y, Sato S, Obi S,
Teratani T, Imamura M, Yoshida H, Shiina S and Omata M:
Des-gamma-carboxy prothrombin as a useful predisposing factor for
the development of portal venous invasion in patients with
hepatocellular carcinoma: a prospective analysis of 227 patients.
Cancer. 91:561–569. 2001. View Article : Google Scholar
|
|
31.
|
Sharma B, Srinivasan R, Chawla YK, Kapil
S, Saini N, Singla B, Chakraborthy A, Kalra N, Duseja A and Dhiman
RK: Clinical utility of prothrombin induced by vitamin K absence in
the detection of hepatocellular carcinoma in Indian population.
Hepatol Int. 4:569–576. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Abe A, Inoue K, Tanaka T, Kato J, Kajiyama
N, Kawaguchi R, Tanaka S, Yoshiba M and Kohara M: Quantitation of
hepatitis B virus genomic DNA by real time detection PCR. J Clin
Microbiol. 37:2899–2903. 1999.PubMed/NCBI
|
|
33.
|
Mizokami M, Nakano T, Orito E, Tanaka Y,
Sakugawa H, Mukaide M and Robertson BH: Hepatitis B virus genotype
assignment using restriction fragment length polymorphism pattern.
FEBS Lett. 450:66–71. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Sato T, Tateishi R, Yoshida H, et al:
Ultrasound surveillance for early detection of hepatocellular
carcinoma among patients with hepatitis C. Hepatol Int. 3:544–550.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Forner A, Vilana R, Ayuso C, et al:
Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis:
prospective validation of the noninvasive diagnostic criteria for
hepatocellular carcinoma. Hepatology. 47:97–104. 2008. View Article : Google Scholar
|
|
36.
|
Nguyen MH, Garcia RT, Simpson PW, Wright
TL and Keeffe EB: Racial differences in effectiveness of
alpha-fetoprotein for diagnosis of hepatocellular carcinoma in
hepatitis C virus cirrhosis. Hepatology. 36:410–417. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Takano S, Honda I, Watanabe S, Soda H,
Nagata M, Hoshino I, Takenouchi T and Miyazaki M:
PIVKA-II-producing advanced gastric cancer. Int J Clin Oncol.
9:330–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Takahashi Y, Inoue T and Fukusato T:
Protein induced by vitamin K absence or antagonist-II-producing
gastric cancer. World J Gastrointest Pathophysiol. 1:129–136. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Durazo FA, Blatt LM, Corey WG, Lin JH, Han
S, Saab S, Busuttil RW and Tong MJ: Des-gamma-carboxy prothrombin,
alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis,
cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol.
23:1541–1548. 2008. View Article : Google Scholar
|
|
40.
|
Iwatsuki S, Dvorchik I, Marsh JW,
Madariaga JR, Carr B, Fung JJ and Starzl TE: Liver transplantation
for hepatocellular carcinoma: a proposal of a prognostic scoring
system. J Am Coll Surg. 191:389–394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Hemming AW, Cattral MS, Reed AI, Van Der
Werf WJ, Greig PD and Howard RJ: Liver transplantation for
hepatocellular carcinoma. Ann Surg. 233:652–659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Mazzaferro V, Chun YS, Poon RT, Schwartz
ME, Yao FY, Marsh JW, Bhoori S and Lee SG: Liver transplantation
for hepatocellular carcinoma. Ann Surg Oncol. 15:1001–1007. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Yao FY, Ferrell L, Bass NM, Bacchetti P,
Ascher NL and Roberts JP: Liver transplantation for hepatocellular
carcinoma: comparison of the proposed UCSF criteria with the Milan
criteria and the Pittsburgh modified TNM criteria. Liver Transpl.
8:765–774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Yao FY: Liver transplantation for
hepatocellular carcinoma: beyond the Milan criteria. Am J
Transplant. 8:1982–1989. 2008. View Article : Google Scholar : PubMed/NCBI
|