Introduction
Hepatitis B virus (HBV) infection is one of the most
significant public health problems worldwide. Approximately
one-third of the world’s 6.0 billion population is estimated to be
infected with HBV (1), including
350–400 million chronic carriers of this virus (2). The HBV vaccine applied at present is
primarily composed of HBsAg (S protein) expressed by Chinese
hamster ovary (CHO) cells and beer yeast. A total of 10–20% of the
population is known to be unresponsive or weakly responsive to the
HBV vaccine or even not to produce antibodies at all (3,4).
Vaccination is crucial in the prevention of HBV infection and there
are no specific therapies to manage it. Therefore, it is crucial to
develop a hepatitis B vaccine with a better penetrating and
responsive rate.
Hepatitis B vaccine made by recombinant DNA
techniques has the same degree of safety as the recombinant subunit
vaccine and the same efficacy to induce immune response as live
attenuated vaccine. It is relatively simple to clone and is a
purified DNA with no need for a synthetic protein in vitro,
and can sustain long-term immune efficacy (5). The recombinant DNA vaccine is also
relatively cost-effective and convenient to transport and preserve,
thus it is a promising approach for the vaccine development. HBV
DNA vaccination may induce the CD8+ T cell as well as
dominant Th1 phenotype among the splenic lymphocytes, to elicit
strong CTL and protective antibody levels (6).
To overcome traditional HBsAg vaccine immunogenicity
defects, HBV large envelope protein (L protein) was selected as a
dominant antigen, while granulocyte-macrophage colony
stimulating factor (GM-CSF) acted as an immune
adjuvant to enforce antibody response and construct an eukaryotic
expression plasmid pVAX1-L-GM containing preS1, preS2
and S genes of the L protein and immune adjuvant GM-CSF.
After the successful expression of the vaccine into the L-02 cell
line, immune responses were stimulated in mice to lay a foundation
for the development of a novel type of hepatitis B DNA vaccine.
Materials and methods
Ethics
The present study was conducted in the Department of
Microbiology and Immunology of the Medical College of the Jinan
University (Guangzhou, China). The Ethics Committee of The First
Affiliated Hospital of the Jinan University (Guangzhou, China)
approved the animal procedures and the experimental protocol.
Construction and identification of
recombinant plasmid
Based on the CDS sequence of the
preS1-preS2-S gene (GI:157091234), designed
the primers 5′-CAGCTAGCATGGGAGGTTGGTCTTCCAAA-3′ upstream)
(NheI) (Takara Bio Inc., Otsu, Japan) and
5′-GGCGGAAGCTTAATGTATACCCAAAGAC-3′ (downstream) (HindIII)
with appropriate restriction endonuclease sites. Hepatitis B DNA
extraction was obtained from the hepatitis B surface
antigen-positive serum (The First Affiliated Hospital of the Jinan
University) using phenol/chloroform extraction methods and used as
a template to amplify HBV preS1 preS2 S region. The coding
sequences of these GM-CSF fragments were synthesized using PCR from
pORF-GM-CSF using specific primers, upstream:
5′-CCAAGCTTGGTGGCGGTGGAAGCGGCGGTGGCGGAAGCGGCGGTGGCGGCAGCTGGCTGCAGAGCCTGCT-3′
(HindIII and Linker), downstream:
5′-CGGAATTCTCACTCCTGGACTGGCTC-3′ (EcoRI), and cloned into
pVAX1 using the standard cloning techniques. PCR and restriction
endonuclease assay were used to screen and identify positive
clones. DNA sequencing analysis (Sangon Biological Engineering and
Technology and Service Company, Shanghai, China) of the recombinant
pVAX1-L-GM identified the sucessful constructions of recombinant
plasmid pVAX1-L-GM.
Cell transfection
L-02 cells were digested with 0.25% trypsin and
diluted to 2×105 cells/ml and plated to 6-well plates
with 2 ml medium per well. Then, when cells were 70–80% confluent,
4 μg purified plasmid were transfected into the prepared cells
using 8 μl Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA,
USA).
Immunocytochemical staining
Non-transfected cells were considered as the blank,
while pVAX1-transfected cells as the negative comparison.
Immunocytochemical staining was performed according to the
manufacturer’s instructions (Boster Biological Technology, Ltd.,
Wuhan, China), and mouse anti-HBsAg antibody was used as the
primary antibody.
Western blot analysis
Western blot analysis of fusion proteins was
performed according to the standard procedure. The purified protein
was separated on 12% SDS-PAGE and transferred to nitrocellulose
membrane. Anti-HBsAg mAb antibody (Beijing Biosynthesis
Biotechnology Co., Beijing, China) at a dilution of 1:1000 or
Anti-GM-CSF mAb was used as the primary antibody to detect the
presence of protein. Blots were developed using the ECL (Thermo
Fisher Scientific, Inc., Rockford, IL, USA) method with HRP-labeled
rabbit anti-mouse IgG at a dilution of 1:5000.
ELISA assay protein levels of GM-CSF
Double-antibody sandwich ELISA (DAS-ELISA) was used
to detect the GM-CSF protein level according to the manufacturer’s
instructions (R&D Inc., Minneapolis, MN, USA). The results were
presented as the mean ± standard deviation (SD), and statistically
significant differences between values were analyzed using the SPSS
13.0 software. P<0.05 was considered to indicate a statistically
significant difference.
Animal immunization
Female BALB/c mice (n=30; 6–8 weeks old) were
purchased from the Experimental Animal Center of the Jinan
University (Guangzhou, China) and divided equally into 3 groups
(n=10/group). Mice were immunized intramuscularly individually with
pVAX1-L-GM, pVAX1 and pVAX1HBsAg. The mice were then injected with
a dose of 100 μg/100 μl plasmid pVAX1-L-GM, pVAX1 and pVAX1HBsAg, 3
times every second week. Blood was obtained from the tail each week
after immunization. The spleens of each mouse in the vaccinated
groups were removed aseptically at week 13 after the first
immunization.
Detection of specific anti-HBsAb
antibodies using the ELISA test
After the first-immunization, serum was collected
every week using the tail vein bleeding method. Absorbance at 450
nm was measured in a microplate reader, according to the
manufacturer’s instruction of the Mouse HBsAb ELISA kit (Wuhan
EIAab Science Co., Ltd., Wuhan, China). Levels of serum antibody in
immunized mice were monitored for 12 weeks.
Proliferation of splenocytes
Lymphocyte proliferation of immunized mice was
measured by MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide]. At week 13 following the first immunization,
mice were sacrificed, their spleens were aseptically removed and
splenocytes were prepared as single-cell suspensions. The cells
were cultured in triplicate using 96-well round-bottom plates at
2×107 cells per well. RPMI-1640 (Gibco, Paisley, UK)
supplemented with 10% fetal calf serum (FCS) was added to each
well, and stimulated with HBsAg at a final concentration of 10
μg/ml. Lymphocytes stimulated with the medium alone were used as
the negative control. The cells were incubated for 48 h at 37°C in
a humid atmosphere of 5% CO2, then 20 ml of MTT (5
mg/ml) (Sigma-Aldrich, Shanghai, China) was added to each well.
Following additional incubation for 4 h, the supernatant was
carefully aspirated and 200 μl of dimethylsulfoxide (DMSO) was
added into each well and absorbance of the soluble formazan was
measured at 570 nm using an automatic micro-plate reader (Bio-Rad,
Hercules, CA, USA).
Cytotoxicity assay
Splenocytes obtained from mice at week 13, following
the first immunization were cultured in 24-well plates with
complete culture RPMI-1640 medium [with 10% FBS, 50 μM
2-mercaptoethanol, 10 mM HEPES, 2 mM L-glutamine, 100 units of
penicillin per ml and 100 μg of streptomycin per ml]. Complete
culture RPMI-1640 medium was used containing 5 μg/ml Concanavalin A
(Dingguo Biotech, Beijing, China) and 10 U/ml of IL-2 (Pepro Tech,
London, UK) to culture splenocytes in vitro for 2 days as
the effector. The stimulator cells, harvested from naive mice, were
pulsed with the final concentration of 20 μg/ml of HBV-specific
peptide for 4 h at 37°C in 5% CO2, and were then treated
with 80 μg/ml mitomycin C for another 2 h. The cells were washed
extensively with RPMI-1640 medium. The effector cells
(4×107 cells) were incubated with stimulator cells at an
effector-stimulator ratio of 10:1 for 7 days in culture medium
containing 10 U/ml recombinant IL-2 (Peprotech, Rocky Hill, NJ,
USA) at 37°C in 5% CO2. The target cells were prepared
by P815 cells (mouse mastocytoma cell line, Shanghai Institute of
Biochemistry and Cell Biology of the Chinese Academy of Sciences)
pulsed with HBV-specific peptide for 4 h at 37°C in 5%
CO2. The cytotoxic activity was tested by
non-radioactive LDH release assay. The assays were performed in
triplicate with 1×104 target cells/well incubated with
effector cells at various effector cell/target cell (E:T) ratios of
100:1, 50:1, 25:1 and 12.5:1 in 96-well round-bottom plates,
according to the Non-Radioactive Cytotoxicity Assay Kit (Promega,
Madison, WI, USA). The absorbance values from the supernatants were
recorded at 490 nm using an ELISA microplate reader.
Analysis of the molecules of
CD4+ and CD8+ on the surface of T cell
At week 13 following immunization, the mice were
sacrificed and their spleens were removed aseptically.
Phosphate-buffered saline (PBS) buffer (0.1 mmol/l) was used to
wash the spleen cells and cell suspension was collected. The
CD4+/CD8+ detection kit (Beckman Coulter,
Inc., Brea, CA, USA) required the volume of 100 μl of each sample
intake, in order to detect the number of CD4+,
CD8+ molecules on the surface of spleen T cells using
the Epics XL flow cytometry (Beckman Coulter, Miami, FL, USA).
Cytokines of IFN-γ and IL-2 secretion
assays
The splenocytes of immunized mice were cultured
following the same procedure in the proliferation assays for 72 h.
Following incubation, the supernatant from each well was removed
for evaluation of secreted IFN-γ and IL-2 levels using ELISA. The
concentrations of IFN-γ and IL-2 in the culture supernatant were
measured using murine cytokine ELISA kits (R&D Systems,
Minneapolis, MN, USA). The limit of the detection was 2 pg/ml.
Statistical analysis of data
Measurement data show the mean ± SD. The statistical
software SPSS was used to perform statistical analysis. Differences
between groups were analyzed using stochastic analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
In vitro expression of the recombinant
plasmid
To determine whether or not the recombinant plasmid
pVAX1-L-GM was expressed in vitro, L-02 cells were
transiently transfected with pVAX1-L-GM or pVAX1 and their
expression at a protein level was detected using western blot
analysis and ELISA assay, respectively. Western blot analysis
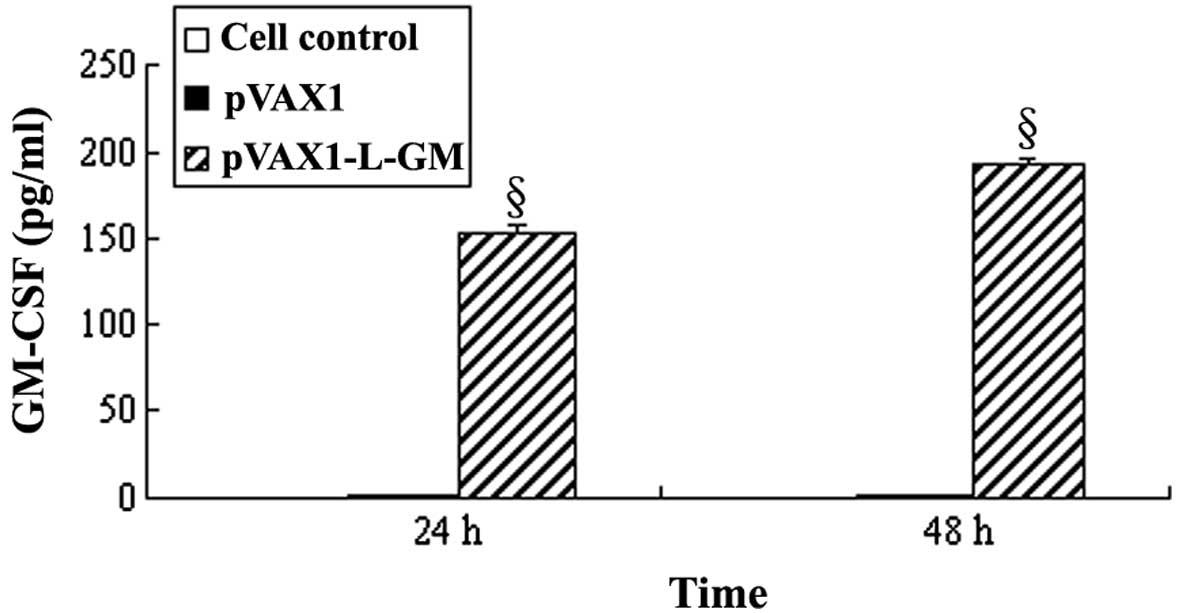
indicated a protein level of ∼64 kDa, as expected (Figs. 1 and 2). Expression values of GM-CSF proteins in
the pVAX1 control group were 1.382±0.081 pg/ml and 1.382±0.081
pg/ml (mean ± SD, n=3). While the expression values of the GM-CSF
proteins of the pVAX1-L-GM-transfected group were 153.073±4.20 and
193.124±3.943 pg/ml, compared to the negative control groups (cell
control and pVAX1), the results were considered statistically
significant (P<0.05) (Fig.
3).
Detection of specific anti-HBsAb
antibodies using ELISA
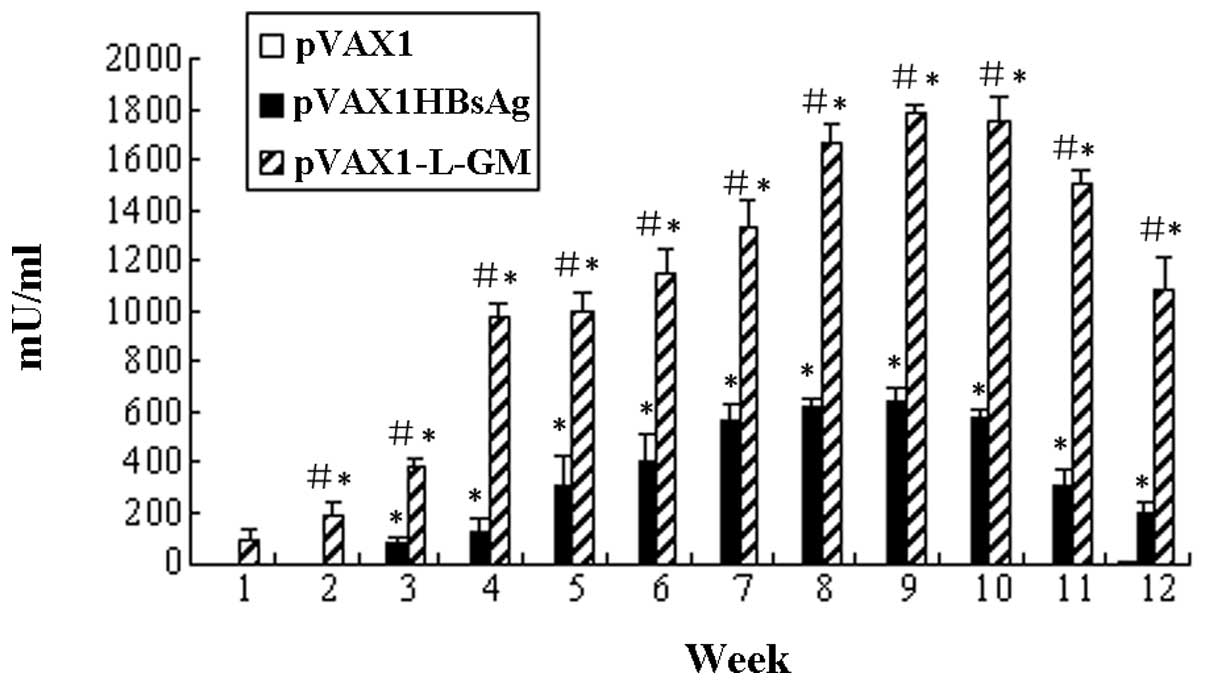
To assess the effect of the pVAX1-L-GM DNA vaccine
on the humoral responses in mice, blood samples were collected
using the tail vein bleeding method each week following the first
immunization, and the sera were isolated. The presence of
anti-HBsAg-specific antibodies in sera was analyzed by ELISA.
Specific antibody response was detectable in the pVAX1-L-GM and
pVAX1HBsAg groups (Fig. 4).
Statistical analysis of antibody levels of pVAX1-L-GM and
pVAX-1HBsAg groups was performed and an enhanced antibody response
was observed in the pVAX1-L-GM group, and the difference was
statistically significant (P<0.05). The pVAX1-L-GM group
produced antibody 2 weeks earlier than the control plasmid pVAX1
and pVAX1HBsAg groups.
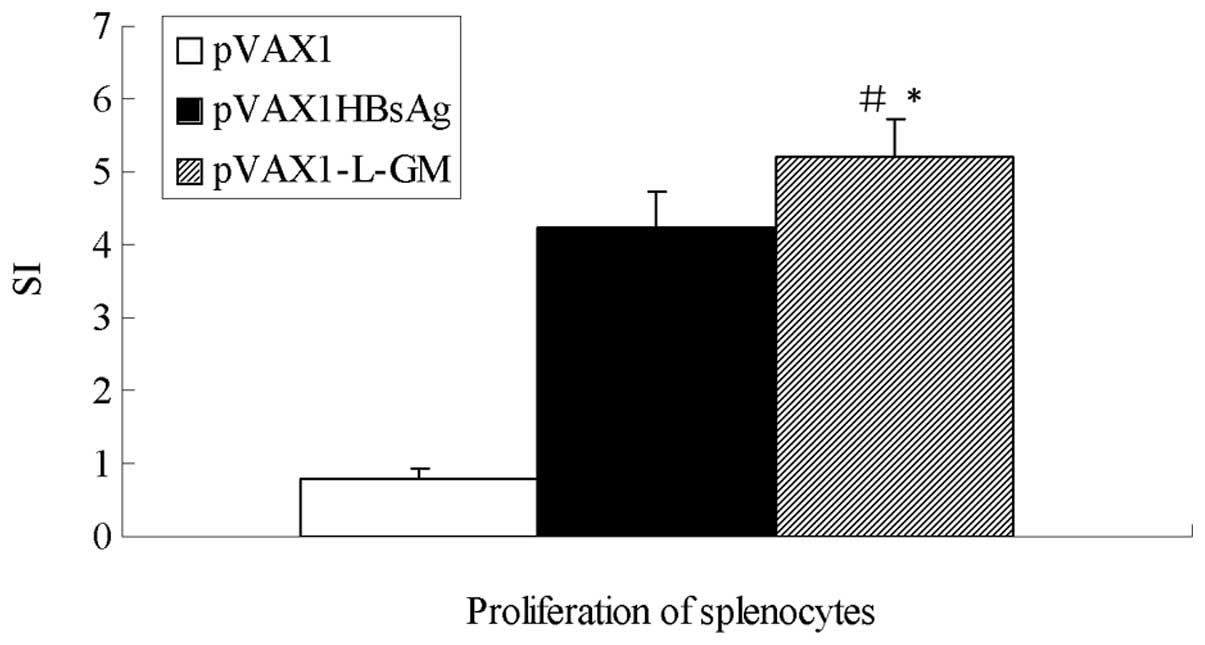
Proliferation of splenocytes
To determine whether or not the pVAX1-L-GM DNA
vaccine influenced cell-mediated immunity, a single-cell suspension
of lymphocytes was prepared from immunized mice at week 13 after
immunization. As shown in Fig. 5,
mice immunized with pVAX1-L-GM elicited the highest level of
splenocyte T-cell proliferation compared to the pVAX1HBsAg and
pVAX1 groups (P<0.05).
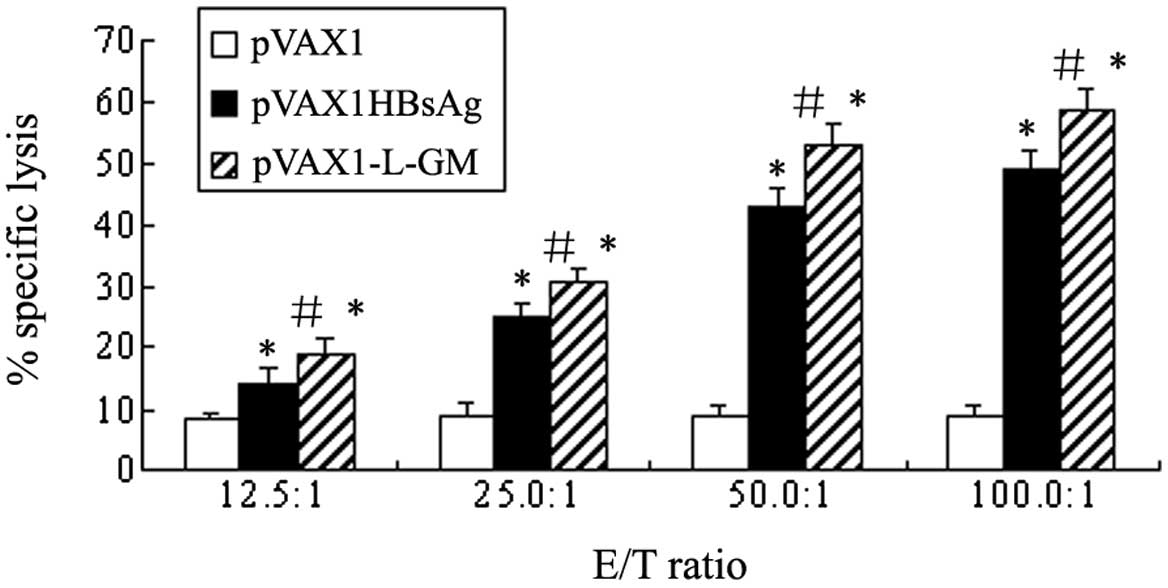
Cytotoxicity assay
In the protection against or eradication of viruses
or other intracellular pathogens, specific cytotoxic responses have
been previously demonstrated to be a key factor. To analyze the
ability of recombinant pVAX1-L-GM to enhance the HBsAg-specific CTL
response, splenic cells, derived from the immunized mice 13 weeks
after immunization, were restimulated specifically by naive mice
splenocytes pulsed with HBsAg-specific peptides in vitro for
7 days. P815 cells pulsed with HBsAg-specific peptides were used as
target cells. The cytotoxic activity was tested using
non-radioactive LDH release assay and the specific lysis rates are
shown in Fig. 6. HBsAg-specific CTL
was detectable in the mice immunized with the HBV DNA vaccine
pVAX1-L-GM plasmids compared to the pVAX1HBsAg or pVAX1 groups at
the E/T ratio of 100:1 (P<0.05). The specific CTL activities
increased significantly in the pVAX1-L-GM and pVAX1HBsAg groups
compared to the pVAX1 group, while the strongest CTL response was
detected at the E/T ratio of 100:1 (P<0.01). The results
demonstrated that cellular immunity was markedly enhanced by
pVAX1-L-GM DNA and pVAX1HBsAg DNA vaccine plasmids.
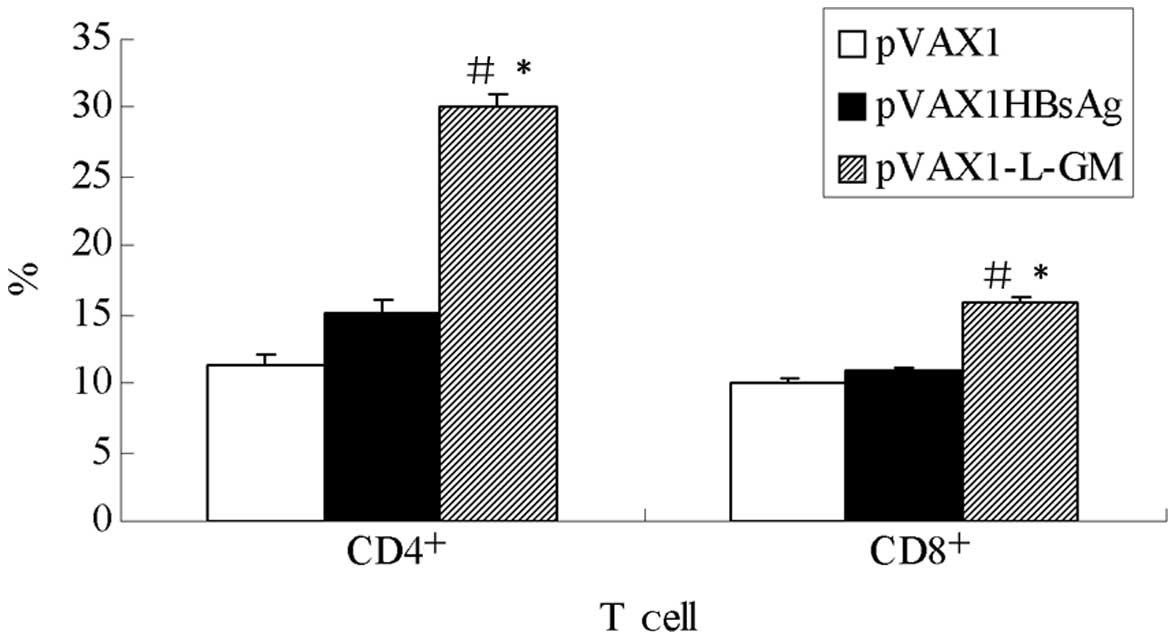
Analysis of the molecules of
CD4+, CD8+ on the surface of T cell
To evaluate the subsets of T cells, total T cells
were isolated at week 13 following immunization and re-stimulated
in culture with HBsAg. These cells were then analyzed using FACS
with a gate set on CD4+ and CD8+ T cells. As
shown in Fig. 7, the number of
CD4+ and CD8+ molecules on the surface of
spleen T cell produced by the pVAX1-L-GM group was higher compared
to the pVAX1HBsAg group, and the difference was statistically
significant (P<0.05), indicating that the GM-CSF
gene enhanced cell immune function.
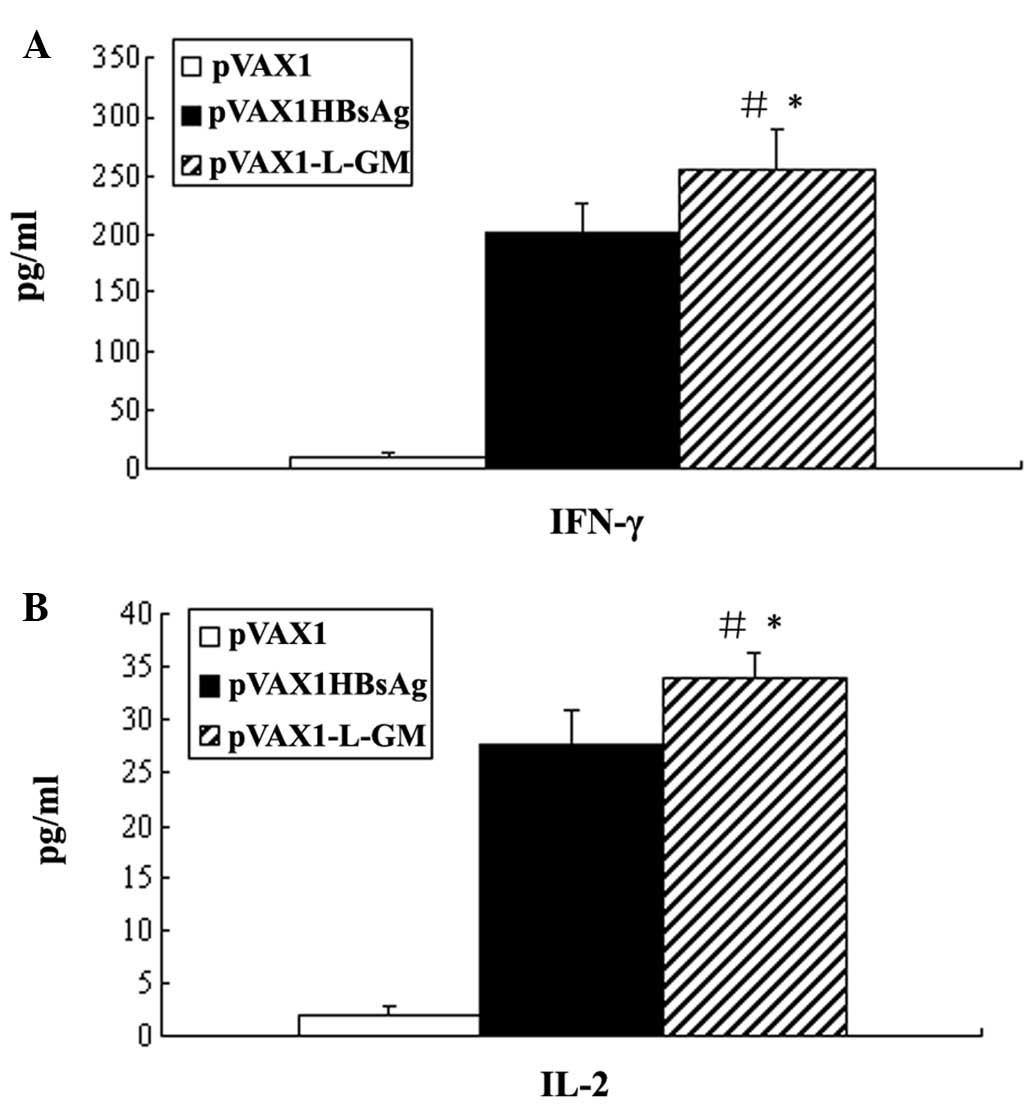
Cytokines of IFN-γ and IL-2 secretion
assays
We quantified the production of the cytokines IFN-γ
and IL-2 released from splenocytes from immunized mice
re-stimulated with HBsAg in vitro. Mice immunized with DNA
vaccine pVAX1-L-GM elicited a significant enhancement of IFN-γ and
IL-2 production, even significantly higher compared to the
pVAX1HBsAg group (P<0.05) (Fig.
8). This finding suggested that this DNA vaccine resulted in
stronger Th1-type cell immune response.
Discussion
In this study, the L protein was used as an antigen.
Compared to the gene of the S protein, the gene of L protein has
more preS1 and preS2 gene. Previous studies
demonstrated that the preS1 (21–47aa) is the liver cell
receptor binding site, which binds the HBV into the host cell
membrane. Previous studies have also demonstrated that the
preS1 peptide has several T- and B-cell epitopes, and that
anti-preS1 serum is able to neutralize the toxicity of HBV
and protect against HBV infection gorillas (7–9).
PreS2 (120–145aa) sequence also contains multiple epitopes
which are able to neutralize the virus. It is able to mediate HBV
adhesion and the invasion of liver cells, and with strong
immunogenicity, is able to induce the generation of neutralizing
antibodies and protective immunity. Containing the specific T and B
lymphocytes binding site, it is able to break the body immune
tolerance of existing HBsAg vaccine (10–12).
There has been an increasing demand for gene
adjuvant therapy both in the treatment and prevention of HBV in
recent years, thus GM-CSF as a preponderant adjuvant
is important in activating endotheliocytes and macrophagocytes
through various mechanisms. It can also regulate the amount and
function of antigen-presenting cells and enhance the cyto-activity
of CTL and NK, thereby strengthening the immune level of the
hepatitis B vaccine. Several clinical trials showed that
GM-CSF is able to increase antibody levels in
subjects in a rapid, effective and constant manner (13–15).
Therefore, GM-CSF was used as an immune adjuvant, for
studies of gene adjuvant in the field of DNA vaccine immunopotency
improvement.
In this study, we used vector-pVAX1, which was
approved by the Food and Drug Administration (FDA) and could be
used in the late stages of human disease, to clone a recombinant
plasmid which containing the gene of the L protein and the
GM-CSF gene, through Linker to connect the fusion
gene consisting of glycine (G) and serine (S) to five peptides
(16). Thus, the recombinant
plasmid was transfected into L-02 cells using liposome transfection
methods, confirming the successful construction of pVAX1-L-GM by
restriction enzyme digestion and sequencing, and also confirming
the stable expression of fusion protein using western blot analysis
and ELISA.
To determine whether or not the pVAX1-L-GM plasmid
has any effect on the cell and humoral immune responses in BALB/c
mice, three groups of mice were immunized in the study with
negative control plasmid (pVAX1 group), positive control plasmid
(pVAX1HBsAg group) and recombinant plasmid (pVAX1-L-GM group). The
level of antibody was then detected and the results showed that the
antibody level of the experimental pVAX1-L-GM group was higher
compared to the empty vector pVAX1 and positive control pVAX1 HBsAg
groups. The difference was statistically significant (P<0.05).
Additionally, studies have demonstrated that the presence of
antibodies in the pVAX1-L-GM group was recorded two weeks earlier
compared to the pVAX1HBsAg group. Splenocytes from mice in the
three groups were evaluated for antigen-specific proliferation as
well as the CTL component of immune response by the specific
killing of syngeneic target cells pulsed with a recognized CTL
epitope peptide. The number of CD4+ and CD8+
molecules on the surface of spleen T cells and the level of IFN-γ
and IL-2 was also detected. The strongest HBsAg-specific
proliferative activity in the pVAX1-L-GM group was in concordance
with the highest level of CTL activity and CD4+,
CD8+ molecules and IFN-γ, IL-2 production in the two
groups. The level of CD8+ on cytotoxic T lymphocyte was
increased, indicating that cell immunity was significantly enhanced
after immunization by the fusion L-GM gene. The
results indicated that the plasmid pVAX1-L-GM is able to enhance
the specific cell and humoral immune responses in mice.
Possible reasons for these results are that the L
protein has several advantageous features of its antigens, compared
to the commercially available hepatitis B HBsAg vaccine whose
component contains only S antigen. GM-CSF as an adjuvant is able to
stimulate the activation of antigen-presenting cells, promote the
secretion of IL-2 and the proliferation of CD4+ and
CD8+ cells, thus it is likely to be important in
enhancing the DNA vaccine induction of humoral and cell immune
responses.
In summary, the results suggest that L protein
stimulated the cell and humoral immune response following
immunization of mice with L-GM fusion gene. The
immune effect of vaccine pVAX1-L-GM was superior to that of
pVAX1HBsAg vaccine since cell immunity is considered to be the most
essential host immune response to eradicating the virus. Therefore,
this study provided a novel method to enhance the effect of
hepatitis B DNA vaccine, as well as an effective means to develop
hepatitis B DNA vaccine as a prevention and treatment vaccine.
Additional investigations into the immune protection of the vaccine
may have better prospects.
Acknowledgements
The present study was supported by the
Research Fund of the Science and Technology Plan of Guangdong
Province (no. A20101006-2006) and the Major National Science and
Technology (S&T) Special Projects (no. 2008ZX10002-009).
References
|
1
|
Poorolajal J, Mahmoodi M, Majdzadeh R,
Nasseri-Moghaddam S, Haghdoost A and Fotouhi A: Long-term
protection provided by hepatitis B vaccine and need for booster
dose: a meta-analysis. Vaccine. 28:623–631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen T, Yan XM, Zou YL, Gao JM and Dong H:
Virologic characteristics of hepatitis B virus in patients infected
via maternal-fetal transmission. World J Gastroenterol.
14:5674–5682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kubba AK, Taylor P, Graneek B and Strobel
S: Non-responders to hepatitis B vaccination: a review. Commun Dis
Public Health. 6:106–112. 2003.PubMed/NCBI
|
|
4
|
Jin L, Wang G, Zhao X, et al:
Characterization and immune effect of the hepatitis B-BCG combined
vaccine for using a needle innoculation. Vaccine. 28:6041–6051.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanif SN, Al-Attiyah R and Mustafa AS: DNA
vaccine constructs expressing Mycobacterium tuberculosis-specific
genes induce immune responses. Scand J Immunol. 72:408–415. 2010.
View Article : Google Scholar
|
|
6
|
Qing Y, Chen M, Zhao J, et al:
Construction of an HBV DNA vaccine by fusion of the GM-CSF gene to
the HBV-S gene and examination of its immune effects in normal and
HBV-transgenic mice. Vaccine. 28:4301–4307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JH, Cho EW, Lee YJ, Shin SY and Kim
KL: Determination of the protective effects of neutralizing
anti-hepatitis B virus (HBV) immunoglobulins by epitope mapping
with recombinant HBV surface-antigen proteins. Microbiol Immunol.
44:703–710. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lian M, Zhou X, Chen B, Li C, Gu X, Luo M
and Zheng X: Identification of the critical regions in hepatitis B
virus preS required for its stability. J Pept Sci. 14:307–312.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petersen J, Dandri M, Mier W, et al:
Prevention of hepatitis B virus infection in vivo by entry
inhibitors derived from the large envelope protein. Nat Biotechnol.
26:335–341. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JH, Lee MK, Kim HS, Kim KL and Cho
EW: Targeted destruction of the polymerized human serum albumin
binding site within the preS2 region of the HBV surface antigen
while retaining full immunogenicity for this epitope. J Viral
Hepat. 10:70–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin Y, Lin F, Zhuang Q, Liu L and Qian C:
Generation of full-length functional antibody against preS2 of
hepatitis B virus in hepatic cells in vitro from bicistrons
mediated by gutless adenovirus. Bio Drugs. 23:391–397.
2009.PubMed/NCBI
|
|
12
|
Salyaev RK, Stolbikov AS, Rekoslavskaya
NI, Shchelkunov SN, Pozdnyakov SG, Chepinoga AV and Hammond RV:
Obtaining tomato plants transgenic for the preS2-S-HDEL gene, which
synthesize the major hepatitis B surface antigen. Dokl Biochem
Biophys. 433:187–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cruciani M, Mengoli C, Serpelloni G, Mazzi
R, Bosco O and Malena M: Granulocyte macrophage colony-stimulating
factor as an adjuvant for hepatitis B vaccination: a meta-analysis.
Vaccine. 25:709–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizza P, Ferrantini M, Capone I and
Belardelli F: Cytokines as natural adjuvants for vaccines: where
are we now? Trends Immunol. 23:381–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasaki MG, Foccacia R and de
Messias-Reason IJ: Efficacy of granulocyte-macrophage
colony-stimulating factor (GM-CSF) as a vaccine adjuvant for
hepatitis B virus in patients with HIV infection. Vaccine.
21:4545–4549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gustavsson M, Lehtiö J, Denman S, Teeri
TT, Hult K and Martinelle M: Stable linker peptides for a
cellulose-binding domain-lipase fusion protein expressed in
Pichia pastoris. Protein Eng. 14:711–715. 2001. View Article : Google Scholar : PubMed/NCBI
|