Introduction
The capsid of the lentiviral vector (LV) is
disassembled shortly after LV is endocytosed into the cytoplasm and
the retrotranscription process is initiated, producing a
double-strand viral DNA. This double-strand viral DNA, together
with matrix protein (MA), integrase (IN), HIV-1 viral protein R
(Vpr) and cellular factors, form the preintegration complex (PIC).
Subsequently, PIC binds to importin α (Imα) through the interaction
of Imα with the nuclear location signal (NLS) located in MA and IN
(1–4). Furthermore, Vpr was shown to increase
the Imα affinity for NLS (5).
The Imα carrying PIC is then combined with importin
β, forming the Imα/β heterodimer. The Imα/β interacts with a
nucleoporin of phenylalanine-glycine repeats, leading to PIC import
(6). Subsequently, PIC is
disassembled in the nucleus through the binding of RanGTP to
importin β, resulting in the separation of PIC from Imα. The
double-strand viral DNA is then integrated into the target cell
genome to achieve LV infection.
The glucocorticoid receptor (GR) is also combined
with Imα and transported to the nucleus, even in the absence of
glucocorticoid (3). GR has two NLSs
and one nuclear export signal. The first NLS (NLS-1), which is
located in the DNA-binding domain and is similar to the SV40 NLS,
binds Imα. The second NLS (NLS-2), which is located in the
ligand-binding domain, binds glucocorticoid. GR is localized to the
cytoplasm in the absence of hormone and localizes to the nucleus
following hormone binding (3).
Since GR is able to bind Imα, GR retention in the nucleus may
theoretically result in Imα redistribution, affecting PIC
import.
In the cytoplasm, GR transports transcription
factors to the nucleus and alters their activity (e.g., nuclear
factor NF-κB), triggering gene transcription modulation (7). In the nucleus, GR may directly bind to
a specific DNA sequence, referred to as glucocorticoid response
element (GRE), which is a short sequence of DNA within the promoter
of a gene that is able to bind the GR complex and regulate
transcription. Depending on the cell line, glucocorticoids may
differentially affect HIV-1 expression (8).
Despite the well-known effect of dexamethasone (Dex)
on HIV-1 gene transcription, it has not thus far been determined
whether the binding of Dex to GR affects the Imα redistribution.
This study was designed to confirm the Imα redistribution induced
by Dex through detecting the luciferase (Luci) activity (LucA) in
cells transfected with the Luci reporter LV. The Imα redistribution
affects LV infection, as well as other nucleophilic imports.
Materials and methods
Luci expression vector construct
The Luci gene was obtained from the pGl3 plasmid
(Promega, Madison, WI, USA) by a polymerase chain reaction and
cloned into pcDNA™6.2-GW/miR (Invitrogen, Carlsbad, CA, USA) to
form pcDNA™6.2-GW/Luci. pcDNA6.2-GW/Luci was then recombined with
pDONR™221 (Invitrogen) to generate a pDONR™Luci entry clone (BP
recombination). The pDONR Luci entry clone was again recombined
with pLenti6/V5-DEST™ (Invitrogen) to construct pLenti6/Luci (LP
recombination). Subsequently, the products of the LP recombination
were treated with proteinase K and then transformed into One
Shot® Stbl3™ Chemically Competent E. coli to
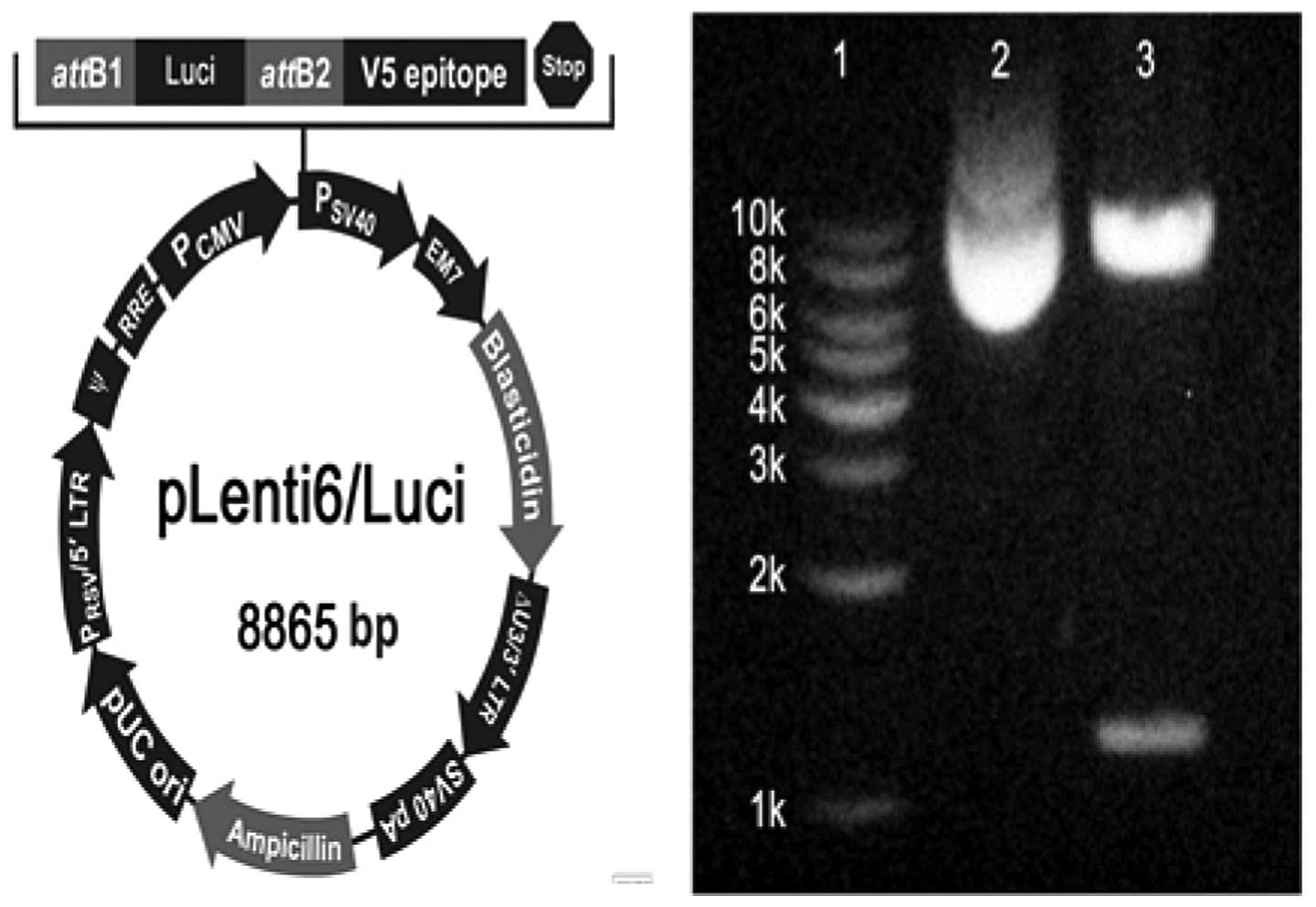
obtain pLenti6/Luci (Fig. 1).
pLenti6/Luci was identified with nucleic acid electrophoresis and
LucA assay.
LV preparation
Preparation of the DNA complex
A total of 9 μg of ViraPower™ Packaging Mix
(Invitrogen) and 3 μg of pLenti6/Luci were added to 1.5 ml
Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St. Louis, MO,
USA) without serum and mixed gently.
Preparation of the GenEscort III
complex
A total of 36 μl GenEscort III (Wisegen
Biotechnology Corp., Nanjing, China) were diluted in 1.5 ml DMEM
without serum, mixed gently and incubated for 5 min at room
temperature.
Preparation of the transfection
complex
The DNA complex was added to the GenEscort III
complex, mixed gently and incubated for 20 min at room temperature.
At the same time, the 293T cells were resuspended in DMEM at a
density of 1.2×106 cells/ml. Subsequently, DNA-GenEscort
III was added to a 10-cm tissue culture plate containing 5 ml DMEM
supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 0.1 mM
minimum essential medium (MEM) non-essential amino acids and 1 mM
MEM sodium pyruvate. The 293T cell suspension (5 ml) was added to
the plate and mixed by gentle rocking. Finally, the cells were
incubated overnight at 37°C in a CO2 incubator. The
following day, the medium containing DNA-GenEscort III was removed
and replaced with complete culture medium. The supernatants were
harvested 48–72 h after transfection, centrifuged at 1,000 × g for
5 min at 4°C and stored at −80°C. The LV was titered immediately
prior to use. All the operations applied to Biosafety Level 2.
Cell culture and pretreatment
The 293T cells (1×103/well) were
maintained in 96-well plates containing 200 μl DMEM (as described
above) and each well was repeated 3 times. RU486 (1×10−6
M; Sigma) was used for stimulating GR shuttling to the nucleus
(9). Bimax1 (2.5×10−8 M;
Shanghai Science Peptide Biological Technology Co., Ltd, Shanghai,
China) was used for inhibiting Imα (10,11).
Pretreatment with Dex (Sigma) was classified into 0-, 5-, 15-, 30-,
60- and 120-min groups (12); the
grouping for Dex treatment was the same as that for Dex
pretreatment. The 30-min group of Dex pretreatment was again
classified into two dose groups of 1×10−7 and
1×10−6 M (Dex-1 and Dex-2, respectively) (13). All the cells were used for LucA
assay 72 h after pretreatment or treatment.
Animals
An amount of 0.5 mg/10 g Dex and LV
(titer=106) was administered by intraperitoneal
injection (14). A total of 42
Kunming mice of clean grade (half of the animals were female,
although the gender of the animal was not considered a significant
factor) were randomly assigned into 7 groups as follows: LV-nC
(LucA-negative control); LV-1C [20 μl LV/mouse, normal saline (NS)
pretreatment control]; LV-2C (100 μl LV/mouse, NS pretreatment
control); LV-1/P-1 (20 μl LV/mouse, 30-min Dex pretreatment);
LV-2/P-1 (100 μl LV/mouse, 30-min Dex pretreatment); LV-1/P-2 (20
μl LV/mouse, 15-h Dex pretreatment); LV-2/P-2 (100 μl LV/mouse,
15-h Dex pretreatment). Seven days after the injection of LV, the
mice were anaesthetized with 0.4% pentobarbital sodium and
sacrificed and their livers were immediately excised. The mice were
maintained and handled in accordance with the National and
International Guiding Principles for Biomedical Research Involving
Animals, as promulgated by the Society for the Study of
Reproduction.
LucA assay
The 293T cells and 0.2 g of mouse liver were lysed
in cell culture lysis reagent (Promega) at −80°C for 30 min and
then immersed in 37°C water bath for 5 min. Subsequently, the
lysates were collected and centrifuged at 13,800 × g for 2 min at
4°C to remove cell debris. The LucA in 100 μl of lysate was
assessed by the addition of 100 μl Luci assay reagent (Promega).
After 30 min of pre-incubation, the produced light was measured for
10 sec with the GloMax®-Multi Jr Single Tube Multimode
Reader (Promega).
Statistical analysis
Data were analyzed for significant differences by
the independent samples t-test. A P<0.05 was considered to
indicate a statistically significant difference. The tests were
performed with SPSS software, version 10.0 for Windows (SPSS, Inc.,
Chicago, IL, USA). All the values are presented as means ± standard
deviation.
Results
Effect of Dex on LucA (Fig. 2)
Considering that Dex results in GR retention in the
nucleus, it may be hypothesized that cytoplasmic Imα is decreased
due to the ability of GR to bind Imα, which suggests that Dex
pretreatment may inhibit PIC import. To determine whether Dex
affects PIC import, the 293T cells were pretreated with Dex. As a
result, the LucA in the Dex pretreatment group was significantly
higher compared to that in the LV alone group (P≤0.01).
Furthermore, the LucA in the 30-min Dex pretreatment group was the
highest, with a significant difference between the 30-min Dex
pretreatment group and the 15- or the 60-min groups (P≤0.01).
However, these results were not sufficient to determine whether
cytoplasmic Imα is decreased even if Dex improves LV transcription.
To verify that Dex improves LV transcription, the 293T cells were
treated with Dex following transfection. The LucA in the Dex
treatment groups was significantly higher compared to that in the
LV alone group (P≤0.01), with the LucA in the 5-min Dex treatment
group being the highest. Of note, the LucA in the 5-min Dex
treatment group was higher compared to that in the 5-min Dex
pretreatment group; however, the LucA in 30-min Dex treatment group
was lower compared to that in the 30-min pretreatment group
(P≤0.01), which suggests that pretreatment for 30 min with Dex
improves PIC import as well as LV transcription, whereas treatment
for 30 min with Dex only improves LV transcription.
Effect of Bimax1 on Imα alleviated by Dex
(Fig. 3)
The increased LucA may be partly attributed to the
increasing levels of cytoplasmic Imα. Thus, LucA may be decreased
by Bmax1 and the decreased LucA may be elevated through increasing
the Dex dose. To verify the above, the 293T cells were pretreated
with Bmax1, followed by the addition of Dex-1 and Dex-2 30 min
prior to transfection. The LucA in the Bmax1 group was
significantly lower compared to that in the LV alone group
(P≤0.01), suggesting that Bimax1 was able to block PIC import
through inhibiting Imα. In comparison with the Bmax1 group, the
LucA in the Dex-1 and Dex-2 groups was significantly elevated
(P≤0.01), with the LucA present in the Dex-2 group being higher
compared to that in the Dex-1 group (P≤0.01). These results suggest
that the elevated LucA may be attributed to the Imα increase
mediated by Dex, as well as to the transcription enhanced by
Dex.
Effect of RU486 on Imα (Fig. 4)
To exclude the effect of transcription on LucA, Dex
was replaced with RU486. The 293T cells, which were pretreated with
RU486, followed by the addition of Bmax1 30 min prior to
transfection, were transfected with LV. Compared to the RU486 alone
group, the LucA in the Bmax1 group was significantly decreased
(P≤0.01), further confirming that the increased LucA was associated
with the increasing cytoplasmic Imα levels.
Effect of Dex on LucA in vivo (Fig. 5)
The mice were pretreated with Dex either 30 min or
15 h prior to LV transfection. The 30-min pretreatment aimed to
increase cytoplasmic Imα levels, whereas the 15-h pretreatment
aimed to suppress immune and inflammatory responses. Compared to
the LV-1C and LV-2C groups, the LucA in the LV-1/P-1 and LV-2/P-1
groups, respectively, was not increased (P≥0.05), although the LucA
in the LV-1/P-2 and LV-2/P-2 groups was significantly elevated
(P≤0.01). These findings suggest that the efficiency of LV
transfection depends mainly on suppressing immune and inflammatory
responses in vivo.
Discussion
To the best of our knowledge, this study was the
first to demonstrate that the effect of Dex on LV infection is
associated with Imα, suggesting that Imα may be a novel pathway
mediating the effects of Dex on HIV-1 infection.
Dex increases cytoplasmic Imα levels. Imα may bind
to the NLS-1 of GR in the absence of gluocorticoid (3). Thus, the cytoplasm contains two types
of Imα, namely Imα and Imα-GR. Following Dex pretreatment, Dex is
combined with the NLS-2 of GR to form two new complexes, GR-GR-Dex
and Imα-GR-Dex (15). Therefore,
the cytoplasm contains Dex, GR, Imα, Imα-GR, Imα-GR-Dex and
GR-GR-Dex, in a dynamic balance. Dex promotes GR import, thus
resulting in the nuclear retention of GR (7,16–18).
Subsequently, the GR-GR dimer changes into GR in order to bind GRE,
triggering LV gene transcription modulation (19). This may be a mechanism through which
Dex pretreatment leads to elevated LucA. Furthermore, the nuclear
retention of GR-GR results in decreased cytoplasmic GR levels.
According to the balance theory, cytoplasmic GR attenuation
inhibits the binding of GR to Imα, resulting in increased
cytoplasmic Imα levels and improving PIC import. This may be
another mechanism underlying the association of elevated LucA with
Dex pretreatment. In addition, Dex induces the downregulation of GR
(20), which may also help increase
cytoplasmic Imα levels.
Bimax1, a peptide with a NLS-like sequence
exhibiting a high affinity for Imα (11), may block the import of the nucleus
accumbens-associated protein 1 by the Imα pathway (10). In our study, Bimax1 impaired the PIC
import that was induced by Dex, resulting in decreased LucA.
However, increasing the Dex dose elevated LucA, which was
attributed to promoting PIC import and LV gene transcription. RU486
binds to the co-activator pocket of GR (GR1), with domain swapping
of the GR3 between the subunits of the GR dimer (21), inducing GR shuttling to the nucleus,
without stimulating subsequent events (22). In the present study, Dex was
replaced by RU486. LucA was elevated by RU486, but decreased by
Bimax1, further suggesting that the change in cytoplasmic Imα
levels affects LV infection.
Increased cytoplasmic Imα levels may also result
from a change in the GR affinity for Imα. The conformational change
of the GR D-loop and second helix was shown to confer a change in
the GR affinity for Imα (23,24),
which was, however, not investigated in the present study.
Increasing cytoplasmic Imα levels favor PIC
shuttling to the nucleus. The interaction of Imα with IN and MA
results in PIC import (2,25–27),
which is followed by the integration of double-strand LV DNA into
the target cell genome. Subsequently, the GR accumulated in the
nucleus is combined with LV GRE to improve Luci transcription,
resulting in increased LucA. Several previous experiments also
confirmed that Dex improves LV gene transcription (28–30).
Of note, the inactivation of NLS within IN and MA
was previously reported to inhibit viral infection (2,26).
Considering that PIC import depends on Imα (31–33),
the inactivation of NLS within Imα may also inhibit HIV-1
infection. The majority of the currently available drugs for the
treatment of HIV-1 have focused on inhibiting viral entry, viral
genome replication and virus-specific proteolysis. However,
numerous viruses are able to exploit cellular kinases, facilitating
subcellular targeting during infection to achieve drug resistance
(34). Accordingly, blocking PIC
import may suppress HIV-1 infection through the Imα pathway,
although it remains unclear whether inhibiting Imα leads to HIV-1
drug resistance due to the HIV-1 accumulation in the cytoplasm.
In vivo, increasing LucA depends mainly on
immunosuppression and anti-inflammation. Following intraperitoneal
injection, LV must go through several processes to reach the target
cell. During these processes, LV may be sequestered in bypass
organs and destroyed by opsonization, immune and inflammatory
responses, resulting in significantly impaired gene delivery. Dex
is one of the most frequently used immunosuppressive and
anti-inflammatory drugs. The binding of Dex to GR suppresses the
transcription of numerous cytokines, adhesion molecules and
proinflammatory genes via the NF-κB pathway (35). Vpr is a coactivator of GR and its
effects, including suppressing IL-12 transcription in human
monocytes and inducing the apoptosis of human CD4+ T
cells and thymocytes, are enhanced by the binding of Dex to GR
(36–38). Megadose Dex may preserve lysosomal
membrane integrity by rapid non-genomic effects and long-term
receptor-dependent genomic events (14). In our study, it was demonstrated
that Dex promotes LV infection via the Imα pathway.
In conclusion, LucA may be increased through
improving PIC import and LV transcription. The improved PIC import
by Dex is associated with Imα, suggesting that Imα may be a novel
pathway mediating the effects of Dex on HIV-1 infection.
Acknowledgements
This study was funded by a grant from the Less
Developed Region of the National Science Foundation of China (no.
81060273). The authors would like to thank Professor Bijun Peng and
Wei Dong for their help.
References
|
1
|
Jayappa KD, Ao Z, Yang M, Wang J and Yao
X: Identification of critical motifs within HIV-1 integrase
required for importin α3 interaction and viral cDNA nuclear import.
J Mol Biol. 410:847–862. 2011.PubMed/NCBI
|
|
2
|
Levin A, Armon-Omer A, Rosenbluh J,
Melamed-Book N, Graessmann A, Waigmann E and Loyter A: Inhibition
of HIV-1 integrase nuclear import and replication by a peptide
bearing integrase putative nuclear localization signal.
Retrovirology. 6:1122009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carrigan A, Walther RF, Salem HA, Wu D,
Atlas E, Lefebvre YA and Hache RJ: An active nuclear retention
signal in the glucocorticoid receptor functions as a strong inducer
of transcriptional activation. J Biol Chem. 282:10963–10971. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lange A, McLane LM, Mills RE, Devine SE
and Corbett AH: Expanding the definition of the classical bipartite
nuclear localization signal. Traffic. 11:311–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Popov S, Rexach M, Ratner L, Blobel G and
Bukrinsky M: Viral protein R regulates docking of the HIV-1
preintegration complex to the nuclear pore complex. J Biol Chem.
273:13347–13352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Iaco A and Luban J: Inhibition of HIV-1
infection by TNPO3 depletion is determined by capsid and detectable
after viral cDNA enters the nucleus. Retrovirology. 8:98–116.
2011.PubMed/NCBI
|
|
7
|
Ou XM, Chen K and Shih JC: Glucocorticoid
and androgen activation of monoamine oxidase A is regulated
differently by R1 and Sp1. J Biol Chem. 281:21512–21525. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russo FO, Patel PC, Ventura AM and Pereira
CA: HIV-1 long terminal repeat modulation by glucocorticoids in
monocytic and lymphocytic cell lines. Virus Res. 64:87–94. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitra D, Sikder SK and Laurence J: Role of
glucocorticoid receptor binding sites in the human immunodeficiency
virus type 1 long terminal repeat in steroid-mediated suppression
of HIV gene expression. Virology. 214:512–521. 1995. View Article : Google Scholar
|
|
10
|
Okazaki K, Nakayama N, Nariai Y, Nakayama
K, Miyazaki K, Maruyama R, Kato H, Kosugi S, Urano T and Sakashita
G: Nuclear localization signal in a cancer-related transcriptional
regulator protein NAC1. Carcinogenesis. 33:1854–1862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marfori M, Lonhienne TG, Forwood JK and
Kobe B: Structural basis of high-affinity nuclear localization
signal interactions with importin-α. Traffic. 13:532–548.
2012.PubMed/NCBI
|
|
12
|
Tanaka M, Nishi M, Morimoto M, Sugimoto T
and Kawata M: Yellow fluorescent protein-tagged and cyan
fluorescent protein-tagged imaging analysis of glucocorticoid
receptor and importins in single living cells. Endocrinology.
144:4070–4079. 2003. View Article : Google Scholar
|
|
13
|
Xu X, Zhou X, Zhou XW, Zhang Z, Liao MJ,
Gao Q and Luo HM: Schizandrin prevents dexamethasone-induced
cognitive deficits. Neurosci Bull. 28:532–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hinz B and Hirschelmann R: Dexamethasone
megadoses stabilize rat liver lysosomal membranes by non-genomic
and genomic effects. Pharm Res. 17:1489–1493. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bledsoe RK, Montana VG, Stanley TB, Delves
CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson
TM, Lambert MH, Moore JT, Pearce KH and Xu HE: Crystal structure of
the glucocorticoid receptor ligand binding domain reveals a novel
mode of receptor dimerization and coactivator recognition. Cell.
110:93–105. 2002. View Article : Google Scholar
|
|
16
|
Johnston PA, Shinde SN, Hua Y, Shun TY,
Lazo JS and Day BW: Development and validation of a high-content
screening assay to identify inhibitors of cytoplasmic
dynein-mediated transport of glucocorticoid receptor to the
nucleus. Assay Drug Dev Technol. 10:432–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Watson AM, Williamson CD, Rahimi
M, Liang C, Colberg-Poley AM and Rose MC: Glucocorticoid receptor
and histone deacetylase-2 mediate dexamethasone-induced repression
of MUC5AC gene expression. Am J Respir Cell Mol Biol. 47:637–644.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Charmandari E, Kino T, Ichijo T, Jubiz W,
Mejia L, Zachman K and Chrousos GP: A novel point mutation in helix
11 of the ligand-binding domain of the human glucocorticoid
receptor gene causing generalized glucocorticoid resistance. J Clin
Endocrinol Metab. 92:3986–3990. 2007. View Article : Google Scholar
|
|
19
|
Mikuni S, Tamura M and Kinjo M: Analysis
of intranuclear binding process of glucocorticoid receptor using
fluorescence correlation spectroscopy. FEBS Lett. 581:389–393.
2007. View Article : Google Scholar
|
|
20
|
Carey KT, Tan KH, Ng J, Liddicoat DR,
Godfrey DI and Cole TJ: Nfil3 is a glucocorticoid-regulated gene
required for glucocorticoid-induced apoptosis in male murine T
cells. Endocrinology. 154:1540–1552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kauppi B, Jakob C, Farnegardh M, Yang J,
Ahola H, Alarcon M, Calles K, Engstrom O, Harlan J, Muchmore S,
Ramqvist AK, Thorell S, Ohman L, Greer J, Gustafsson JA,
Carlstedt-Duke J and Carlquist M: The three-dimensional structures
of antagonistic and agonistic forms of the glucocorticoid receptor
ligand-binding domain: RU-486 induces a transconformation that
leads to active antagonism. J Biol Chem. 278:22748–27754. 2003.
View Article : Google Scholar
|
|
22
|
Nelson G, Wilde GJ, Spiller DG, Kennedy
SM, Ray DW, Sullivan E, Unitt JF and White MR: NF-kappaB signalling
is inhibited by glucocorticoid receptor and STAT6 via distinct
mechanisms. J Cell Sci. 116:2495–2503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stockner T, Sterk H, Kaptein R and Bonvin
AM: Molecular dynamics studies of a molecular switch in the
glucocorticoid receptor. J Mol Biol. 328:325–334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Necela BM and Cidlowski JA: A single amino
acid change in the first zinc finger of the DNA binding domain of
the glucocorticoid receptor regulates differential promoter
selectivity. J Biol Chem. 279:39279–39288. 2004. View Article : Google Scholar
|
|
25
|
Armon-Omer A, Graessmann A and Loyter A: A
synthetic peptide bearing the HIV-1 integrase 161–173 amino acid
residues mediates active nuclear import and binding to importin
alpha: characterization of a functional nuclear localization
signal. J Mol Biol. 336:1117–1128. 2004.PubMed/NCBI
|
|
26
|
Haffar OK, Popov S, Dubrovsky L, Agostini
I, Tang H, Pushkarsky T, Nadler SG and Bukrinsky M: Two nuclear
localization signals in the HIV-1 matrix protein regulate nuclear
import of the HIV-1 pre-integration complex. J Mol Biol.
299:359–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hearps AC and Jans DA: HIV-1 integrase is
capable of targeting DNA to the nucleus via an importin
α/β-dependent mechanism. Biochem J. 398:475–484. 2006.PubMed/NCBI
|
|
28
|
Agudo J, Ruzo A, Kitur K, Sachidanandam R,
Blander JM and Brown BD: A TLR and non-TLR mediated innate response
to lentiviruses restricts hepatocyte entry and can be ameliorated
by pharmacological blockade. Mol Ther. 20:2257–2267. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karner E, Unger C, Cerny R,
Ahrlund-Richter L, Ganss B, Dilber MS and Wendel M: Differentiation
of human embryonic stem cells into osteogenic or hematopoietic
lineages: a dose-dependent effect of osterix over-expression. J
Cell Physiol. 218:323–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gerolami R, Uch R, Jordier F, Chapel S,
Bagnis C, Brechot C and Mannoni P: Gene transfer to hepatocellular
carcinoma: transduction efficacy and transgene expression kinetics
by using retroviral and lentiviral vectors. Cancer Gene Ther.
7:1286–1292. 2000. View Article : Google Scholar
|
|
31
|
Resa-Infante P, Jorba N, Zamarreno N,
Fernandez Y, Juarez S and Ortin J: The host-dependent interaction
of α-importins with influenza PB2 polymerase subunit is required
for virus RNA replication. PLoS One. 3:e39042008.
|
|
32
|
Reid SP, Leung LW, Hartman AL, Martinez O,
Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST and Basler CF: Ebola
virus VP24 binds karyopherin α1 and blocks STAT1 nuclear
accumulation. J Virol. 80:5156–5167. 2006.
|
|
33
|
McBride KM, Banninger G, McDonald C and
Reich NC: Regulated nuclear import of the STAT1 transcription
factor by direct binding of importin-alpha. Embo J. 21:1754–1763.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alvisi G, Rawlinson SM, Ghildyal R,
Ripalti A and Jans DA: Regulated nucleocytoplasmic trafficking of
viral gene products: a therapeutic target? Biochim Biophys Acta.
1784:213–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamazaki T, Tukiyama T and Tokiwa T:
Effect of dexamethasone on binding activity of transcription
factors nuclear factor-κB and activator protein-1 in SW982 human
synovial sarcoma cells. In Vitro Cell Dev Biol Anim. 41:80–82.
2005.
|
|
36
|
Mirani M, Elenkov I, Volpi S, Hiroi N,
Chrousos GP and Kino T: HIV-1 protein Vpr suppresses IL-12
production from human monocytes by enhancing glucocorticoid action:
potential implications of Vpr coactivator activity for the innate
and cellular immunity deficits observed in HIV-1 infection. J
Immunol. 169:6361–6368. 2002. View Article : Google Scholar
|
|
37
|
Tomasicchio M, Avenant C, Du Toit A, Ray
RM and Hapgood JP: The progestin-only contraceptive
medroxyprogesterone acetate, but not norethisterone acetate,
enhances HIV-1 Vpr-mediated apoptosis in human CD4+T
cells through the glucocorticoid receptor. PLoS One. 8:e628952013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Biswas R, Roy T and Chattopadhyay U:
Prolactin induced reversal of glucocorticoid mediated apoptosis of
immature cortical thymocytes is abrogated by induction of tumor. J
Neuroimmunol. 171:120–134. 2006. View Article : Google Scholar : PubMed/NCBI
|