Introduction
Bisphenol A (BPA) is a xenoestrogen, which is
commonly used in food storage plastics (1) and distributed in the environment
(2,3), making it a cause for concern. BPA is
one of the most widely spread endocrine-disrupting chemicals, which
can be found in the carbohydrate-rich foods; thus, BPA was
classified as a probable human carcinogen (4–6). In
their study, Braniste et al(7) demonstrated that BPA induced
estrogen-like activities in the intestine of rats orally exposed to
carcinogens. No adverse effects were observed, while BPA decreased
the basal epithelial permeability of the colon and strengthened
nociceptive responses by binding to estrogen receptors (ERs). The
study by Hideaki et al(8)
showed that exposure to BPA during embryonic/fetal life and infancy
induces tissue oxidative stress and peroxidation, ultimately
leading to under-development of the brain, kidney and testis.
Furthermore, BPA exposure caused morphological changes in the
developing seminiferous cords, Sertoli cells and Leydig cells at
gestation days 16–20 (9). Takahashi
and Oishi (10) investigated the
impact of BPA on the male reproductive organs. Their results showed
a significant decrease in testis, epididymis, prostate and seminal
vesicle weights and the testicular daily sperm production in
Jcl:Wistar rats.
Fetal exposure to BPA exerts transient effects in
rat testes. Moreover, the changes observed at postnatal day 3 (PND
3) did not correlate with relevant changes in germ cell
populations, Leydig cell function or fertility in the adult
(11). Experimental data of Ema
et al(12) indicated that
oral doses of BPA did not cause significant changes in the
reproductive or developmental parameters over two generations in
rats. Kato et al(13)
demonstrated that BPA administered during the neonatal period has
little effect on the reproductive function of male rats.
Although numerous studies have been conducted on
BPA, its effects on the reproductive system remain controversial.
The aim of this study was to examine the toxicological effects and
mechanism of action of BPA on the reproductive system by observing
morphological changes and protein expression in mouse testis.
Materials and methods
Instruments
TSJ-1A Automatic organizations dehydration machine
(Tianli Aviation Electrical Co. Ltd., Tianjin, China) was used for
manufacturing testicular organization wax block. Leica paraffin
slicing machine (RM2135; Solms, Germany) was used for making
testicular tissue sections. A BM-II pathological tissue embedding
machine (Electric Power Research Institute, Anhui, China) was used
for tissue embedding and a JEM-1230 transmission electron
microscope (JEOL, Tokyo, Japan) was used for observing
morphological changes in mouse testes.
Materials
BPA (CAS no. 80-05-7; Sigma-Aldrich, St. Louis, MO,
USA) was dissolved in dimethyl sulfoxide (DMSO) (Shanghai Chemical
Reagents, Shanghai, China). The concentration of DMSO did not
exceed 0.01%. Rabbit anti-mouse caspase-3 monoclonal antibody and a
secondary antibody immunohistochemistry kit (Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) were used to detect
the expression of caspase-3 protein. A Hoechst 33258 staining kit
(Beyotime Institute of Biotechnology, Shanghai, China) was utilized
to detect cell apoptosis.
Animals and BPA treatment
Sixty female and 30 male 9-week-old ICR mice were
obtained from the Animal Center of Anhui Province and maintained in
a temperature-controlled room (25±2°C). The animals were
acclimatized for one week in a 12-h light/dark cycle. The mice
(male:female, 2:1) were then placed in a cage at 9:00 p.m. and the
vaginal plug, which is a sign of pregnancy, was checked at 7:00
a.m. the following morning. On day 0 of pregnancy the ICR mice were
randomly divided into blank control, solvent control and three
BPA-exposed dose groups (10, 100 and 1,000 nmol/l). Pregnant ICR
mice were given water containing BPA dissolved in DMSO from
gestational day 0 to the end of lactation. To maintain the BPA
concentration in drinking water, the water was changed every 2–3
days. This study was approved by the Animal Care and Protection
Committee of Anhui Medical University, Anhui, Hefei, China.
Specimens and preservation
Each group was randomly assigned 8 male pups from
PND 21. The male pups were first weighed, administered
intraperitoneal anesthesia with 1% 0.1–0.2 ml sodium pentobarbital
and then the abdominal cavity was rapidly opened and the testis
tissue removed. Simultaneously, testis tissue was weighed and the
organ coefficient was calculated. One side of the testicular tissue
was placed in 4% paraformaldehyde (pH 7.2–7.4) for 24 h at room
temperature and rinsed in running water overnight, then sections
were embedded in paraffin. The other side of the testicular tissue
was fixed in 2–4% glutaraldehyde and observed under an electron
microscope.
General parameters of reproduction and
development
The number of offspring produced by each pregnant
mouse, the male:female ratio of the offspring in each group and the
coefficient of testis were counted.
Ultrastructural observation of cells
The morphological changes of testicular tissue were
observed by electron microscopy. The fresh testicular tissues were
fixed in 2–4% glutaraldehyde and cut into thin sections with
double-staining acetate uranium and lead citrate. The tissues were
then observed under a JEM-1230 transmission electron microscope
(Jeol Ltd.).
Detection of testicular apoptosis
Hoechst 33258 staining was used to detect testicular
apoptosis. Paraffin sections were conventionally dewaxed,
dehydrated, washed twice in phosphate-buffered saline (PBS) for 3
min and rehydrated. Then, 0.5 ml Hoechst 33258 staining solution
was added to the slices, which were stained after 10 min and washed
three times for 5 min in PBS. The sections were then added to
anti-fluorescence quenching liquid, covered with a clean coverslip
and observed by fluorescence microscopy.
Analysis of caspase-3 protein
expression
Conventional immunohistochemistry was used to
analyze caspase-3 protein expression. Caspase-3 monoclonal antibody
was diluted at 1:100.
Image analysis
Caspase-3-positive cells were counted using Image
Pro Plus 6.0 image analysis software (Warrendale, PA, USA). The
number of cells with caspase-3 expression in five random fields of
each section was recorded for the statistical analysis.
Statistical analysis
SPSS 13.0 statistical software was used for
statistical analysis. One-way ANOVA was introduced for the
comparison between groups. P<0.05 was considered to indicate a
statistically significant result.
Results
General parameters of reproduction and
development
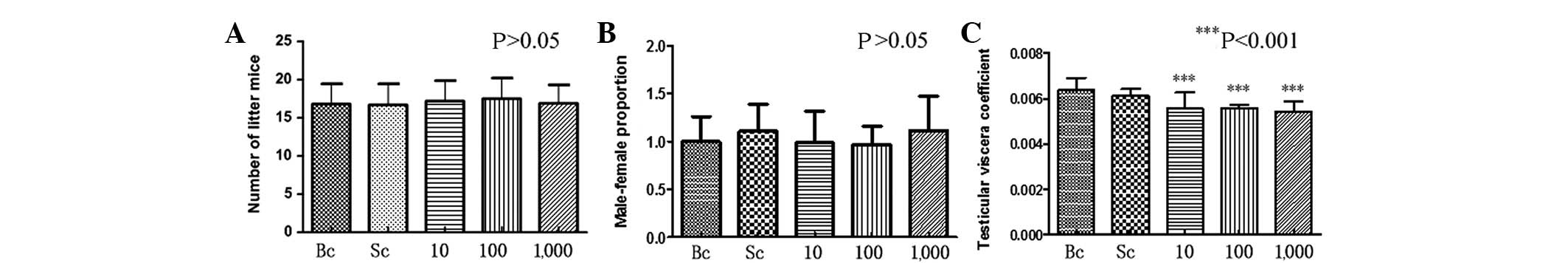
Compared with the control group, there was no
significant difference in the number of litter mice and the
male:female ratio of the BPA-exposed pregnant mice (P>0.05)
(Fig. 1A and B). A slight
difference was detected between the testicular viscera coefficient
in the BPA-exposed male offspring mice compared with the control
group. This difference was statistically significant (P<0.001)
(Fig. 1C).
Ultrastructural results
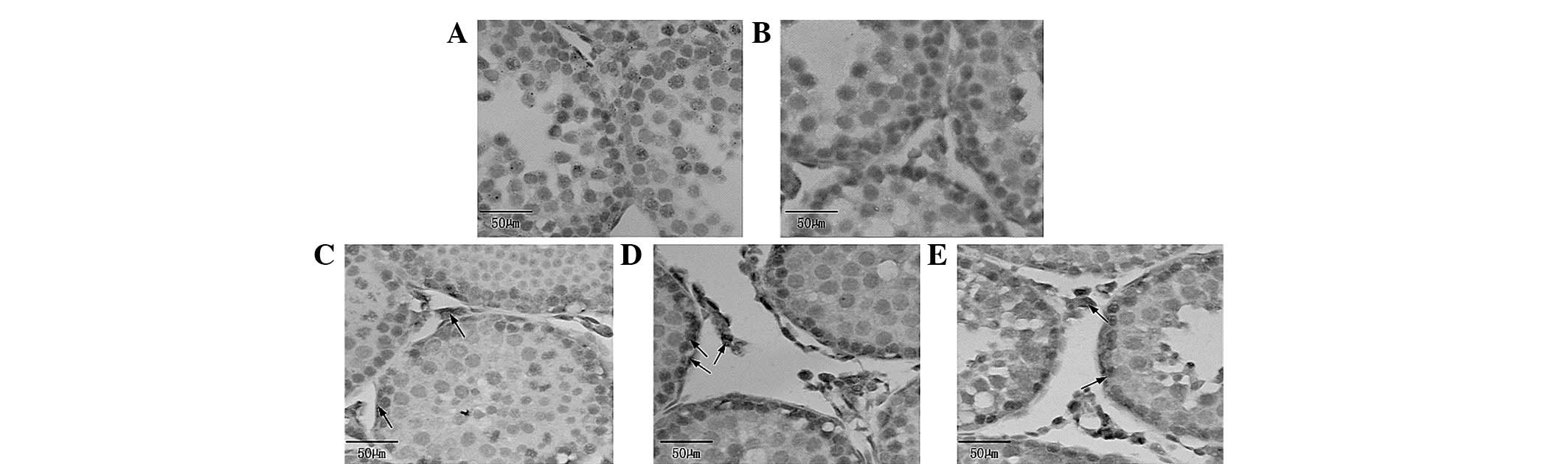
Fig. 2A shows that
the spermatogenous cells were normal in the blank control group,
the nuclear membrane and nucleolus were visible and the structure
of the mitochondria and other organelles was clear. However,
Fig. 2A1 shows that the nuclear
membrane was blurred, while some mitochondria revealed alterations
in vacuoles and mild expansion of the endoplasmic reticulum in the
BPA (100 nmol/l) group. In Fig. 2B
it is evident that the Sertoli cells of the blank control group
were normal, with clear nuclear membrane and no evident
morphological abnormalities in mitochondria and other organelles.
By contrast, experimental results in Fig. 2B1 show that some mitochondria had
alterations in vacuoles and mild expansion in the endoplasmic
reticulum in the BPA (100 nmol/l) group. Fig. 2C shows that Leydig cells in the
blank control group exhibited clear nuclear membrane, normal
morphological organelles and had a few lysosomes. By contrast,
Fig. 2C1 demonstrates that
mitochondria had alterations in the vacuoles and that additional
and larger lysosomes were present. Fig.
2D reveals that peritubular myoid cells in the blank control
group had no evident abnormalities and thickness of the basement
membrane was uniform. However, Fig.
2D shows a large number of vacuolated mitochondria in
peritubular myoid cells of the BPA (100 nmol/l) group.
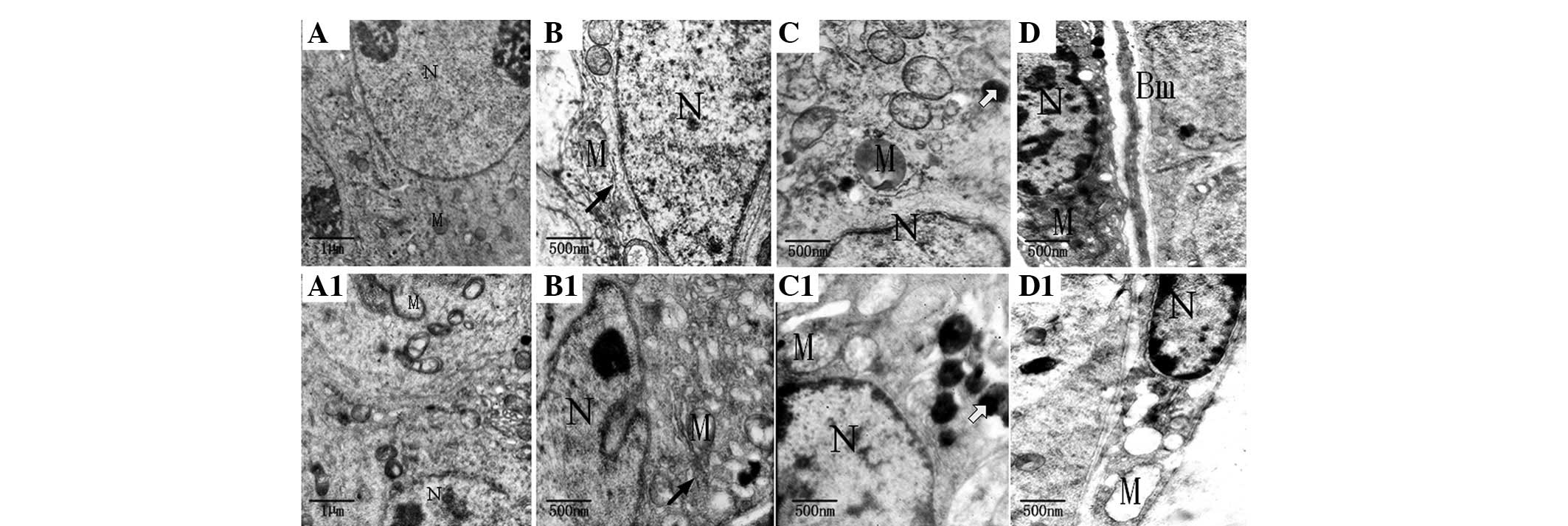 | Figure 2Transmission electron microscopy. (A)
Spermatogenous, (B) Sertoli, (C) Leydig and (D) peritubular myoid
cells in the blank control group had no evident morphological
abnormalities. (A1–D1) In the bisphenol A (100 nmol/l) groups, the
(A1) Spermatogenous, (B1) Sertoli, (C1) Leydig and (D1) peritubular
myoid cells exhibited alterations in the vacuoles. (B1) Smooth
endoplasmic reticulum showing different degrees of expansion. (C1)
There were additional and larger lysosomes compared with the blank
control group. M, mitochondria; N, nucleus; ↗ indicates smooth
endoplasmic reticulum; ⇦ indicates lysosomes; (A and A1)
magnification, ×6,000; (B–D, B1–D1) magnification, ×12,000. |
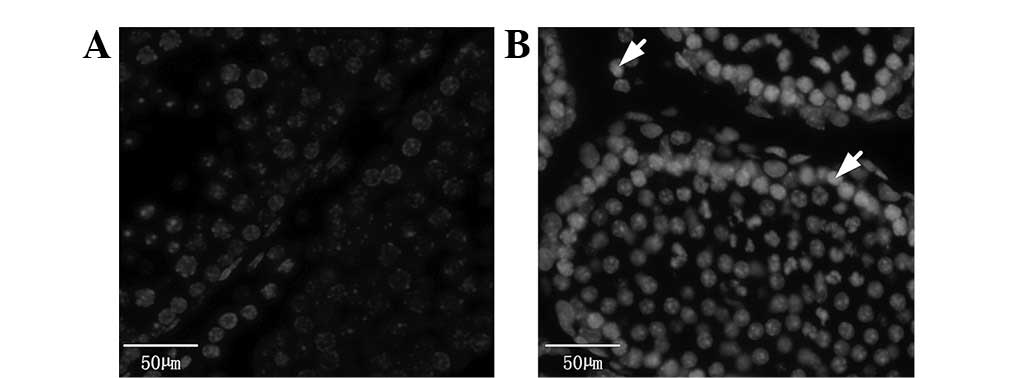
Hoechst staining results
Spermatogenous, Sertoli, Leydig and peritubular
myoid cells exhibited normal blue staining in the blank control
group (Fig. 3A). However, the
nuclei of these cells showed pyknosis, strong staining and appeared
white (Fig. 3B) in the BPA (100
nmol/l) group.
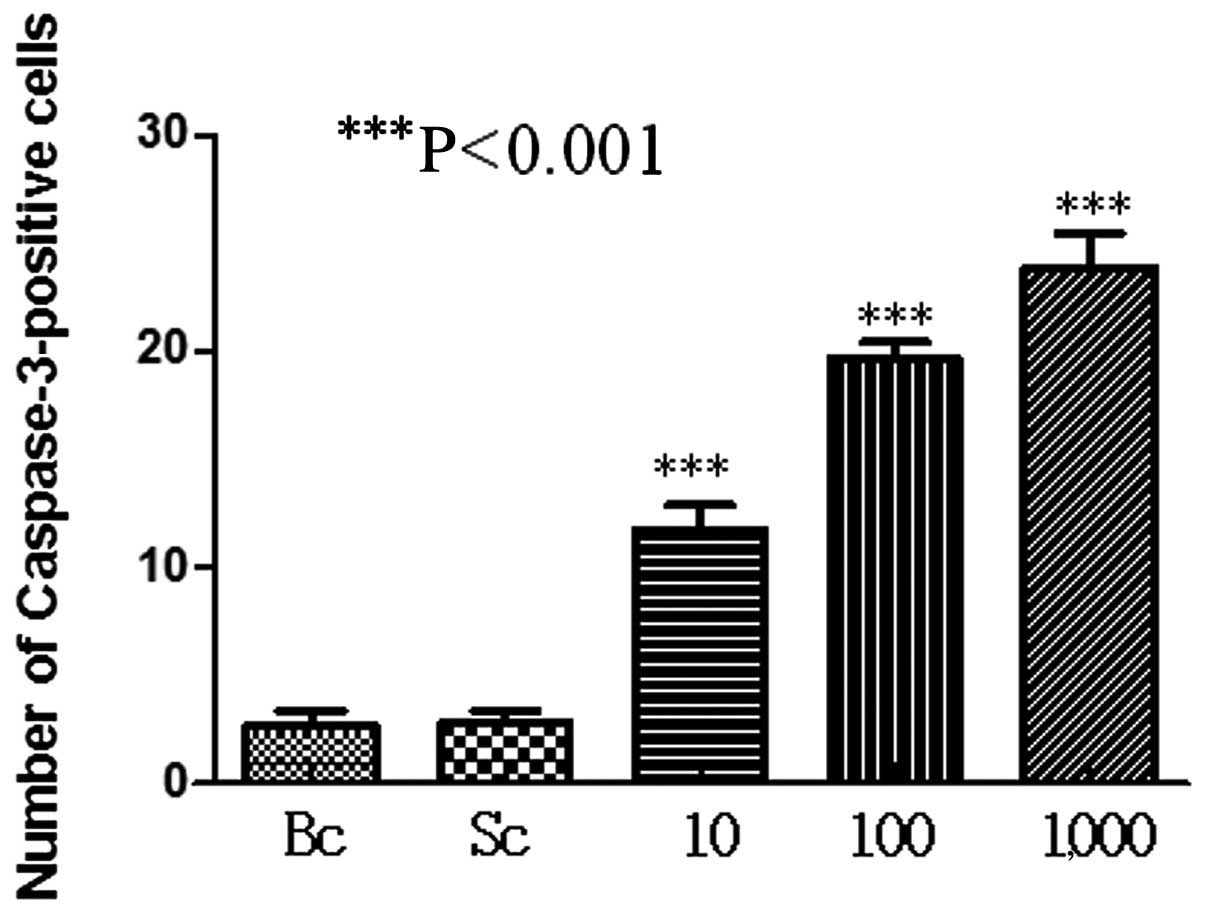
Caspase-3 expression in testicular
tissue
Immunohistochemical results showed that the
expression of caspase-3 in the spermatogenous, Sertoli, Leydig and
peritubular myoid cells was more apparent with an increased dose in
the experimental test groups. However, the expression of caspase-3
was not evident in the control group. Differences were
statistically significant compared with the control group
(P<0.001) (Figs. 4 and 5).
Discussion
BPA is widely used in daily life, ultimately
becoming a part of the food chain as an environmental estrogen
pollutant (14). Studies have shown
that daily sperm production is reduced significantly and the
structure of sperm cells undergoes significant changes in adult
mice fed BPA (15). Testicular
function is two-fold; producing sperm in the process of
spermatogenesis, and involvement in the synthesis of steroid
hormones, which consist mainly of testosterone and a small amount
of estrogen (16). Testicular
tissue with normal structure and function is a prerequisite to
maintain reproductive capacity. BPA increased the sperm abnormality
rate of the tested mice and interfered with the growth and
developmental process of the sperm by crossing the blood-testis
barrier. This effect was enhanced with an increase in exposure time
(17).
Experimental results showed no significant
difference in the number of pups among each litter and the
proportion of males and females. The morphological observations of
electron microscopy suggested that certain vacuole changes occurred
in the mitochondria of the spermatogenous, Sertoli, Leydig and
peritubular myoid cells in PND 21 pups of the experimental groups.
These changes indicated alterations in the function of these cells.
Mitochondria provide energy for the cell and the endoplasmic
reticulum is associated with the protein and lipid synthesis.
Spermatogenous cells, which are close to the basement membrane of
seminiferous tubules, are spermatogenic in origin, and are based on
a diploid cell differentiation pathway (18,19).
Spermatogenesis is a process whereby germ cells generate
proliferation, differentiation and deformation in the epithelium
lining the seminiferous tubules and is vulnerable to the
interference of external factors such as drugs, radiation and
reproductive and somatic pathologies. Other external factors,
including seasonal breeding, temperature and environmental
pollutants, are also likely to increase the constitutive levels of
apoptosis in germ cells (20).
Sertoli cells of the blood-testis barrier are injured early
following exposure to environmental toxic substances (including
BPA) (21). Sertoli cells are
involved in the composition of the blood-testis barrier through
tight junctions between 10 and 16 days of age in mice and are
important in maintaining sperm formation and the micro-environment
around the germ cells. They also provide a number of growth factors
and nutrients (22–24), such as lactic acid, which are energy
sources for germ cell meiosis. In addition, Sertoli cells aid the
maturation of sperm that enter into the lumen. In this study,
morphological changes of the Sertoli cells in the experimental
groups affected the nutritional intake and normal maturation of
spermatogenic cells at various stages.
The activity of luteinizing hormone leads Leydig
cells to synthesize and generate androgens that promote
spermatogenesis and male reproductive development, maintain
secondary sexual characteristics and sexual function. Androgen is
essential for meiosis and sperm differentiation and its receptors
are mostly distributed in Sertoli, Leydig and peritubular myoid
cells. Lack of androgen receptor would cause male mice to undergo
testicular feminization syndrome (25). In this study, Leydig cells in the
experimental group had additional and larger lysosomes as compared
with the control group. It can be speculated from the lysosome
function that there are some senescent organelles, biological
macromolecules or pathogens in Leydig cells. These changes of
Leydig cells likely inhibit synthesis and secretion of androgens,
affecting development of the genitals and thereby the male
reproductive system.
Peritubular myoid cells maintain the morphology of
the seminiferous tubules by connecting to Sertoli cells and
participating in the formation of the basement membrane. Their
contraction was an effective means of sperm transportation and
seminiferous tubule fluid flowing to the rete testis (26). Additionaly, peritubular myoid cells
provide nutrition for germ cell development by synthesizing and
secreting the extracellular matrix. Electron microscopy results
showed that vacuole changes appeared in the mitochondria of
peritubular myoid cells in the experimental group, affecting the
function of the mitochondria, therefore the spermatogenic function
of the testes and sperm transport were ultimately influenced.
Signs of apoptosis include DNA fragmentation,
caspase activation and phosphatidylserine externalization (27,28).
Methods such as Hoechst 33258 staining are used for detecting cell
apoptosis according to the cell morphological changes. Only a small
amount of fluorescent dye is able to cross the normal cell
membrane, thus the normal cells produce low-intensity blue
fluorescence. However, Hoechst 33258 enters apoptotic cells to a
larger extent compared with normal cells due to the membrane
permeability of apoptotic cells being enhanced, thus the apoptotic
cells were dyed white. In this study, experimental results showed
that BPA exposure resulted in the apoptosis of the spermatogenous,
Sertoli, Leydig and peritubular myoid cells. Moreover, cell
apoptosis near the basement membrane was more evident, which was
consistent with the other results of the experiments.
The caspase-3 gene (CASP-3) was originally cloned in
human Jurkat-T lymphocytes and plays a crucial role in apoptosis.
The caspase-3 gene resulted in apoptosis when transferred to Sf9
cells of insects (Spodoptera frugiperda) and the extract
lost its ability to induce apoptosis when the caspase-3 was removed
from the cell extract. The extract recovered the function of
apoptosis induction when purified caspase-3 was added (29). Caspase-3 is considered one of the
most important apoptosis executors in the caspase family and the
main effect factor in the apoptotic process (27). Apoptosis allows inactive caspase to
become active and the activation of caspase-3 results in apoptosis
reaching an irreversible stage. Apoptosis has two pathways: the
extracellular pathway (the death-receptor pathway) and
intracellular pathway (mitochondria and endoplasmic reticulum
pathway), with both pathways activating caspase-3. This study has
shown that the caspase-3 expression of testicular seminiferous
tubules was mostly located at cells near the basement membrane and
Leydig cells in the experimental group. The caspase-3 expression
was dose-dependent, indicating that the number of cells positive
for caspase-3 expression increased with the dose increase of BPA
exposure. The expression site of caspase-3 protein has also
demonstrated that the ultrastructural changes of these cells may be
associated with apoptosis and the reason for the absence of
apoptotic bodies may be associated with the dose of BPA.
In conclusion, this study has shown that male
offspring exhibited early apoptosis in spermatogenous, Sertoli,
Leydig and peritubular myoid cells. It was found that BPA was able
to promote testicular cell apoptosis of pups through the placental
barrier and breast milk and affect the reproductive function.
Therefore, studies should focus on how to prevent the occurrence of
adverse effects and reproductive toxicity of reversible BPA due to
its being widely used. In the future, more specific and in-depth
indicators should be further investigated.
Acknowledgements
The authors would like to acknowledge
Xiaoyu Chen and other members of the Department of Histology and
Embryology, Anhui Medical University (Anhui, China). This article
is distributed under the terms of i) the Natural Science Foundation
of Higher Education Institutions of Anhui Province, China
(KJ2010A183); ii) the Natural Science Foundation of Anhui, China
(1208085MC53); iii) Shanghai Municipal Natural Science Foundation
(12ZR1429900), which permits any non-commercial use, distribution
and reproduction in any medium, provided the original author(s) and
source are credited.
References
|
1
|
Richter CA, Birnbaum LS, Farabollini F, et
al: In vivo effects of bisphenol A in laboratory rodent studies.
Reprod Toxicol. 24:199–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le HH, Carlson EM, Chua JP, et al:
Bisphenol A is released from polycarbonate drinking bottles and
mimics the neurotoxic actions of estrogen in developing cerebellar
neurons. Toxicol Lett. 176:149–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi A, Higashino F, Aoyagi M, et al:
Bisphenol A from dental polycarbonate crown upregulates the
expression of hTERT. J Biomed Mater Res B Appl Biomater.
71:214–221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Besaratinia A and Pfeifer GP: A review of
mechanisms of acrylamide carcinogenicity. Carcinogenesis.
28:519–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
World Health Organization: FAO/WHO
Consultation on the Health Implications of Acrylamide in
Food-Summary Report. Geneva, Switzerland: WHO; pp. 1–12. 2002
|
|
6
|
Stadler RH, Blank I, Varga N, et al:
Acrylamide from Maillard reaction products. Nature. 419:449–450.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braniste V, Jouault A, Gaultier E, et al:
Impact of oral bisphenol A at reference doses on intestinal barrier
function and sex differences after perinatal exposure in rats. Proc
Natl Acad Sci USA. 107:448–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hideaki K, Amakawa M and Shishibori T:
Exposure to bisphenol A during embryonic/fetal life and infancy
increases oxidative injury and causes underdevelopment of the brain
and testis in mice. Life Sci. 74:2931–2940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horstman KA, Naciff JM and Overmann GJ:
Effects of transplacental 17-α-ethynyl estradiol or bisphenol A on
the developmental profile of steroidogenic acute regulatory protein
in the rat testis. Birth Defects Res B Dev Reprod Toxicol.
95:318–325. 2012.
|
|
10
|
Takahashi O and Oishi S: Testicular
toxicity of dietarily or parenterally administered biaphenol A in
rats and mice. Food Chem Toxicol. 41:1035–1044. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thuillier R, Manku G, Wang Y and Culty M:
Changes in MAPK pathway in neonatal and adult testis following
fetal estrogen exposure and effects on rat testicular cells.
Microsc Res Tech. 72:773–786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ema M, Fujii S, Furukawa M, et al: Rat
two-generation reproductive toxiccity study of bisphenol A. Reprod
Toxicol. 15:505–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato H, Furuhashi T, Tanaka M, et al:
Effects of bisphenol A given neonatally on reproductive functions
of male rats. Reprod Toxicol. 22:20–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan HH and Cheng CY: Blood-testis barrier
dynamics are regulated by an engagement/disengagement mechanism
between tight and adherens junctions via peripheral adaptors. Proc
Natl Acad Sci USA. 102:11722–11727. 2005. View Article : Google Scholar
|
|
15
|
Toyama Y, Suzuki-Toyota F, Maekawa M, et
al: Adverse effects of bisphenol A to spermiogenesis in mice and
rats. Arch Histol Cytol. 67:373–381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hermo L, Pelletier RM, Cyr DG and Smith
CE: Surfing the wave, cycle, life history, and genes/proteins
expressed by testicular germ cells. Part1: background to
spermatogenesis, spermatogonia, and spermatocytes. Microsc Res
Tech. 73:241–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minamiyama Y, Ichikawa H, Takemura S, et
al: Generation of reactive oxygen species in sperms of rats as an
earlier marker for evaluating the toxicity of endocrine-disrupting
chemicals. Free Radic Res. 44:1398–1406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Rooij DG and Russell LD: All you wanted
to know about spermatogonia but were afraid to ask. J Androl.
21:776–798. 2000.PubMed/NCBI
|
|
19
|
Oatley JM and Brinster RL: Regulation of
spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev
Biol. 24:263–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tripathi R, Mishra DP and Shaha C: Male
germ cell development: turning on the apoptotic pathways. Reprod
Immunol. 83:31–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiorini C, Tilloy-Ellul A, Chevalier S, et
al: Sertoli cell junctional proteins as early targets for different
classes of reproductive toxicants. Reprod Toxicol. 18:413–421.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharpe RM, Mckinnell C, Kivlin C and
Fisher JS: Proliferation and functional maturation of Sertoli
cells, and their relevance to disorders of testis function in
adulthood. Reproduction. 125:769–784. 2003. View Article : Google Scholar
|
|
23
|
Yan HH, Mruk DD and Cheng CY: Junction
restructuring and spermatogenesis: the biology, regulation, and
implication in male contraceptive development. Curr Top Dev Biol.
80:57–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mruk DD and Cheng CY: Sertoli-Sertoli and
Sertoli-germ cell interactions and their significance in germ cell
movement in the seminiferous epithelium during spermatogenesis.
Endocr Rev. 25:747–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeh S, Tsai MY, Xu Q, et al: Generation
and characterization of androgen receptor knockout (ARKO) mice: an
in vivo model for the study of androgen functions in selective
tissues. Proc Natl Acad Sci USA. 99:13498–13503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romano F, Tripiciano A, Muciaccia B, et
al: The contractile phenotype of peritubular smooth muscle cells is
locally controlled: possible implications in male fertility.
Contraception. 72:294–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fadeel B, Ottosson A and Pervaiz S: Big
wheel keeps on turning: apoptosome regulation and its role in
chemoresistance. Cell Death Differ. 15:443–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|