Introduction
Prostate cancer (PCa) is the leading cause of new
cancer cases and the second most common cause of cancer-related
mortality in men in the US (1).
Radiotherapy is commonly employed as curative therapy for locally
confined PCa and is also used for salvage therapy in individuals
who have undergone a failed radical prostatectomy. However,
radiorecurrent PCa and a poor long-term prognosis are experienced
by numerous PCa patients, as ~30% of those individuals treated with
potentially curative doses develop radioresistance (2). Current strategies employed to improve
outcomes include The addition of androgen deprivation therapy (ADT)
and radiation dose escalation are two strategies currently being
used to improve the outcome for PCa patients. These techniques have
successfully been able to reduce the biochemical failure rates.
However, even with these improvements, the rates of biochemical
failure remain poor for those individuals with higher risk
localized and locally advanced diseases (3).
NF-κB plays a significant role in tumorigenesis and
resistance to therapy-induced cytotoxicity (4). Thus, inhibition of this protein
complex is being contemplated as a target to enhance the efficacy
of conventional radiotherapy and chemotherapy (5). In our previous study, it was
demonstrated that the RNA interference of RelB could enhance the
radiosensitivity of the PCa RM-1 cell line and induce apoptosis
(6). Anti-apoptotic Bcl-xl is
significant in tumor progression, development and radioresistance
(7,8). Ionizing radiation (IR) has previously
been shown to activate Bcl-xl in PC-3 cells, and upregulation of
this protein results in decreased cell radiosensitivity (9). Thus, the disruption of anti-apoptotic
pathways may be a novel target for overcoming radioresistance in
PCa. The aims of the present study were to determine if there was
an association between the RelB/p52 alternative NF-κB pathway and
Bcl-xl in the mouse PCa RM-1 cell line, and to analyze the
association between Bcl-xl expression and radiosensitivity in the
RM-1 cells.
Materials and methods
Cell culture and treatment
RM-1 is an androgen-independent PCa cell line
(10), which was purchased from the
Cell Culture Collection, Chinese Academy of Sciences (Beijing,
China) and maintained in Dulbecco’s modified Eagle’s medium
(HyClone™; Thermo Scientific, Logan, UT, USA), supplemented with
10% fetal bovine serum (Gibco, Grand Island, NY, USA). The RM-1
cells were plated in 6-well plates at a density of 5×105
cells/well. A lentiviral vector expressing siRelB
(pLentilox-sh-RelB) was constructed, as described previously
(11). The transfection efficiency
of >95% was used for the subsequent experiments.
Radiation exposure
The RM-1 cells in the 6-well plates were irradiated
via a 6 MeV linear accelerator (Varian Clinac 21EX; Varian Medical
Systems, Inc., Palo Alto, CA, USA) at room temperature. Each type
of cell received a single dose of 2, 4, 6, or 8 Gy per treatment.
Dose rates were given at 2.25 Gy/min. The distance between the
radiation source and the cells was 100 cm.
Clonogenic survival assay
Subsequent to radiation exposure, the RM-1 cells
from each well were trypsinized and grown in triplicate in 60-mm
culture dishes with different densities (20, 40, 100, 200 and 400
per dish) for 14 days. Once the cells had been fixed in
methanol/acetic acid (3:1) for 30 min, the cell clones were counted
under a microscope (>50 cells/clone). Plating efficiency (PE)
was calculated by dividing the average number of colonies per plate
by the amount of cells plated and multiplying by 100. Survival
fractions (SFs) were calculated using the formula SF = colony
number / (cell number cultured × PE). A one-hit multi-target model
was fitted to the cell survival curve [SF = 1 - 1 (1 -
e−KD)N, SF = 1 - (1 -
e−D/D0)N(D0 = 1 / K)] to determine
the dose quasithreshold (Dq), the mean lethal dose
(D0), the 2 Gy SF (SF2), the N-value and the
sensitization enhancement ratio (SER).
Measurement of apoptosis by flow
cytometry
Once the RM-1 cells had been exposed to radiation at
a single dose of 6 Gy, the cells were incubated in normal medium
for 48 h. The cells were then collected ([according to the Annexin
V-FITC apoptosis detection kit (Byotime, Nantong, China)] and
analyzed immediately on a FSCAN flow cytomer (BD Biosciences,
Franklin Lakes, NJ USA).
Western blot analysis
For each treatment group, proteins from the cytosol
and nucleus were isolated from the RM-1 cells. subsequent to being
blocked in 5% milk for 1 h, the membrane was incubated with the
primary antibody, followed by the corresponding secondary antibody.
Total proteins (50 μg) were separated using 10% SDS-PAGE and were
transferred onto a nitrocellulose membrane. The RelB protein was
detected with a primary rabbit RelB monoclonal antibody (1:1,000;
Invitrogen Life Technologies, Carlsbad, CA, USA) and a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (KPL, Inc.,
Gaithersburg, MD, USA).
Quantitative polymerase chain reaction
(qPCR)
Total RNA (1 μg) isolated from the RM-1 cells using
TRIzol reagent (Invitrogen Life Technologies) was
reverse-transcribed using oligo-dT primers with reverse
transcription reagents (Toyobo, Co., Ltd., Osaka, Japan).
Reverse-transcribed RNA was amplified with SYBR-Green PCR Master
mix (Toyobo) plus 0.4 μM of gene-specific upstream and downstream
primers during 40 cycles on an Applied Biosystems 7500 Fast
Realtime Cycler (Applied Biosystems, Carlsbad, CA, USA). Each cycle
consisted of denaturation at 95°C for 15 sec, annealing at 55°C for
15 sec and extension at 72°C for 45 sec. The primer sequences were
as follows: β-actin forward, 5′-CCGTGAAAA GATGACCCAG-3′ and
reverse, 5′-TAGCCACGCTCGGTC AGG; and Bcl-xl forward,
5′-CCCAGAAAGGATACAGCT GG-3′ and reverse,
5′-GCGATCCGACTCACCAATAC-3′. Data were analyzed by relative
quantification using the 2−ΔΔCt method (12).
Statistical analysis
Statistical analysis was performed using a one-way
analysis of variance for multiple group comparisons using SPSS 11.5
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Adenovirus-mediated small interfering RNA
(siRNA) targeting RelB inhibits the expression of RelB protein in
RM-1 cells
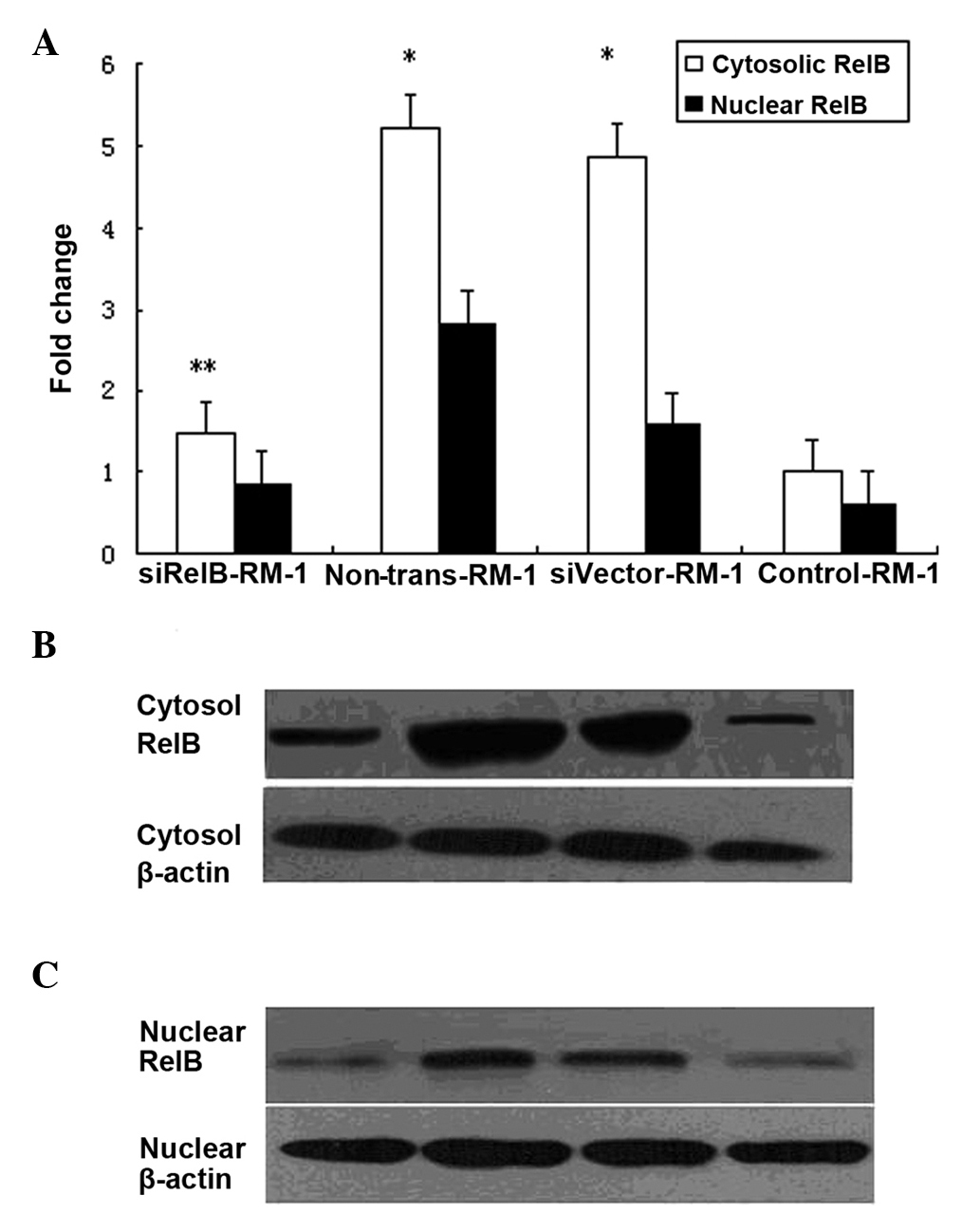
Western blot analyses were performed to determine
the expression level of the RelB protein prior to and following IR
in the murine PCa RM-1 cell line. The RM-1 cells transfected with
the empty vector (siVector-RM-1) and the non-transfected RM-1 cells
(non-trans-RM-1) were treated with a 6 Gy dose. Following
treatment, the total RelB proteins from the varying transfected
cells were isolated and subjected to western blot analysis using
cell lysate from the cytosolic and nuclear fractions. The results
showed that the expression levels of RelB protein were markedly
higher in the siVector-RM-1 and non-trans-RM-1 cells than in the
non-irradiated RM-1 cells (control-RM-1). Compared with the level
of the control-RM-1 cells, the RelB protein levels of the
non-trans-RM-1 cells were higher by ~5.2- and 2.8-fold in the
cytosolic and nuclear fractions, respectively. Similarly, the RelB
protein levels of the siVector-RM-1 cells were higher by ~4.9- and
1.6-fold in the cytosolic fractions and nuclear fractions
(P<0.05) (Fig. 1). There was no
difference in the expression levels of the RelB protein between the
non-trans-RM-1 and siVector-RM-1 cells. Thus, it was concluded that
IR may promote the expression of RelB in RM-1 cells.
To further analyze the effect of the
adenovirus-mediated siRNA-targeting of RelB on the expression of
the RelB protein in the RM-1 cell line,
pLentilox-sh-RelB-transfected RM-1 cells (siRelB-RM-1) were treated
with a 6 Gy dose. The RelB protein levels in the siRelB-RM-1 cells
were ~71.8 and 70.4% less in the cytosolic and nuclear fractions
compared with that of the non-trans-RM-1 cells (P<0.01). in
comparison to the level in the non-trans-RM-1, the RelB protein
levels in the siRelB-RM-1 cells exhibited similar manifestations
(Fig. 1). These data showed that
adenovirus-mediated siRNA targeting RelB could specifically and
significantly inhibit the expression of RelB protein in RM-1
cells.
pLentilox-sh-RelB significantly increases
the sensitivity of RM-1 cells to IR in vitro
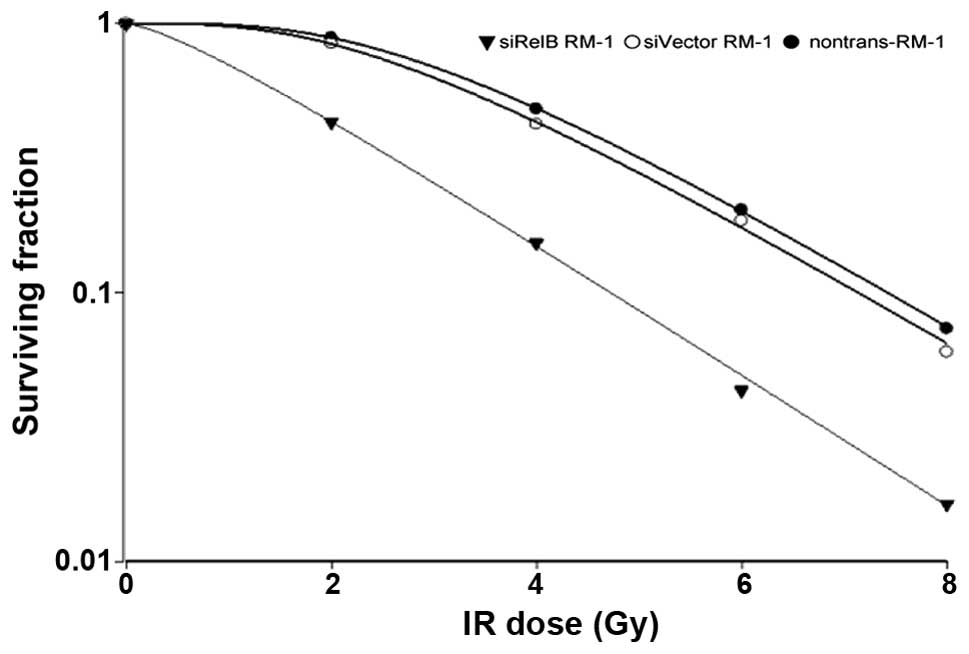
siRelB-RM-1, siVector-RM-1 and non-trans-RM-1 cells
were subjected to radiation exposure at 2, 4, 6 and 8 Gy doses. A
clonogenic survival assay was performed, as aforementioned. As
shown in Fig. 2, the SF values from
the siRelB-RM-1 cells (0.8, 0.11, 0.06 and 0.02) were less than
that of the siVector-RM-1 cells (0.95, 0.8, 0.12 and 0.09) at the
corresponding dosage of 2, 4, 6 and 8 Gy respectively, indicating
that the siRelB-RM-1 cells were more sensitive to radiation-induced
cell death compared with the controls. Increases in D0
indicate higher cell radiation resistance, while Dq
represents the ability of a cell to recover from sublethal damage.
Table I shows that the values of
D0 (1.68) and Dq (0.60) in the siRelB-RM-1
cells were significantly lower than those of the non-trans-RM-1
(1.02, 3.08, 0.89 and 4.97) and siVector-RM-1 (1.93, 2.76, 0.84 and
4.17) cells, indicating that the RelB siRNA-transfected cells had
lower radiation resistance and a weakened damage-recovery
ability.
 | Table IValues of the varying parameters of
the one-hit multi-target model fitted to the non-trans-RM-1,
siVector-RM-1 and siRelB-RM-1 cells following radiation
treatment. |
Table I
Values of the varying parameters of
the one-hit multi-target model fitted to the non-trans-RM-1,
siVector-RM-1 and siRelB-RM-1 cells following radiation
treatment.
| Cells | D0 | Dq | N | SF2 | SER
(D0) | SER
(Dq) |
|---|
| Non-trans-RM-1 | 1.92 | 3.08 | 4.97 | 0.89 | | |
| siVector-RM-1 | 1.93 | 2.76 | 4.17 | 0.84 | 0.99 | 1.12 |
| siRelB-RM-1 | 1.68 | 0.60 | 1.43 | 0.43 | 1.14 | 5.13 |
RelB siRNA transfection of RM-1 cells
increases radiation-induced apoptosis by inhibiting the expression
of the Bcl-xl gene
Once the siRelB-RM-1, siVector-RM-1 and
non-trans-RM-1 cells had been exposed to X-rays at a 6 Gy dose, the
cells were incubated for another 48 h. These cells were collected
along with the RM-1 cells without radiation treatment. Apoptosis
was measured using the Annexin V/PI flow cytometry assay, as
aforementioned. The results showed that the siRelB-RM-1 cells had a
much higher apoptosis rate (15.27±1.62) than the siVector-RM-1
(8.40±0.69) and non-trans-RM-1 (7.90±1.50%) cells.
Next, to determine whether the adenovirus-mediated
siRNA-targeting of RelB changes the radiosensitivity of PCa cells
due to modulation of Bcl-xl gene expression, the levels of mRNA
from the Bcl-xl gene was quantified by qPCR (Fig. 3). As shown in Fig. 3, the expression levels of the Bcl-xl
mRNA in the non-trans-RM-1 or siVector-RM-1 cells was increased by
~2.3- or 2.2-fold, compared with those in control-RM-1 cells, as
observed by qPCR (P<0.05). Consistent with its inhibition of
RelB (Fig. 1), siRNA targeting RelB
specifically and significantly eliminated irradiation-dependent
increases in the levels of the Bcl-xl mRNA in the RM-1 cells. These
data showed that adenovirus-mediated siRNA targeting RelB could
significantly inhibit the expression of the Bcl-xl gene in the RM-1
cells (Fig. 3). The expression
levels of the Bcl-xl mRNA in the siRelB-RM-1 cells were decreased
by ~75.3 or 73.1% compared with those of the non-trans-RM-1 or
siVector-RM-1 cells (P<0.01; Fig.
3). Overall, these results indicate that inactivation of Bcl-xl
using siRNA targeting RelB may be a significant mechanism for the
radiosensitization effects of siRNA targeting RelB on the survival
of PCa cells.
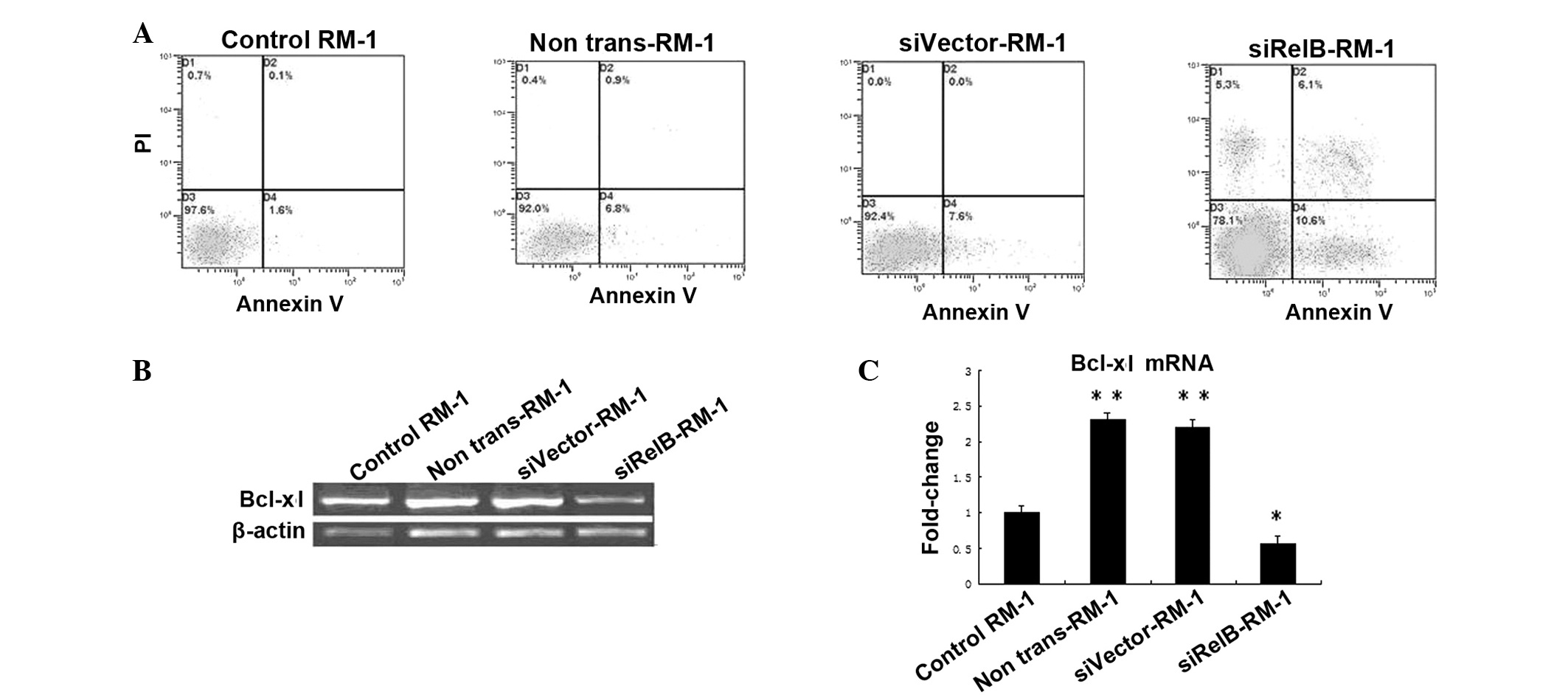 | Figure 3(A) Apoptosis induction with radiation
in siRelB-RM-1, siVector-RM-1 and non-trans-RM-1 cells. The
apoptosis rate was measured with Annexin V/PI flow cytometry in the
various RM-1 cells that were treated with 6 Gy radiation, along
with the untreated control RM-1 cells. Radiation treatment induced
apoptosis in all the treated cells compared with the control cells.
(B) Irradiation activates Bcl-xl in RM-1 cells. The cells were
pretreated with siRelB prior to irradiation. The RM-1 cells were
subjected to qPCR assay. Total RNA isolated from irradiated cells
and untreated control RM-1 cells. (C) Bcl-xl mRNA levels were
measured by qPCR assay normalized by the level of β-actin.
Significant differences were observed, as indicated, when compared
with the untreated groups (*P<0.05
and**P<0.01 vs. control). si, small interfering;
control-RM-1, non-irradiated RM-1 cells; non-trans-RM-1,
non-transfected RM-1 cells; siVector-RM-1, RM-1 cells transfected
with empty vector; siRelB-RM-1, pLentilox-sh-RelB-transfected RM-1
cells; qPCR, quantitative polymerase chain reaction. |
Discussion
Despite the fact that the incidence and mortality
rate of PCa is low in China, a significant increase was recorded
between 2003 and 2007 (13). The
mainstay method for the treatment for early-stage PCa includes
active surveillance, surgery, external beam radiation therapy and
brachytherapy, while the management of more advanced localized
disease is generally via a combination of methods and frequently
includes the addition of ADT. Radiotherapy is a commonly used
treatment for various malignancies and has a prominent role in the
care of PCa patients. Attempts to improve the therapeutic ratio of
radiation via technological and pharmacological methods has
resulted in significant progress in cancer care. Previous studies
have indicated that only a minority of patients achieve a complete
pathologic response to therapy due to the radioresistance of these
tumors, and PCa is no exception (14).
The androgen-independent PCa RM-1 cell line is
derived by the transformation of cells from the genital ridge of
embryonic C57BL/6 mice with ras and myc oncogenes. Given their
characteristic histopathology, RM-1 cells have been considered to
be suitable for PCa studies (15).
Numerous studies have been undertaken to establish an improved
understanding of the mechanisms by which the inhibition of the
alternative NF-κB pathway increases radiation sensitivity in PCa
(4,16,17).
From these experimental data, it was concluded that the RelB-based
alternative NF-κB pathway plays a significant role in protecting
PCa cells against IR, and that selective inhibition of RelB may be
effective for enhancing the susceptibility of PCa cells with high
Gleason scores to IR (18). In our
previous study, RelB siRNA-expressing lentiviral vectors targeting
the RelB gene were conducted with the molecular biological
technique to silence the RelB expression in RM-1 cells (6). In the present study, the RM-1 cells
were transfected with pLentilox-sh-RelB followed by irradiation,
and western blot analysis was subsequently employed to detect the
expression of RelB in the cells. It was demonstrated that RelB was
overexpressed in the non-trans-RM-1 and siVector-RM-1 cells
following IR treatment compared with the control-RM-1 cells without
irradiation. Moreover, pLentilox-sh-RelB targeting RelB
significantly downregulated the expression of the RelB protein in
the siRelB-RM-1 cells following IR treatment.
The development of the resistance of PCa to
radiation is a significant complication in treating this disease.
Eliminating these radioresistant cancer cells is perhaps the most
effective method for decreasing the recurrence of cancer following
radiotherapy. The radioresistance of the RM-1 cell line was
determined using a colony-forming assay in the present study.
Subsequently, it was observed that RelB inhibition by
pLentilox-sh-RelB significantly inhibited colony formation in the
mouse PCa RM-1 cell line following treatment with pLentilox-sh-RelB
for 48 h. In line with the observations of a previous study
(18), the present study
demonstrated that mouse PCa RM-1 cell SF number and other survival
parameters following IR treatment were lower in the siRelB-RM-1
cells compared with the siVector-RM-1 cells. These data indicated
that pLentilox-sh-RelB enhanced the radiosensitivity of the RM-1
cells and made them more likely to be killed by radiation
treatment.
In the present study, pLentilox-sh-RelB was able to
increase the radiosensitivity of the RM-1 cells in vitro,
which may be mainly associated with apoptosis enhancement. As a
consequence, apoptosis may be considered as the most suitable
method of anticancer therapy (19).
The main aim of apoptoaia-based treatment is to cause tumoral cell
death while limiting the cytotoxic effects on healthy tissues. This
may be achieved by, for example, promoting the expression of
pro-apoptotic factors at the same time as reducing the expression
of anti-apoptotic factors in the tumor cells only. The present
study found that the apoptosis rate was much higher in the
siRelB-RM-1 cells compared with the siVector-RM-1 and
non-trans-RM-1 cells. Mineva et al (20) demonstrated that RelB-knockdown using
siRNA promoted apoptosis in WEHI 231B lymphoma cells, which is
accordant with the present results. In the present study,
pLentilox-sh-RelB was able to reverse the radioresistance of the
RM-1 cells by increasing the level of radiation-induced apoptosis.
The level of radiation-induced apoptosis increased to a significant
extent, which indicated a significant role for RelB in the control
of irradiated PCa cell survival, possibly involving the activation
of the anti-apoptotic factors. However, the manner in which RelB
was able to affect the apoptosis in the RM-1 cells remains unclear
and requires further elucidation. A key mechanism by which NF-κB
controls cell survival is through the enhancement of the
transcription of various anti-apoptotic genes, including
Bcl-xl.
Bcl-xl is an important novel member of the Bcl-2
family, an anti-apoptotic group that has been reported to be vital
in tumor progression, development and chemo- or radioresistance
(21). Strick et al
(22) showed that the expression of
proteins (Bcl-xl and BAX) from the Bcl-2 family was able to
modulate radiosensitivity in human glioma cells. Moreover, Li et
al (23) proposed that, in
order to effectively overcome the acquired radioresistance of
cancer cells, the overexpression of Bcl-2 and Bcl-xl may be
targeted. Additionally, it has previously been reported that Bcl-xl
is overexpressed in PCa and involved in radioresistance, which is
also altered by modulating RelB level in cells (17,24).
In the present study, following the irradiation of the RM-1 cells,
the expression levels of Bcl-xl were relatively high. qPCR analysis
indicated that Bcl-xl was expressed in the murine hormone-resistant
PCa RM-1 cells and that the expression of Bcl-xl was upregulated in
the non-trans-RM-1 and siVector-RM-1 cells following irradiation
compared with the control-RM-1 cells without irradiation. Moreover,
the expression of Bcl-xl was downregulated in the siRelB-RM-1 cells
treated with pLentilox-sh-RelB compared with the siVector-RM-1 and
non-trans-RM-1 cells. Overall, the results indicated that IR
induces the expression of Bcl-xl in PCa cells to protect the cells
against IR, and that RelB-specific siRNA leads to a decrease in the
radiation-induced expression of Bcl-xl mRNA. The inhibition of
Bcl-xl may participate in the reduction of responses to IR, which
could efficiently enhance the efficacy of radiotherapy.
This is the first study to show that
pLentilox-sh-RelB downregulates the expression of Bcl-xl in RM-1
PCa cells in vitro. The downregulation of Bcl-xl by
pLentilox-sh-RelB may at least partly explain its ability to
reverse the radioresistance of RM-1 cells. Further studies are
required to determine the mechanisms underlying this phenomenon.
The present study data indicated that the decreased radioresistance
of the RM-1 cells could be attributed to the promotion of apoptosis
by the downregulation of Bcl-xl expression, and also revealed the
potential benefit of pLentilox-sh-RelB treatment in conjunction
with radiotherapy for PCa treatment. In summary, the present
results indicate that the alternative NF-κB pathway appears to be
important for radiation resistance in PCa cells, and that the
inhibition of Bcl-xl with pLentilox-sh-RelB and the promotion of
apoptosis may reverse the radioresistance of RM-1 cells in
vitro.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie BX, Zhang H, Yu L, et al: The
radiation response of androgen-refractory prostate cancer cell line
C4-2 derived from androgen-sensitive cell line LNCaP. Asian J
Androl. 12:405–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alcorn S, Walker AJ, Gandhi N, et al:
Molecularly targeted agents as radiosensitizers in cancer therapy -
focus on prostate cancer. Int J Mol Sci. 14:14800–14832. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holley AK, Xu Y, St Clair DK and St Clair
WH: RelB regulates manganese superoxide dismutase gene and
resistance to ionizing radiation of prostate cancer cells. Ann NY
Acad Sci. 1201:129–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Fang F, St Clair DK and St Clair WH:
Inverse relationship between PSA and IL-8 in prostate cancer: an
insight into a NF-κB-mediated mechanism. PLoS One.
7:e329052012.PubMed/NCBI
|
|
6
|
Zhu B, Yang LY, Zhao XK, et al: RNA
interference of RelB enhances the radiosensitivity of prostate
cancer cell line RM-1 in mice. Zhonghua Nan Ke Xue. 18:595–599.
2012.(In Chinese).
|
|
7
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: the sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov.
8:33–40. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Y, Fang F, Sun Y, et al: RelB-dependent
differential radiosensitization effect of STI571 on prostate cancer
cells. Mol Cancer Ther. 9:803–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ribeiro AM, Andrade S, Pinho F, et al:
Prostate cancer cell proliferation and angiogenesis in different
obese mice models. Int J Exp Pathol. 91:374–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu HC, Tao T and Liu XH: Construction and
identification of mouse RelB siRNA-expressing lentiviral vectors.
Sci Res Essays. 6:777–783. 2011.
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
13
|
Han RQ, Wu M, Chen WQ, et al: Analysis on
incidence and mortality of prostate cancer in China during
2003–2007. China Cancer. 21:805–811. 2012.(In Chinese).
|
|
14
|
Dass K, Ahmad A, Azmi AS, Sarkar SH and
Sarkar FH: Evolving role of uPA/uPAR system in human cancers.
Cancer Treat Rev. 34:122–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang AL and Russell PJ: Paclitaxel
suppresses the growth of primary prostate tumours (RM-1) and
metastases in the lung in C57BL/6 mice. Cancer Lett. 233:185–191.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Fang F, St Clair DK, et al: SN52, a
novel nuclear factor-kappaB inhibitor, blocks nuclear import of
RelB:p52 dimer and sensitizes prostate cancer cells to ionizing
radiation. Mol Cancer Ther. 7:2367–2376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, Fang F, St Clair DK, et al:
Suppression of RelB-mediated manganese superoxide dismutase
expression reveals a primary mechanism for radiosensitization
effect of 1alpha,25-dihydroxyvitamin D(3) in prostate cancer cells.
Mol Cancer Ther. 6:2048–2056. 2007. View Article : Google Scholar
|
|
18
|
Josson S, Xu Y, Fang F, et al: RelB
regulates manganese superoxide dismutase gene and resistance to
ionizing radiation of prostate cancer cells. Oncogene.
25:1554–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Russo A, Terrasi M, Agnese V, et al:
Apoptosis: a relevant tool for anticancer therapy. Ann Oncol.
17(Suppl 7): vii115–vii123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mineva ND, Rothstein TL, Meyers JA, et al:
CD40 ligand-mediated activation of the de novo RelB NF-kappaB
synthesis pathway in transformed B cells promotes rescue from
apoptosis. J Biol Chem. 282:17475–17485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llambi F and Green DR: Apoptosis and
oncogenesis: give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strik H, Deininger M, Streffer J, et al:
BCL-2 family protein expression in initial and recurrent
glioblastomas: modulation by radiochemotherapy. J Neurol Neurosurg
Psychiatry. 67:763–768. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JY, Li YY, Jin W, et al: ABT-737
reverses the acquired radioresistance of breast cancer cells by
targeting Bcl-2 and Bcl-xL. J Exp Clin Cancer Res. 31:1022012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Sun M, Zhang A, et al:
Adenovirus-mediated siRNA targeting Bcl-xL inhibits proliferation,
reduces invasion and enhances radiosensitivity of human colorectal
cancer cells. World J Surg Oncol. 9:1172011. View Article : Google Scholar
|