Introduction
Aβ is a peptide consisting of 36–43 amino acids and
is processed from the amyloid precursor protein (APP). APP is
commonly processed by two competing pathways; one pathway involves
cleavage by α-secretase and then by γ-secretase, whereas the other
involves processing by β-secretase and then by γ-secretase. The
former pathway produces sAPPα and p3 and the latter pathway
produces sAPPβ and Aβ (1,2). It was previously demonstrated that the
two pathways exert antagonistic effects on the generation of Aβ
(3). β-secretase cleavage of APP
produces Aβ, whereas α-secretase cleavage of APP within the Aβ
domain precludes the generation of Aβ (3). Although best known as a component of
senile plaques in the brain of patients with Alzheimer’s disease,
significant amounts of Aβ are produced in the periphery (4). It was recently reported that the
presence of Aβ in the blood is intimately involved in the
inflammatory pathology of atherosclerosis (AS) (5,6).
It was demonstrated that Aβ peptides present in the
atherosclerotic lesions are mainly derived from activated platelets
(5). Platelets contain abundant APP
and proteases involved in APP processing (α-, β- and γ-secretases)
(4). Platelet APP and APP
proteolytic products (sAPPα, sAPPβ and Aβ) are stored in α-granules
and are released by agents that induce platelet degranulation, such
as collagen (4). Platelets produce
up to 90% of the Aβ in circulation, mainly the Aβ40 peptide
(4). Therefore, platelet-derived Aβ
may serve as a target for anti-AS therapy.
Tanshinone IIA (Tan IIA) is a pharmacologically
active component isolated from the rhizome of the Chinese herb
Salvia miltiorrhiza Bunge (Danshen) (7–9). Since
Tan IIA has a quinoid structure and is easy to be oxidized and
reduced, it may participate in various biochemical reactions in the
human body. Emerging experimental studies and clinical trials
demonstrated that Tan IIA is able to prevent atherogenesis,
possibly through its antioxidant and anti-inflammatory actions
(7–9). However, the precise mechanisms
underlying the vasoprotective effect of Tan IIA have not been
clearly determined. Tan IIA was recently identified as a new member
of the phytoestrogen family (7,10). Tan
IIA at 5 μM was found to increase estrogen response
element-luciferase activity in an estrogen receptor (ER)-dependent
manner in HeLa cells (7). The
non-genomic action of ER is involved in a number of the
anti-inflammatory actions of Tan IIA (7,10). For
example, in lipopolysaccharide-treated RAW 264.7 cells, Tan IIA was
shown to inhibit the expression of inducible nitric oxide synthase,
the production of nitric oxide and the release of inflammatory
cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis
factor-α via an ER-dependent pathway (10). In our previous studies, we
demonstrated that ER-mediated PI3K/Akt signaling promotes
α-secretase cleavage of APP and inhibits the production of Aβ
(1,11). In this study, we aimed to assess
whether Tan IIA modulates platelet APP metabolism via an
ER-dependent pathway.
Materials and methods
Reagents
Tan IIA was purchased from Sigma (St. Louis, MO,
USA). Phosphospecific antibodies to p-Akt (Ser473 and Thr308) and
total Akt were obtained from Santa Cruz Biotechnology (CA, USA).
The goat anti-rabbit IgG-horseradish peroxidase (HRP) was purchased
from Invitrogen Life Technologies (Grand Island, NY, USA). Unless
stated otherwise, all the chemicals were purchased from Sigma (St.
Louis, MO, USA). Stock solutions (1,000X) of Tan IIA, the ER
antagonist ICI182.780, the ERα-specific antagonist
methyl-piperidinopyrazole (MPP) and the phosphatidylinositol
3-kinase (PI3K) inhibitor LY294002 were prepared in ethanol and
added to the platelet suspension at the indicated concentrations.
Stock solutions of collagen (5 mg/ml, human placental collagen type
I) were prepared in water (the pH was adjusted to 3.0 with acetic
acid). In all the experiments, an equivalent volume of ethanol
(0.1%) was added to the control group.
Subjects
A total of 12 healthy subjects, including 6 men and
6 women of mean age ± SD 57.7±4.6 years (range 50–65 years), were
recruited in this study, all without complaints of cognitive or
memory deficits. Major systemic, psychiatric or neurological
conditions, hypertension, diabetes mellitus, tumors, autoimmune
diseases, hypercholesterolaemia, drug or alcohol abuse and intake
of anticoagulants were precluded for all the subjects. Written
informed consent was obtained from all the participants prior to
study initiation. This study was approved by the Institutional
Board of Guangzhou Medical University (Guangzhou, China).
Platelet preparation and pharmacological
treatments
Peripheral venous blood (10 ml) was collected from
each subject. The anticoagulated blood was immediately centrifuged
at 150 × g for 10 min at room temperature and the supernatant was
the platelet-rich plasma (PRP) (1).
The platelet counts of PRP were adjusted to 1×108/ml by
platelet-free plasma obtained by centrifugation of the blood
samples at 4,000 × g for 20 min. To determine the effect of Tan IIA
on platelet APP metabolism, 5 μM Tan IIA was added to PRP, followed
by incubation with gentle agitation at 37°C for 24 h.
To determine whether Tan IIA affects platelet APP
metabolism via ER-mediated cell signaling, the platelets were
incubated with Tan IIA (5 μM) in the presence or absence of
ICI182.780 (1 μM), MPP (1 μM) or LY294002 (10 μM) at 37°C.
Subsequently, the platelets were collected and resuspended in
modified Tyrode’s buffer (150 mM NaCl, 5 mM
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.55 mM
NaH2PO4, 7 mM NaHCO3, 2.7 mM KCl,
0.5 mM MgCl2 and 5.6 mM glucose). The platelet
suspension was then incubated with gentle agitation at 37°C for
another 1 h, followed by measurement of Aβ and sAPPα released from
inactivated and collagen (10 μg/ml)-activated platelets (1).
sAPPα levels
Platelet secretion of sAPPα was measured using an
sAPPα ELISA kit (IBL International, Hamburg, Germany). Following
platelet treatment as described above, the medium was collected and
sAPPα levels were measured according to the manufacturer’s
protocol. Spectrophotometric data were collected using the Victor-2
Multilabel counter (Perkin Elmer/Wallace, Akron, OH, USA) at a
wavelength of 450 nm.
Aβ levels
Platelet secretion of Aβ was measured using a human
Aβ40 ELISA kit (Invitrogen, Camarillo, CA, USA). Following platelet
treatment as described above, the medium was collected and Aβ
levels were measured according to the manufacturer’s protocol.
Spectrophotometric data were collected using the Victor-2
Multilabel counter at a wavelength of 450 nm.
α-secretase activity
In this study, the α-secretase activity in living
platelets and platelet lysates was measured using an α-secretase
activity kit (R&D Systems, Minneapolis, MN, USA). Following
treatment, the platelets were collected by centrifugation of the
platelet suspension at 1,400 × g for 15 min. Alternatively, the
platelets were lysed in the lysis buffer provided in the kit and
then treated with Tan IIA or vehicle, as described above. The
α-secretase activity in living platelets and platelet lysates was
then measured by fluorescent spectroscopy according to the
manufacturer’s protocol. The cleavage-dependent release of
fluorescence signal was quantified using the Victor-2 Multilabel
counter at an excitation wavelength of 340 nm and an emission
wavelength of 495 nm.
The protein concentration of the platelet lysates
was determined with a detergent-compatible protein assay kit
(Bio-Rad, Hercules, CA, USA). Spectrophotometric data were obtained
using the Victor-2 Multilabel counter at a wavelength of 650 nm.
The enzyme activities were corrected for protein content.
PI3K activity
The PI3K activity was determined using a
commercially available PI3K ELISA kit (Echelon Biosciences Inc.,
Salt Lake City, UT) according to the manufacturer’s instructions.
Briefly, following drug treatment, the platelets were collected and
lysed in ice-cold lysis buffer [137 mM NaCl, 20 mM Tris-HCl (pH
7.4), 1 mM CaCl2, 1 mM MgCl2, 0.1 mM sodium
orthovanadate, 1% NP-40 and 1 mM phenylmethylsulphonyl fluoride].
PI3K was then immunoprecipitated with anti-PI3K antibody and
protein A-sepharose beads. The PI3K activity in the
immunoprecipitates was then assayed by PI3K ELISA according to the
manufacturer’s instructions. The spectrophotometric data were
obtained using the Victor-2 Multilabel counter at a wavelength of
450 nm. The protein concentration in the platelet lysates was
determined as described above. The activity of PI3K was corrected
for protein content.
p-Akt levels
Following drug treatment, the platelets were
collected by centrifugation of the platelet suspension at 1,400 × g
for 15 min. The protein levels of p-Akt were measured by western
blot analysis. Briefly, after boiling in two-fold loading buffer
[100 nM Tris-HCl (pH 6.8) 4% sodium dodecyl sulfate, 200 nM
dithiothreitol, 0.2% bromophenol blue and 20% glycerol] for 5 min,
the proteins (20 μg per well) were subjected to 12% SDS-PAGE at
100V for 3 h and then transferred onto nitrocellulose membranes
using a semi-dry transfer apparatus (Bio-Rad). The transfer of the
proteins was performed for 1 h at 10 V. The blots thus obtained
were rinsed in PBST solution [100 mM phosphate buffer (pH 7.5)
containing 150 mM NaCl and 0.05% Tween-20] and blocked with 5%
non-fat dry milk (Bio-Rad, Hercules, CA, USA) in PBST for 1 h. The
blots were then incubated with primary antibodies against p-Akt
(Ser473 and Thr308) (1:1,000) and total Akt (1:1,000) on a platform
shaker overnight at 4°C. After rinsing with PBST, the blots were
incubated with 1:5,000 goat anti-rabbit IgG-HRP for 2 h and then
rinsed with PBST. The protein band was visualized using X-ray film
and the band intensities were determined with the Bio-Rad GS800
densitometer equipped with ‘Quantity One’ software package.
Statistical analysis
The statistical analysis was performed by SPSS
software, version 15.0 (SPSS Inc., Chicago, IL, USA). After the
Ryan-Joiner test for normal distribution and the Levene’s test of
Equal Variances for variance homogeneity, data were analyzed by
one-way analysis of variance followed by pairwise t-tests.
Results
Tan IIA promotes non-amyloidogenic APP
processing in human platelets
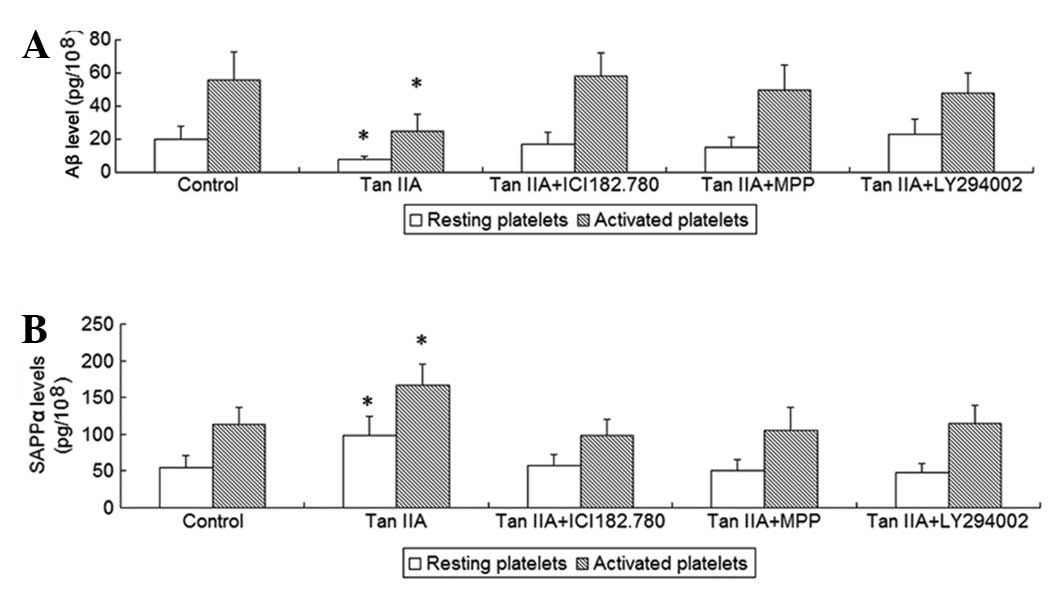
To investigate the effects of Tan IIA on platelet
APP metabolism, the platelets were incubated with 5 μM Tan IIA for
24 h; subsequently, sAPPα and Aβ released from resting and
activated platelets were measured. It was observed that Tan IIA
treatment increased the release of sAPPα and decreased the release
of Aβ from resting as well as from collagen-activated platelets
(P<0.01, Fig. 1), suggesting
that Tan IIA promotes non-amyloidogenic APP processing in
platelets.
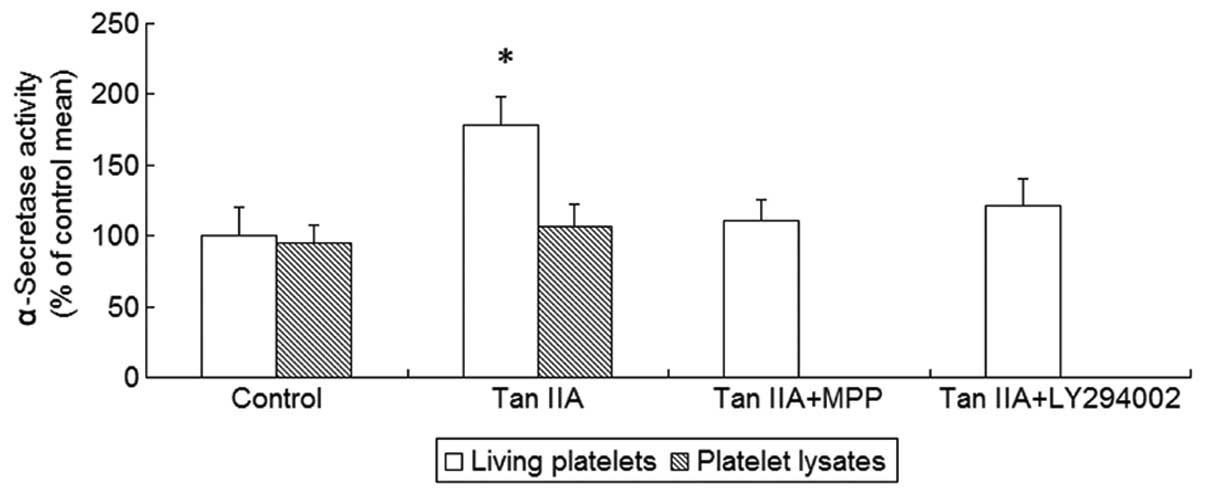
Non-amyloidogenic APP processing is mediated by
α-secretase. Therefore, we next investigated the effect of Tan IIA
on α-secretase activity and observed that Tan IIA enhanced
α-secretase activity in platelets (P<0.01, Fig. 2). We hypothesized that the promotion
of non-amyloidogenic APP processing by Tan IIA required structured
cellular functions, such as signal transduction cascades, rather
than direct activation of α-secretase. To exclude the possibility
of direct activation, the platelet lysates were treated with Tan
IIA (5 μM). However, there was no statistically significant effect
of Tan IIA on α-secretase activity in the platelet lysates
(P>0.05, Fig. 2), suggesting
that α-secretase activation by Tan IIA may not be mediated by a
direct molecular interaction between α-secretase and Tan IIA. These
findings indicate the involvement of signal transduction pathways
in Tan IIA-mediated α-secretase activation.
ER-mediated PI3K/Akt signaling is
involved in the effect of Tan IIA on platelet APP metabolism
It was demonstrated that ER-mediated PI3K/Akt
signaling is involved in APP metabolism (1,11). Tan
IIA is known to be a phytoestrogen and may activate ER-mediated
signaling, such as PI3K/Akt (7,10). To
determine whether Tan IIA modulates APP metabolism via ER
signaling, the platelets were incubated with Tan IIA in the
presence or absence of the ER antagonist ICI182.780 or the
ERα-specific antagonist MPP or the PI3K inhibitor LY294002;
subsequently, sAPPα and Aβ released from resting and activated
platelets as well as the activity of α-secretase were measured. It
was observed that the two ER antagonists and the PI3K inhibitor
were able to abrogate the effects of Tan IIA on APP metabolism and
α-secretase acitivity (P<0.01, Figs.
1 and 2). To determine whether
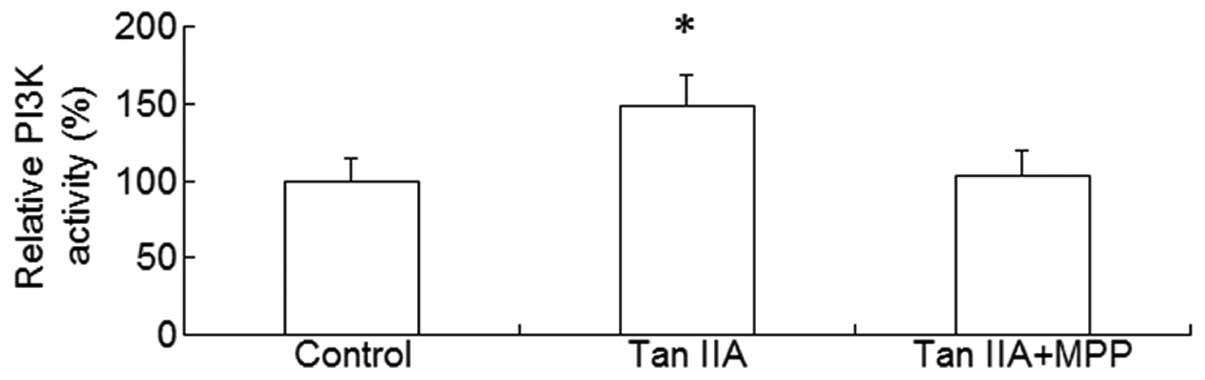
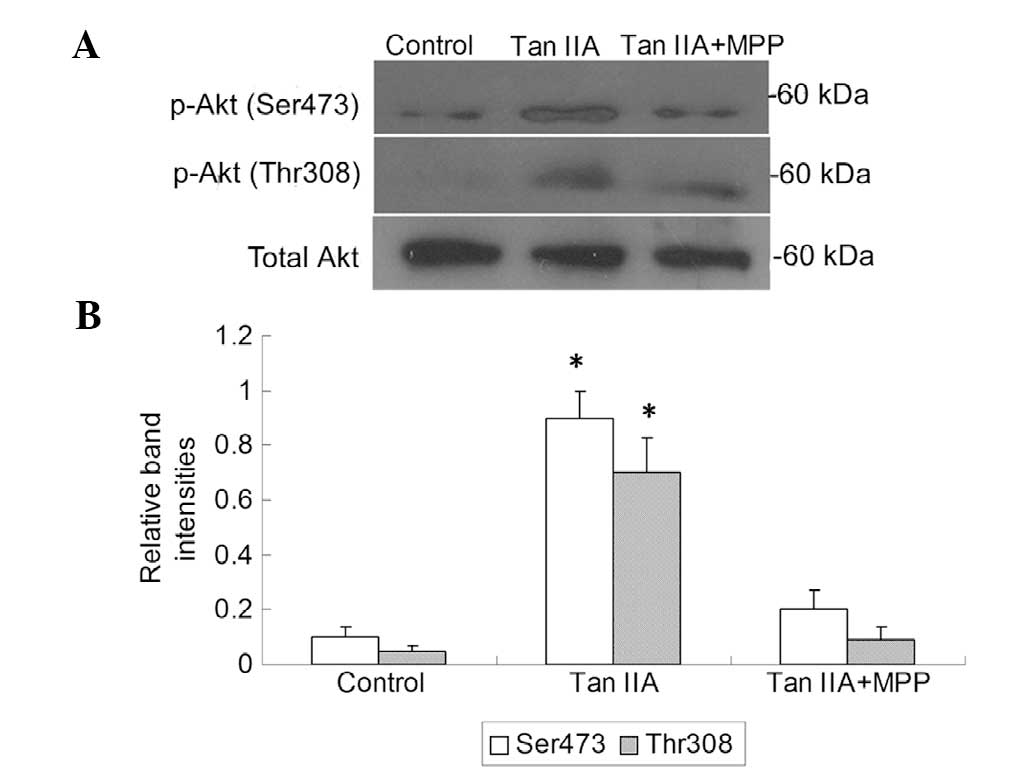
the PI3K/Akt pathway is a downstream effector of activated ER, the
platelets were treated with Tan IIA in the presence or absence of
the ER antagonist MPP; subsequently, the PI3K activity and p-Akt
levels were measured. It was observed that Tan IIA upregulated the
PI3K activity and p-Akt levels and this effect was suppressed in
the presence of MPP (P<0.01, Figs.
3 and 4). These results suggest
that ER-mediated PI3K/Akt signaling may be involved in the effect
of Tan IIA on APP metabolism.
Discussion
It was demonstrated that the aberrant deposition of
Aβ, predominantly Aβ40, in the intima is pathologically significant
in the development of AS (5,6). The
activated platelets were reported to be the principal source of Aβ
in AS lesions (5). Therefore,
platelet APP metabolism may serve as a target for AS therapy. Tan
IIA, the major active ingredient responsible for the beneficial
actions of Danshen, has long been used for the prevention and
treatment of AS (7–9) and was recently identified as a
phytoestrogen (7,10). However, it has not been elucidated
whether Tan IIA intervenes in platelet APP processing and whether
such an intervention is associated with its estrogenic
activity.
In this study, we demonstrated that Tan IIA promoted
the release of sAPPα and inhibited the secretion of Aβ from resting
as well as from activated platelets, suggesting that Tan IIA
enhances non-amyloidogenic APP processing in platelets.
Additionally, the promotion of non-amyloidogenic APP processing by
Tan IIA required activation of PI3K/Akt signaling rather than
direct interaction between α-secretase and Tan IIA. In our previous
studies, we reported that ER-mediated PI3K/Akt signaling is
involved in APP metabolism (1,11),
suggesting that the effect of Tan IIA on APP metabolism may be
associated with its estrogenic activity. In this study, the effect
of Tan IIA on platelet APP metabolism was inhibited blocked by the
ER antagonists and the PI3K inhibitor. In addition, Tan IIA
upregulated PI3K activity and p-Akt levels, an effect that was
suppressed by the ER antagonists. These data suggest that Tan IIA
modulates platelet APP metabolism, possibly through ER signaling to
PI3K/Akt. In accordance with this finding, our previous studies
demonstrated that treatment of HT22 cells, SH-SY5Y cells stably
expressing the Swedish mutant APP and ovariectomized rats with
phytoestrogens, such as ginsenoside Rg1 and bilobalide, increases
the secretion of sAPPα, enhances α-secretase activity and decreases
the production of Aβ via ER-mediated PI3K/Akt signaling (11–13).
It is known that ER regulates cellular function
through at least two signaling pathways, including the nuclear
ER-mediated genomic pathway and the membrane ER-mediated
non-genomic pathway (14). In
platelets, Tan IIA modulates APP metabolism possibly via membrane
ER-mediated non-genomic signaling. It was demonstrated that
membrane ERs reside in the lipid rafts (15,16),
where the ER isoform, ERα, activates PI3K through membrane
recruitment and direct interaction with PI3K (17). ERα binds in a ligand-dependent
manner to the p85α regulatory subunit of PI3K, leading to the
phosphorylation of Akt (18).
Given that blood Aβ is intimately associated with
the inflammatory pathology of AS, the finding that Tan IIA promotes
non-amyloidogenic APP processing in platelets sheds new light on
the mechanism underlying the vasoprotective effect of Tan IIA. Of
note, blood sAPPα and Aβ were demonstrated to exert antagonistic
effects on platelet function (4).
Aβ augments physiological agonist-induced platelet activation and
platelet adhesion, whereas sAPPα inhibits platelet aggregation and
degranulation (4). Therefore, there
may be an enhanced interaction between the released sAPPα and Tan
IIA in the mechanism of Tan IIA-induced reduction in platelet Aβ
release: Tan IIA increases the release of sAPPα from platelets,
possibly leading to a sustained decrease of platelet Aβ release via
inhibition of platelet degranulation.
In conclusion, this study demonstrated, using human
platelets, that Tan IIA promotes the non-amyloidogenic pathway of
APP cleavage via its estrogenic activity. The PI3K/Akt pathway may
be involved in the effect of Tan IIA on APP metabolism as a
downstream effector of ER signaling. Since the APP proteolytic
product, Aβ, is involved in the inflammatory pathology of AS, these
findings may contribute to a better understanding of the
vasoprotective effect of Tan IIA.
Acknowledgements
The authors are grateful for the support from the
National Natural Science Foundation of China (grant nos. 81370395
and 81200846) and the Guangdong Natural Science Foundation (grant
no. S2012040007858 and S2013010014468).
References
|
1
|
Shi C, Na N, Zhu X and Xu J: Estrogenic
effect of ginsenoside Rg1 on APP processing in post-menopausal
platelets. Platelets. 24:51–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gandy S and DeKosky ST: Toward the
treatment and prevention of Alzheimer’s disease: rational
strategies and recent progress. Annu Rev Med. 64:367–383. 2013.
|
|
3
|
Mandel S, Amit T, Reznichenko L, Weinreb O
and Youdim MB: Green tea catechins as brain-permeable, natural iron
chelators-antioxidants for the treatment of neurodegenerative
disorders. Mol Nutr Food Res. 50:229–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li QX, Fuller SJ, Beyreuther K and Masters
CL: The amyloid precursor protein of Alzheimer disease in human
brain and blood. J Leukoc Biol. 66:567–574. 1999.PubMed/NCBI
|
|
5
|
Kokjohn TA, Van Vickle GD, Maarouf CL, et
al: Chemical characterization of pro-inflammatory amyloid-beta
peptides in human atherosclerotic lesions and platelets. Biochim
Biophys Acta. 1812.1508–1514. 2011.PubMed/NCBI
|
|
6
|
Howlett GJ and Moore KJ: Untangling the
role of amyloid in atherosclerosis. Curr Opin Lipidol. 17:541–547.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan GW, Gao XM, Wang H, et al: The
anti-inflammatory activities of tanshinone IIA, an active component
of TCM, are mediated by estrogen receptor activation and inhibition
of iNOS. J Steroid Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu S, Little PJ, Lan T, et al: Tanshinone
II-A attenuates and stabilizes atherosclerotic plaques in
apolipoprotein-E knockout mice fed a high cholesterol diet. Arch
Biochem Biophys. 515:72–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan G, Zhu Y, Guo H, Wang X, Wang H and
Gao X: Direct vasorelaxation by a novel phytoestrogen tanshinone
IIA is mediated by nongenomic action of estrogen receptor through
endothelial nitric oxide synthase activation and calcium
mobilization. J Cardiovasc Pharmacol. 57:340–347. 2011. View Article : Google Scholar
|
|
11
|
Shi C, Zheng DD, Fang L, Wu F, Kwong WH
and Xu J: Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP
via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim
Biophys Acta. 1820.453–460. 2012.PubMed/NCBI
|
|
12
|
Shi C, Wu F, Xu J and Zou J: Bilobalide
regulates soluble amyloid precursor protein release via
phosphatidyl inositol 3 kinase-dependent pathway. Neurochem Int.
59:59–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi C, Zheng DD, Wu FM, Liu J and Xu J:
The phosphatidyl inositol 3 kinase-glycogen synthase kinase 3β
pathway mediates bilobalide-induced reduction in amyloid β-peptide.
Neurochem Res. 37:298–306. 2012.PubMed/NCBI
|
|
14
|
Grove-Strawser D, Boulware MI and
Mermelstein PG: Membrane estrogen receptors activate the
metabotropic glutamate receptors mGluR5 and mGluR3 to
bidirectionally regulate CREB phosphorylation in female rat
striatal neurons. Neurosci. 170:1045–1055. 2010. View Article : Google Scholar
|
|
15
|
Gilad LA and Schwartz B: Association of
estrogen receptor beta with plasma-membrane caveola components:
implication in control of vitamin D receptor. J Mol Endocrinol.
38:603–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chambliss KL, Yuhanna IS, Mineo C, et al:
Estrogen receptor alpha and endothelial nitric oxide synthase are
organized into a functional signaling module in caveolae. Circ Res.
87:E44–E52. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Losel RM, Falkenstein E, Feuring M,
Schultz A, Tillmann HC, Rossol-Haseroth K and Wehling M: Nongenomic
steroid action: controversies, questions, and answers. Physiol Rev.
83:965–1016. 2003.PubMed/NCBI
|
|
18
|
Simoncini T, Hafezi-Moghadam A, Brazil DP,
Ley K, Chin WW and Liao JK: Interaction of oestrogen receptor with
the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature.
407:538–541. 2000. View
Article : Google Scholar : PubMed/NCBI
|