Introduction
Crohn's disease (CD) is a chronic inflammatory
disorder of the gastrointestinal tract and the pathogenesis is not
entirely clear. Several factors are considered to contribute to the
chronic intestinal inflammatory process in patients with CD. These
factors include environmental exposures, a genetic disposition and
an unbalanced immune reaction to the commensal microorganism of the
gut (1,2). The theoretical foundation for using
antibiotics as the main CD treatment is based on increasing
evidence implicating intestinal bacteria in the pathogenesis of the
disease (3,4). Certain clinical trials have
demonstrated the efficacy of antibiotics in inducing and
maintaining CD remission and preventing CD recurrence (5). However, the use of antibacterial
therapy for CD is also controversial, even this approach is
frequently and successfully adopted in clinical practice (6).
The purpose of the present study was to evaluate the
usefulness of ciprofloxacin (cipro), which is a staple antibiotic
for treatment of CD. A meta-analysis was performed involving
randomized controlled trials only.
Materials and methods
Inclusion/exclusion criteria
Studies that were in line with the following
criteria were included: i) Randomized controlled trials that assess
the efficacy of cipro for the treatment of CD; ii) studies that are
published as a full article; and iii) other inflammatory bowel
disease therapy allowed is the same in the two groups of the
trial.
Studies were excluded if: i) Patients were <18
years, pregnant, had systemic disease, or had significant renal or
hepatic disease; ii) specifically evaluate post-operative CD
patients; and iii) the control group was not treated with
placebo.
Search strategy
A search of the PubMed, Embase and Cochrane Library
was performed up to May 2014. A search strategy was constructed
using a combination of the following words: (Ciprofloxacin or
quinolone or antibiotic) and (Crohn's disease or inflammatory bowel
disease). Studies published in any language were included. A manual
search of the references listed in the studies retrieved from the
online databases and from previously published systematic reviews
was also performed to identify further relevant studies.
Data extraction
Two investigators (Wu and Ji) extracted data. Any
difference regarding the study inclusion, data extraction and
interpretation were resolved by consensus prior to the final
analysis. Study variables were gathered in the following
categories: Study design, demographics, interventions, duration,
extent of disease and co-therapy permitted. To avoid the inclusion
of duplicated data in final analysis, retrieved studies were
carefully evaluated and checked by comparison of author names,
geographical locations and period of study.
Assessment of risk of bias in included
studies
The investigators independently, but without being
blinded to the authors or journal, assessed the risk of bias in the
studies that met the inclusion criteria. The Cochrane
Collaboration's tool for assessing risk of bias in randomized
trials (7) was used, which includes
the following criteria: Adequacy random-sequence generation;
allocation concealment; blinding of participants, personnel and
outcome assessors; and incomplete outcome data. In all the cases,
an answer of ‘yes’ indicates a low risk and an answer of ‘no’
indicates a high risk of bias.
Statistical methods
Meta-analysis was carried out by combining the risk
ratio (RR) between the cipro and control groups of the individual
studies in a global RR. The intention-to-treat analysis was
performed that included all patients who started the medication.
Statistical heterogeneity tested was performed using the
χ2 statistic and I2, and an I2
value >50% was considered to have substantial heterogeneity
(8). A fixed-effects model was
selected when the heterogeneity test showed I2 value
<50%, otherwise, a random-effects model was used. Funnel plot
was used as an indicator of publication bias (9). Analyses were conducted using Review
Manager (RevMan software, version 5.3, 2014; The Nordic Cochrane
Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
Search results
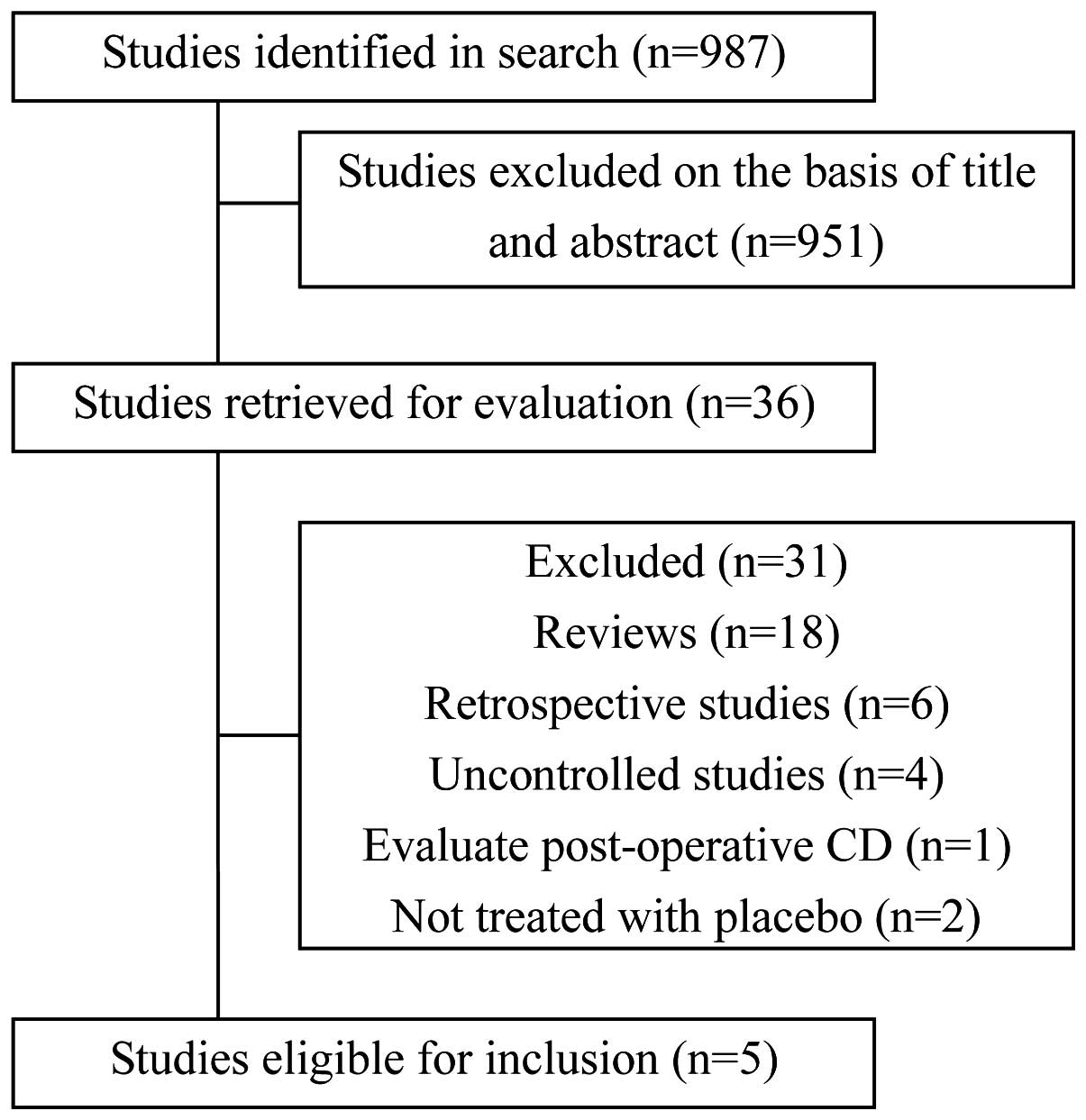
The search identified 987 potentially relevant
studies, of which 951 were excluded following title and abstract
screening. In total, 36 studies were retained for full-text review.
Initially, 18 review studies were discarded. By analyzing detail
data, six retrospective studies and four uncontrolled studies were
excluded. Two controlled studies were excluded as the control group
was treated with non-placebo and another controlled study was
excluded as it specifically evaluated post-operative patients.
Finally, five studies (10–14) were identified involving 293 patients
that fulfilled the inclusion criteria (Fig. 1).
Characteristics of included
studies
The characteristics of included studies and patients
are presented in Table I. Three
studies came from North America, whereas the others were from
Europe. There were 146 (49.8%) males from the available data
regarding the gender in all the studies. The mean age of patients
ranged 30–45.2 years. In two studies, the intervention was
monotherapy and there were 38 patients treated with cipro. In the
other three studies, cipro groups were accepted with combination
therapy. There were 45 patients treated with cipro plus anti-tumor
necrosis factor and 66 patients treated with cipro plus
metronidazole (metro) and budesonide. All the included trials had a
number of methodological limitations (Table II).
 | Table ICharacteristics of the studies
included in the meta-analysis. |
Table I
Characteristics of the studies
included in the meta-analysis.
| Interventions | | |
|---|
|
| | |
|---|
| Study (year) | Country | Study population | Mean age, years | Male, % | Outcome | Cipro | Control | Duration, weeks | (Refs.) |
|---|
| Arnold et al
(2002) | USA | Single-center | 43 | 59.6 | CDAI <150 | Cipro 500 mg bid | Placebo | 12 | (10) |
| Dewint et al
(2014) | The Netherlands | Multicenter | 36 | 52.9 | ≥50% reduction of
fistulas | Cipro 500 mg bid +
adalimumab | Placebo +
adalimumab | 12 | (11) |
| SteinWhart et
al (2002) | Canada | Multicenter | 32 | 42.5 | CDAI<150 +
budesonide | Cipro 500 mg bid
budesonide | Placebo + | 12 | (12) |
| Thia et al
(2009) | USA/Canada | Multicenter | 35 | 67.7 | ≥50% reduction of
fistulas | Cipro 500 mg bid | Placebo | 10 | (13) |
| West et al
(2004) | The Netherlands | Single-center | 34 | 50.0 | ≥50% reduction of
fistulas | Cipro 500 mg bid +
infliximab | Placebo +
infliximab | 12 | (14) |
 | Table IIMethodological quality summary for
each included study. |
Table II
Methodological quality summary for
each included study.
| Study (year) | Adequate sequence
generation | Allocation
concealment | Blinding | Incomplete outcome
data | (Refs.) |
|---|
| Arnold et al
(2002) | Yes | Unclear | No | Yes | (10) |
| Dewint et al
(2014) | Yes | Unclear | Yes | Yes | (11) |
| Steinhart et
al (2002) | Yes | Unclear | Yes | Yes | (12) |
| Thia et al
(2009) | Yes | Unclear | Yes | Yes | (13) |
| West et al
(2004) | No | Unclear | Yes | Yes | (14) |
Efficacy results
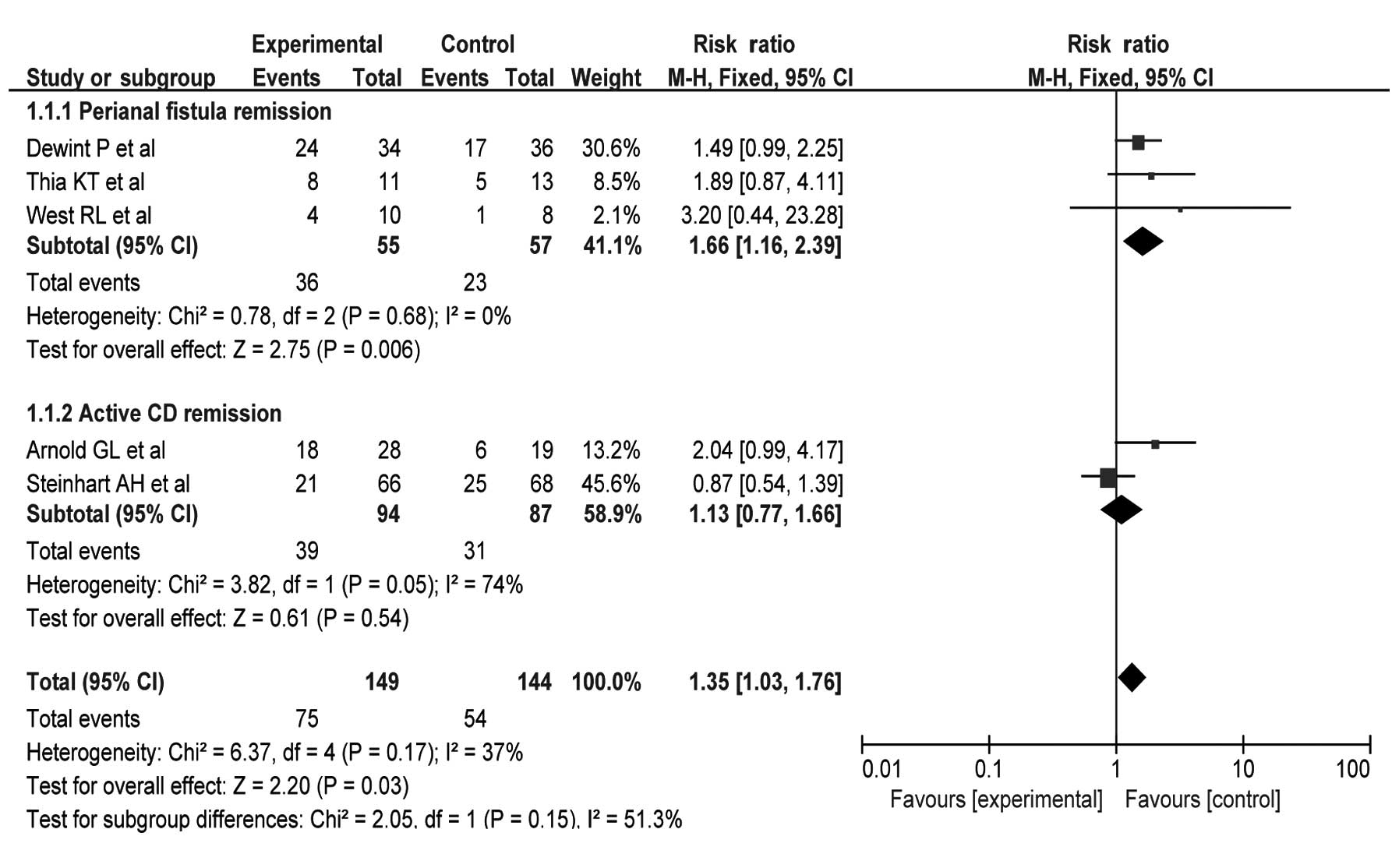
When including all the studies, there was a
statistically significant effect of cipro used for induction of
remission in CD patients at 10–12 weeks [RR=1.35; 95% confidence
interval (CI), 1.03–1.76; P=0.03] (Fig.
2). Subgroup analysis was performed to assess the specific
effects of cipro used for CD with perianal fistula or active CD,
respectively. Three studies (11,13,14)
involving 112 patients indicated that cipro had a specific affect
in achieving remission of CD with perianal fistula (RR=1.66; 95%
CI, 1.16–2.39; P=0.006). Two studies (10,12)
involving 181 patients did not appear to be specifically effective
with regards to cipro inducing remission in active CD (RR=1.13; 95%
CI, 0.77–1.66; P=0.54).
Heterogeneity and publication
bias
The present study did not cause significant
heterogeneity in the meta-analysis (χ2=6.37;
I2=37%; P=0.17) (Fig.
2). Therefore, a fixed-effect model was used for the
meta-analysis. A funnel plot is a helpful tool to estimate
publication bias in a meta-analysis. Inspection of the funnel plot
revealed a reasonably symmetrical distribution of studies.
Accordingly, it was considered that there was no significant
publication bias.
Discussion
With regards to the association between intestinal
bacteria and CD, numerous antibiotic regimens have been performed
as primary therapy to try to modify the intestinal flora and reduce
invasion and colonization of harmful bacteria (15). However, the role of intestinal
bacteria in the pathogenesis of CD is not completely clear. Due to
the complexity of intestinal microbial communities, all the harmful
intestinal bacteria cannot be identified (16). Therefore, broad-spectrum antibiotics
are considered more effective than narrow-spectrum antibiotics for
the treatment of CD (17). Cipro is
a quinolone with good activity against the majority of enteric
bacteria (18) and the emergence of
cipro resistance is relatively infrequent (19). Certain clinical trails have
evaluated the efficacy of cipro for CD treatment.
In a small and uncontrolled study (20) of early stage, four active CD
patients were treated with cipro. Each patient had a significant
improvement in abdominal pain and diarrhea coinciding with the
institution of cipro. A retrospective study (21) involving 233 inpatients with active
CD showed similar achievement rates of a complete or partial
remission obtained between cipro plus metro or cipro alone. An
uncontrolled trial (22) indicated
that cipro in combination with metro was well-tolerated and
beneficial for achieving clinical remission for patients with
active CD, particularly when there was involvement of the colon. A
study (23) in Japan found that
combination therapy of cipro and metro for four weeks significantly
decreased the C-reactive protein in all the patients involved. In a
randomized non-placebo controlled trial (24) involving 40 CD patients, complete
remission was observed in 56% patients treated with cipro and 55%
patients treated with mesalazine. The result indicated that cipro
was as effective as mesalazine in treating mild to moderate
flare-up of CD. Another non-placebo controlled trial (25) involving 41 patients concluded that
cipro plus metro could be an alternative to steroids in treating
the acute phase of CD.
Perianal fistula occurs in ≤40% of CD patients and
its natural history is characterized by chronicity with rarely
spontaneous healing of the fistula tracts (26). Despite enhancive medical and
surgical treatment, the majority of patients continue to suffer
from perianal symptoms and have to accept extensive surgery, such
as proctocolectomy (27). Wolf
(28) reported that five CD
patients of perianal disease were treated with cipro for 1–2
months, which caused relief of symptoms and healing of fissures in
four patients. Following discontinuation of cipro, three of the
four responders underwent recurrence. Uza et al (29) carried out a combination therapy of
cipro, metro and azathioprine to 10 patients with fistulizing CD.
Complete responses were observed in all six patients with external
fistulas, and in only one of four patients with internal fistulas.
Turunen et al (30) treated
10 patients with severe perianal or fistulous CD with cipro for ≥3
months and seven of the 10 patients responded. Two exhibited healed
lesions >2 years and five others relapsed after discontinuing
the antibiotic, but responded again to 1–3 month courses of
cipro.
The present meta-analysis of five randomized
placebo-controlled trials indicates that cipro is effective to
relieve the activity of CD. In a study (12) involving 134 patients, cipro plus
metro was ineffective to patients with active CD of the ileum,
however this combination therapy may improve outcome when there is
involvement of the colon. In another study (10), there was a significantly beneficial
effect of cipro for treatment of active CD. After 3 months of
therapy, remission was observed in 72% of patients treated with
cipro and 50% of patients treated with placebo, respectively, and
after 6 months of therapy the mean Crohn's disease activity index
was 111.7 for the cipro group in contrast to 205.4 for the placebo
group. However, the data of different remission rate according to
the classification of disease location was absent. The other three
studies included 112 patients with fistulizing CD. In two studies
(11,14) involving 45 patients treated with
cipro plus anti-tumor necrosis factor, remission of fistulas was
observed in 71% of patients treated with cipro and 45% of patients
treated with placebo, respectively, and the results demonstrated
that cipro was significantly effective to CD patients with perianal
fistula. Another small sample size study (13) also showed fistula remission occurred
more frequently in patients treated with cipro compared to
treatment with placebo, but the differences were not
significant.
However, the results of the present meta-analysis
are limited. Firstly, the total number of samples in all the
studies was not quite enough for a meta-analysis. Secondly, as the
intervention in certain studies was combination therapy, it was
difficult to accurately evaluate the effects of cipro. Furthermore,
as the adverse events of cipro were not recorded clearly in each
study, the side-effects of cipro used to treat CD could not be
assessed. Therefore, the meta-analysis has specific bias and a
globally multicenter prospective study is required to confirm the
results.
In conclusion, the present meta-analysis showed that
cipro is beneficial for CD patients, particularly with perianal
fistula. However, the study is associated with certain limitations
and it is necessary to conduct further prospective studies of cipro
in CD patients.
Acknowledgements
The present study received financial support from
the National Natural Science Foundation of China (grant no.
81270453).
References
|
1
|
Baumgart DC and Sandborn WJ: Crohn's
disease. Lancet. 380:1590–1605. 2012. View Article : Google Scholar
|
|
2
|
Cho JH and Brant SR: Recent insights into
the genetics of inflammatory bowel disease. Gastroenterology.
140:1704–1712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perencevich M and Burakoff R: Use of
antibiotics in the treatment of inflammatory bowel disease. Inflamm
Bowel Dis. 12:651–664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sartor RB: Therapeutic manipulation of the
enteric microflora in inflammatory bowel diseases: antibiotics,
probiotics, and prebiotics. Gastroenterology. 126:1620–1633. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prantera C: What role do antibiotics have
in the treatment of IBD? Nat Clin Pract Gastroenterol Hepatol.
5:670–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dignass A, Van Assche G, Lindsay JO, et al
European Crohn's and Colitis Organisation (ECCO): The second
European evidence-based Consensus on the diagnosis and management
of Crohn's disease: Current management. J Crohns Colitis. 4:28–62.
2010. View Article : Google Scholar
|
|
7
|
Higgins JP, Altman DG, Gotzsche PC, et al
Cochrane Bias Methods Group; Cochrane Statistical Methods Group:
The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar
|
|
8
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Egger M, Davey Smith G, Schneider M, et
al: Bias in meta-analysis detected by a simple, graphical test.
BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arnold GL, Beaves MR, Pryjdun VO and Mook
WJ: Preliminary study of ciprofloxacin in active Crohn's disease.
Inflamm Bowel Dis. 8:10–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dewint P, Hansen BE, Verhey E, et al:
Adalimumab combined with ciprofloxacin is superior to adalimumab
monotherapy in perianal fistula closure in Crohn's disease: a
randomised, double-blind, placebo controlled trial (ADAFI). Gut.
63:292–299. 2014.PubMed/NCBI
|
|
12
|
Steinhart AH, Feagan BG, Wong CJ, et al:
Combined budesonide and antibiotic therapy for active Crohn's
disease: a randomized controlled trial. Gastroenterology.
123:33–40. 2002. View Article : Google Scholar
|
|
13
|
Thia KT, Mahadevan U, Feagan BG, et al:
Ciprofloxacin or metronidazole for the treatment of perianal
fistulas in patients with Crohn's disease: a randomized,
double-blind, placebo-controlled pilot study. Inflamm Bowel Dis.
15:17–24. 2009. View Article : Google Scholar
|
|
14
|
West RL, van der Woude CJ, Hansen BE, et
al: Clinical and endosonographic effect of ciprofloxacin on the
treatment of perianal fistulae in Crohn's disease with infliximab:
a double-blind placebo-controlled study. Aliment Pharmacol Ther.
20:1329–1336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammer HF: Gut microbiota and inflammatory
bowel disease. Dig Dis. 29:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chassaing B and Darfeuille-Michaud A: The
commensal microbiota and enteropathogens in the pathogenesis of
inflammatory bowel diseases. Gastroenterology. 140:1720–1728. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prantera C and Scribano ML: Antibiotics
and probiotics in inflammatory bowel disease: why, when, and how.
Curr Opin Gastroenterol. 25:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hooper DC and Wolfson JS: Fluoroquinolone
antimicrobial agents. N Engl J Med. 324:384–394. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanders CC, Sanders WE Jr and Goering RV:
Overview of preclinical studies with ciprofloxacin. Am J Med.
82:2–11. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peppercorn MA: Is there a role for
antibiotics as primary therapy in Crohn's ileitis? J Clin
Gastroenterol. 17:235–237. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prantera C, Berto E, Scribano ML and
Falasco G: Use of antibiotics in the treatment of active Crohn's
disease: experience with metronidazole and ciprofloxacin. Ital J
Gastroenterol Hepatol. 30:602–606. 1998.
|
|
22
|
Greenbloom SL, Steinhart AH and Greenberg
GR: Combination ciprofloxacin and metronidazole for active Crohn's
disease. Can J Gastroenterol. 12:53–56. 1998.
|
|
23
|
Ishikawa T, Okamura S, Oshimoto H,
Kobayashi R and Mori M: Metronidazole plus ciprofloxacin therapy
for active Crohn's disease. Intern Med. 42:318–321. 2003.
View Article : Google Scholar
|
|
24
|
Colombel JF, Lémann M, Cassagnou M, et al:
A controlled trial comparing ciprofloxacin with mesalazine for the
treatment of active Crohn's disease. Groupe d'Etudes Therapeutiques
des Affections Inflammatoires Digestives (GETAID). Am J
Gastroenterol. 94:674–678. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prantera C, Zannoni F, Scribano ML, et al:
An antibiotic regimen for the treatment of active Crohn's disease:
a randomized, controlled clinical trial of metronidazole plus
ciprofloxacin. Am J Gastroenterol. 91:328–332. 1996.
|
|
26
|
Lapidus A, Bernell O, Hellers G and
Lofberg R: Clinical course of colorectal Crohn's disease: a 35-year
follow-up study of 507 patients. Gastroenterology. 114:1151–1160.
1998.
|
|
27
|
Schwartz DA, Pemberton JH and Sandborn WJ:
Diagnosis and treatment of perianal fistulas in Crohn disease. Ann
Intern Med. 135:906–918. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wolf J: Ciprofloxacin may be useful in
Crohn's disease. Gastroenterology. 98:(abstract). A2121990.
|
|
29
|
Uza N, Nakase H, Ueno S, et al: The effect
of medical treatment on patients with fistulizing Crohn's disease:
a retrospective study. Intern Med. 47:193–199. 2008. View Article : Google Scholar
|
|
30
|
Turunen U, Farkkila M, Valtonen V, et al:
Longterm outcome of ciprofloxacin treatment in severe perianal or
fistulous Crohn's disease. Gastroenterology. 104:(abstract).
A7931993.
|