Introduction
Intraductal papillary mucinous neoplasms (IPMN) of
the pancreas is a rare pancreatic disease. Since the World Health
Organization (WHO) defined intraductal papillary mucinous tumor
(1) in 1996 [renamed IPMN in 2000
(2)], detection and awareness have
increased. Classification of IPMN as according to the WHO
nomenclature is as follows: Adenoma, borderline tumor, carcinoma
in situ (CIS) and invasive carcinoma (1). However, the classification of IPMN
according to the 2010 WHO criteria is as follows: Low-,
intermediate-, high-grade dysplasia and invasive carcinoma. IPMN
was classified into three subtypes: Branch, main and mixed
duct-type (3,4). Furthermore, much remains unknown
regarding the incidence of malignancy, postoperative prognosis and
natural course of this disease, despite the increasing number of
patients diagnosed with IPMN. An IPMN has malignant potential; it
first transforms from an adenoma to a borderline neoplasm followed
by carcinoma, ultimately becoming invasive (5,6). Tumor
resection is the only curative treatment for malignant and invasive
IPMN. Benign and asymptomatic IPMN may be monitored and do not
require surgical treatment (7–9).
Accurate assessment of the likelihood of malignancy or invasion is
required for appropriate management of IPMN.
Various modalities, including computerized
tomography, endoscopic ultrasound and magnetic resonance, have been
advocated for differentiation of benign from malignant IPMN.
However, accurate diagnosis and preoperative assessment are crucial
(10), even with modern
cross-sectional imaging it remains difficult to predict malignancy
correctly (11). Analysis of cystic
fluid from fine-needle aspiration has also been used to distinguish
malignant tumors and potentially pre-malignant mucinous cystic
neoplasms from pseudocysts and serous cystadenomas (12). Cyst fluid carcinoembryonic antigen
(CEA) and carbohydrate antigen 19-9 (CA19-9) are the most accurate
tests available for the identification of malignant cystic lesions
of the pancreas (13), but CEA and
CA19-9 are of limited value in differentiating malignant from
benign disease (14). In patients
with resectable ductal adenocarcinoma, raised serum levels of
CA19-9 and CEA have been shown to predict the stage and survival
rate (15–17). The role of serum CEA and CA19-9 in
predicting malignant and invasive IPMN remains controversial. The
aim of the present meta-analysis was to determine the diagnostic
precision of serum CEA and CA19-9 in predicting malignant and
invasive IPMN.
Patients and methods
Selection of studies and subgroup
categories
A comprehensive literature search of PubMed and Web
of Knowledge using the terms ‘(IPMN OR Intraductal papillary
mucinous neoplasm) AND Pancrea*’ was performed. Two investigators
(Lufei Zhang and Linghui Chen) independently reviewed each study
for fulfillment of the predefined inclusion criteria.
The inclusion criteria were as follows: i) English
language; ii) full-manuscript publication; iii) publication year,
2001–2013; iv) study design (prospective cohort, retrospective
cohort and case series); v) study population (patients with IPMN
confirmed by histology); and vi) minimum score of 11/14 in the
quality assessment of diagnostic accuracy studies (QUADAS)
checklist. Criteria for exclusion were: i) A specific serum CEA or
CA19-9 cut-off value was not used to evaluate the sensitivity and
specificity of differentiation of benign from malignant IPMN; ii)
insufficient information to construct 2×2 contingency tables; and
iii) editorials, review studies, duplicate publications and case
reports.
Thresholds of 5 ng/ml CEA and 35 ng/ml CA19-9 have
been used in published studies. Studies that included these
thresholds were included in the meta-analysis despite serum CEA and
CA19-9 being continuous variables.
Subgroup analysis
The following information was used to perform
subgroup analysis: Year of publication, study location, number of
patients, number of centers involved, study design and QUADAS
scores. The effects of these variables on the sensitivity and
specificity or inconsistency of CEA and CA19-9 for the diagnosis of
malignant and invasive IPMN were assessed.
Statistical analysis
Outcomes of interest for diagnosis of malignant
invasive in IPMN included: Sensitivity, specificity, likelihood
ratios and area under the summary receiver operating characteristic
curves (sROC) of CEA and CA19-9. Malignant IPMN was defined as CIS
or invasive carcinoma. Data were extracted in the form of the
true-positive, true-negative, false-negative (FN) and
false-positive to construct 2×2 tables.
Data were analyzed using the statistical software
package Meta-DiSc, version 1.4 (Clinical Biostatistics Unit, Ramón
and Cajal Hospital, Madrid, Spain) (18). Pooled estimates of sensitivity,
specificity, positive-likelihood ratio (PLR), negative-likelihood
ratio (NLR), diagnostic odds ratio (DOR) [with a 95% confidence
interval (CI)] were calculated using the random-effects model. sROC
was used to assess the interaction between sensitivity and
specificity. DOR and area under the sROC curve (AUC) were used to
analyze the diagnostic performance of serum CEA, and CA19-9 was
used to distinguish benign from malignant IPMN and to differentiate
non-invasive from invasive IPMN. I2 expresses the
variation across studies according to heterogeneity in the form of
a percentage. The I2 value is 0–100%, with 0% being a
completely homogeneous study. A random-effects pooling method was
used for high I2 (>50%). Threshold analysis was
performed using the Spearman coefficient to identify the
correlations between sensitivity and specificity. ROC curves are
the optimum summary measure of performance in place of pooled
indices when a threshold effect is present.
Heterogeneity was explored by means of a subgroup
analysis. Studies were allocated to pre-specified subgroups
according to location, number of centers, QUADAS score and type of
IPMN. Meta-regression using variables in the subgroup-analysis as
‘dummy’ variables for each category was performed to determine the
main sources of heterogeneity.
Results
Systematic review
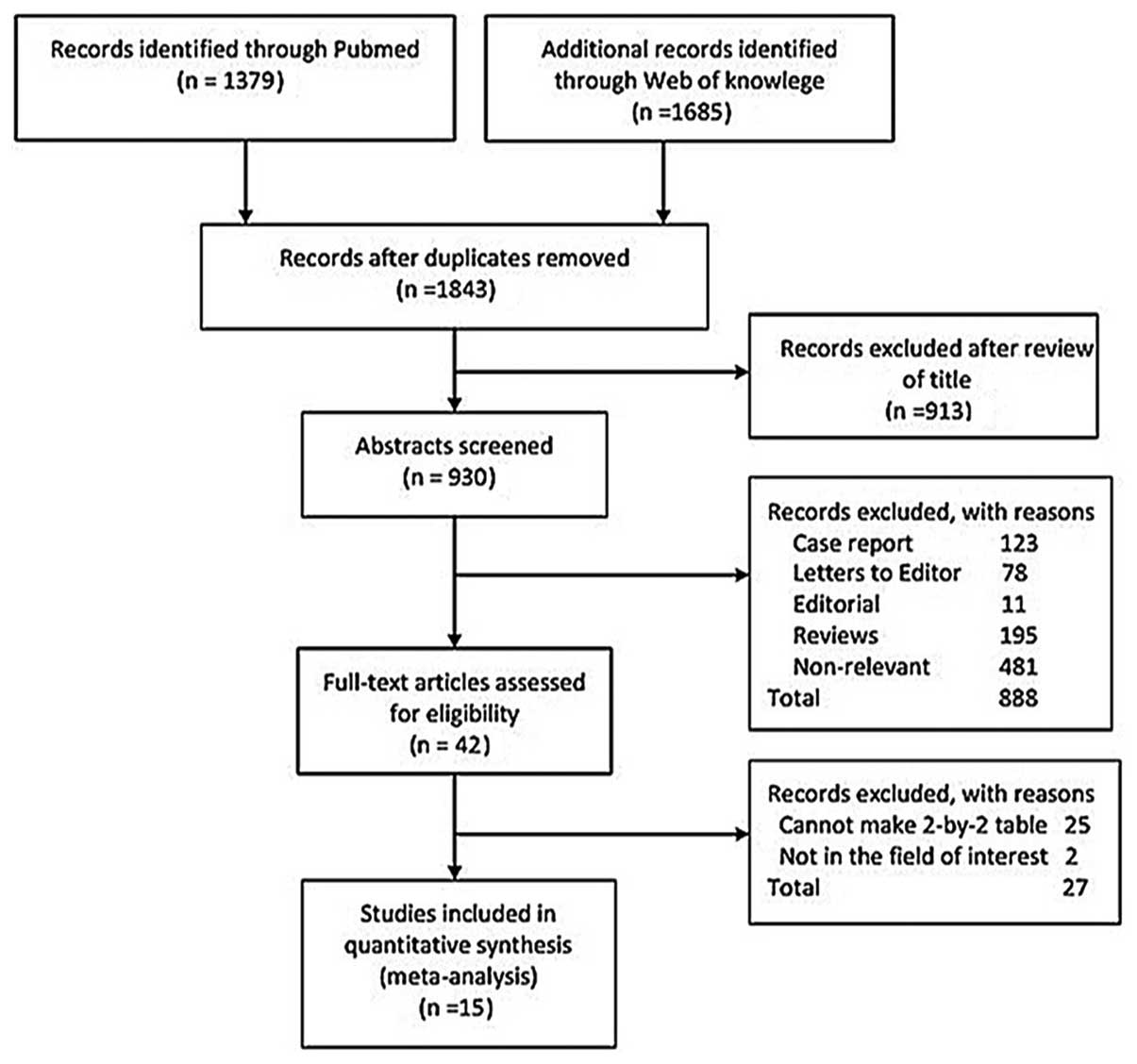
Of the 1,843 studies, 1,801 were excluded following
a preliminary abstract review, leaving 42 for detailed full-text
evaluation. A total of 15 met the inclusion criteria (9,19–32).
The number of studies included is outlined in Fig. 1, as according to the reason for
exclusion at each stage of assessment. The included studies
involved 1,530 patients; the characteristics of the included
studies are shown in Table I.
 | Table ICharacteristics of the included
studies evaluating the performance of serum CEA and CA19-9 for
predicting malignant and invasive IPMN. |
Table I
Characteristics of the included
studies evaluating the performance of serum CEA and CA19-9 for
predicting malignant and invasive IPMN.
| Author | Year | Country | Centres, n | Design | Patients, n | Mean age,
years | Male, n | QUADAS scores | Type of IPMN | (Refs.) |
|---|
| Fujii et
al | 2007 | Japan | 1 | Retrospective | 51 | NA | NA | 11 | Alla | (30) |
| Hirono et
al | 2012 | Japan | 1 | Retrospective | 144 | NA | 74 | 12 | Branchb | (21) |
| Hwang et
al | 2012 | South Korea | 1 | Retrospective | 187 | 63.4 | 114 | 13 | Alla | (20) |
| Sadakari et
al | 2010 | Japan | 1 | Retrospective | 53 | NA | NA | 11 | Branchb | (26) |
| Hwang et
al | 2011 | South Korea | 11 | Retrospective | 237 | 63.1 | 137 | 13 | Branchb | (24) |
| Ohtsuka et
al | 2012 | Japan | 1 | Retrospective | 99 | NA | 60 | 12 | Branchb | (19) |
| Kitagawa et
al | 2003 | USA | 1 | Retrospective | 42 | NA | NA | 11 | Alla | (32) |
| Matsumoto et
al | 2003 | Japan | 1 | Retrospective | 43 | NA | 32 | 12 | Branchb | (31) |
| Lee et
al | 2010 | South Korea | 1 | Retrospective | 119 | NA | NA | 11 | Alla | (27) |
| Sugiyama et
al | 2003 | Japan | 1 | Retrospective | 51 | NA | 39 | 12 | Alla | (9) |
| Xu et
al | 2011 | China | 1 | Retrospective | 86 | NA | 62 | 12 | Alla | (22) |
| Takuma et
al | 2011 | Japan | 1 | Retrospective | 46 | NA | 29 | 12 | Mainb | (23) |
| Nara et
al | 2009 | Japan | 1 | Retrospective | 123 | 64.7 | 70 | 13 | Alla | (28) |
| Hirono et
al | 2009 | Japan | 1 | Retrospective | 54 | NA | 31 | 12 | Alla | (29) |
| Shin et
al | 2010 | Korea | 1 | Retrospective | 195 | NA | NA | 11 | Alla | (25) |
Meta-analysis
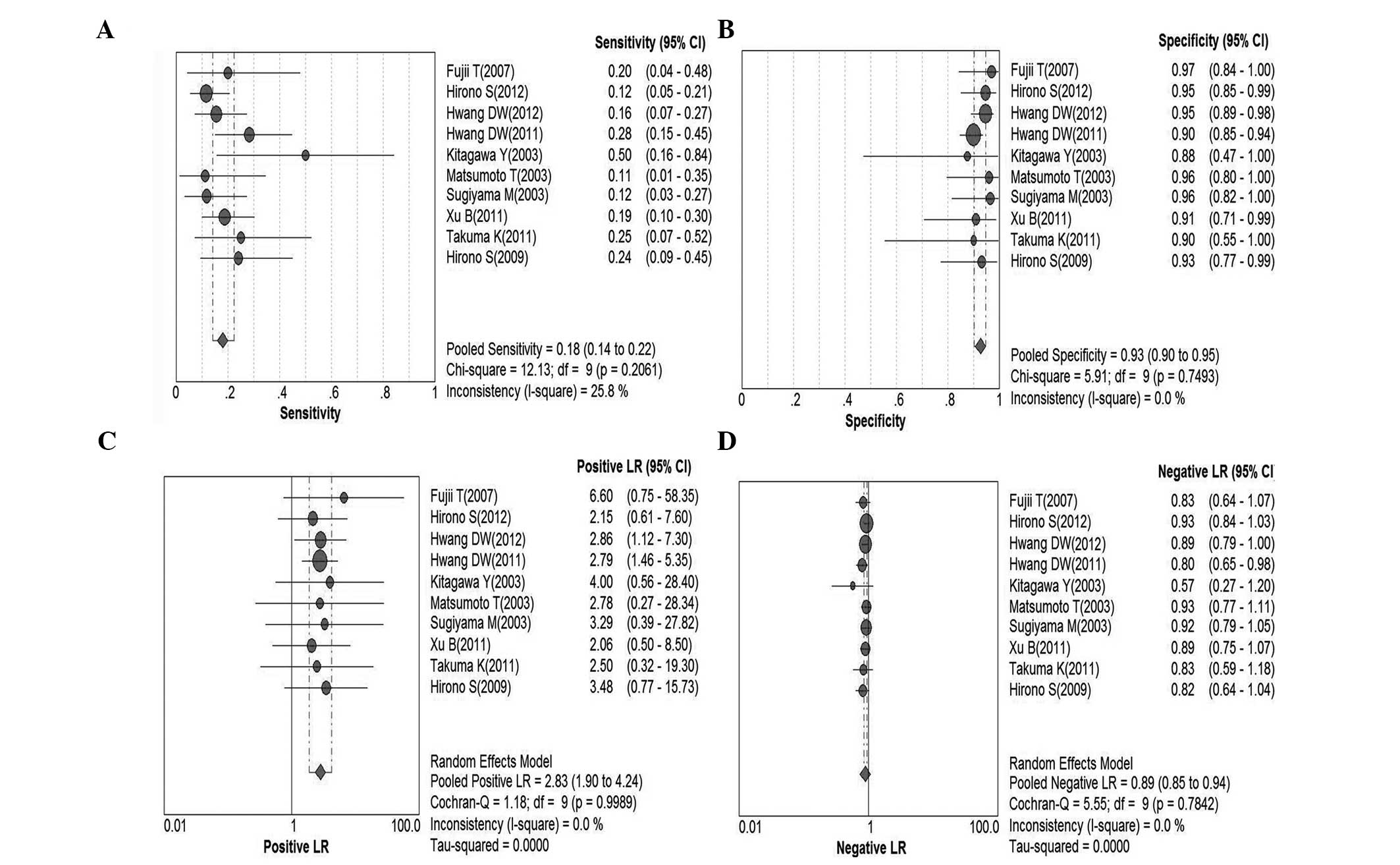
Serum CEA for malignant IPMN
In total, 10 studies were included for analysis
(9,20–24,29–32).
Pooled sensitivity was 18% (95% CI, 14–22%), pooled specificity was
93% (95% CI, 90–95%), PLR was 2.83 (95% CI, 1.90–4.24), NLR was
0.89 (95% CI, 0.85–0.94), DOR was 3.35 (95% CI, 2.09–5.36) and
I2=0.0%. Parameters were homogenous and there was a
significant correlation between sensitivity and specificity,
indicating a threshold effect; therefore, a random-effects method
was used. Forest plots of all indices of diagnostic accuracy are
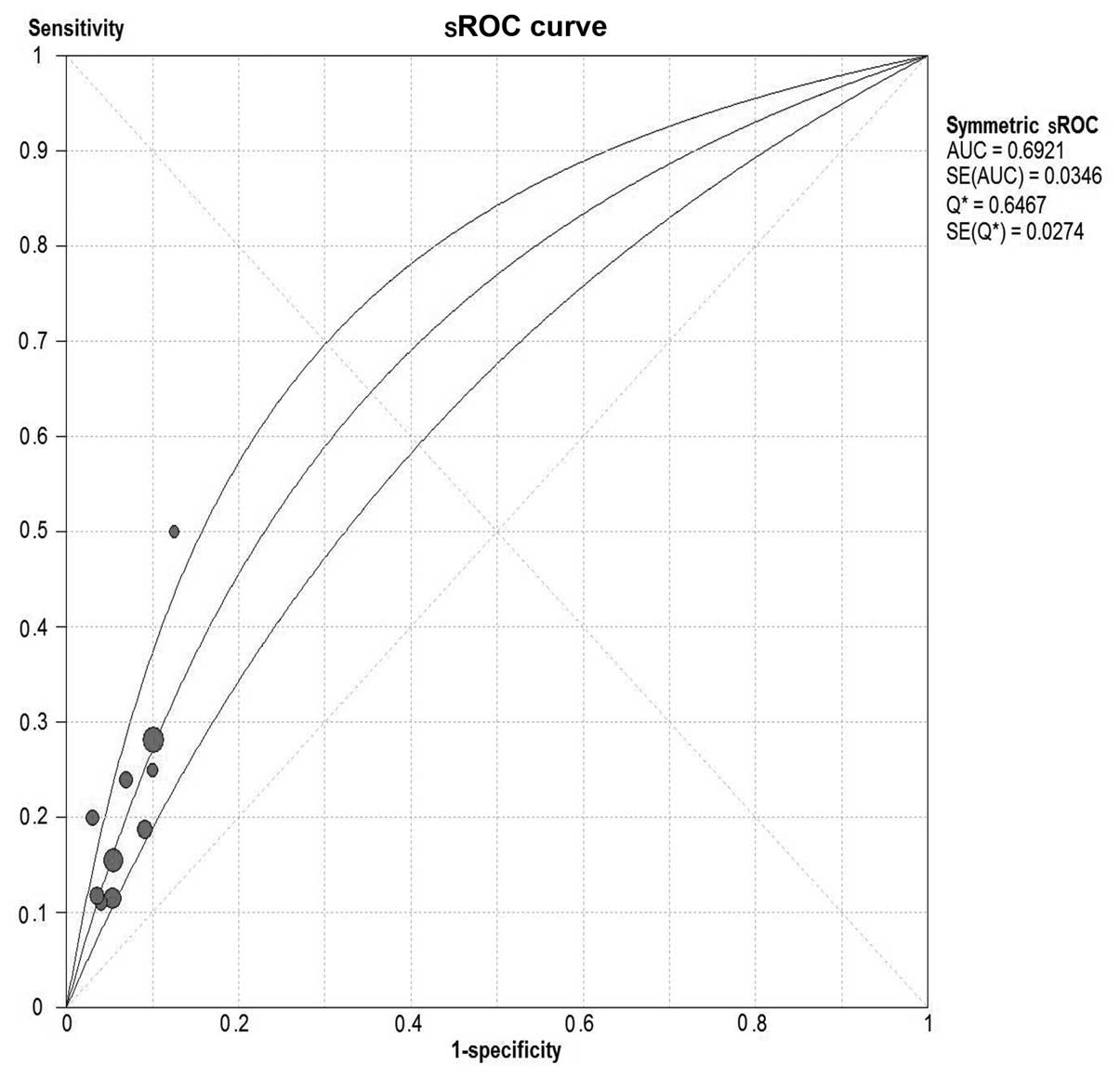
shown in Fig. 2. Results were
plotted as a symmetrical sROC curve (Fig. 3). The AUC was 0.69 [standard error
(SE), 0.03].
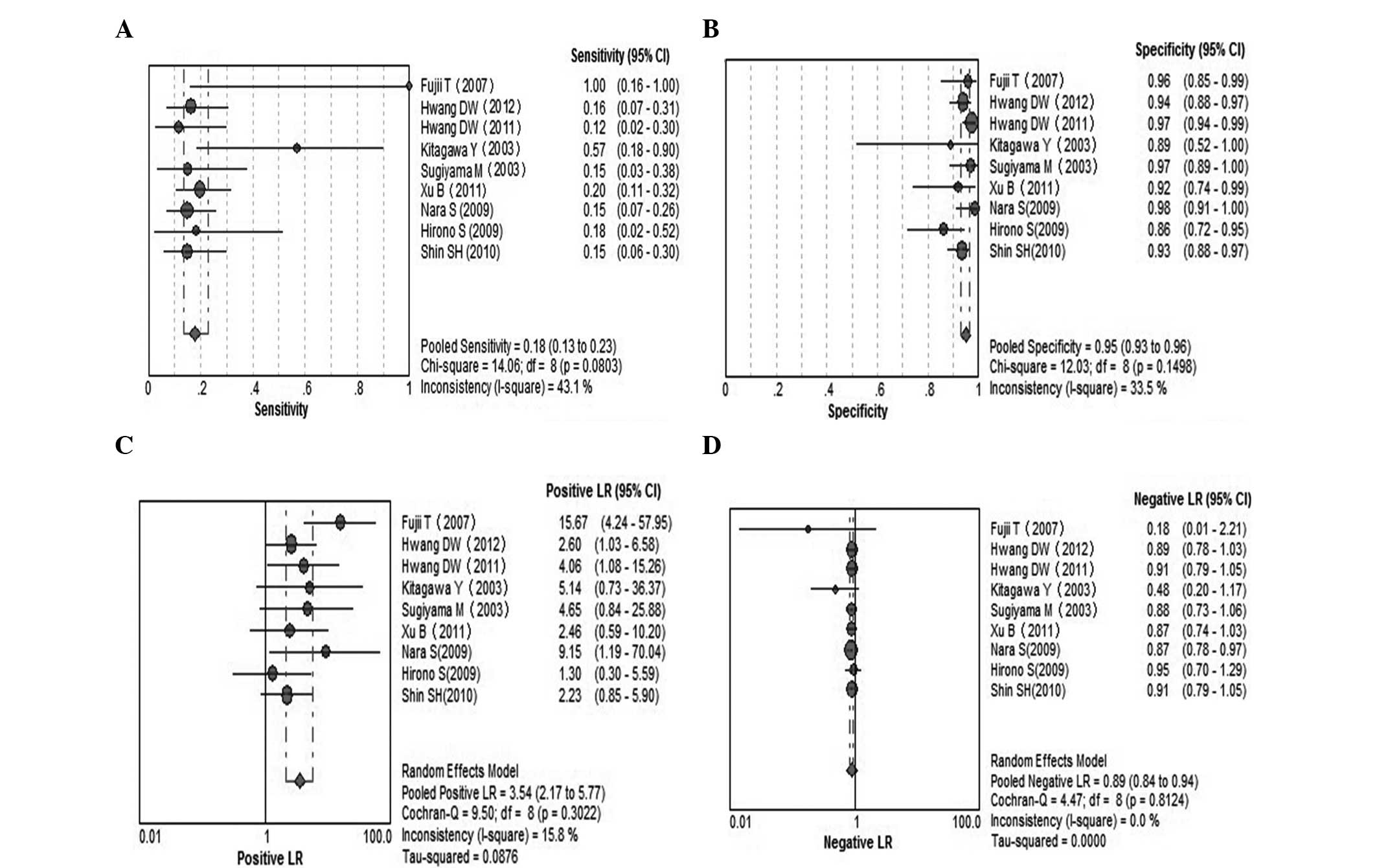
Serum CEA for invasive IPMN
A total of 9 studies were included (9,20,22,24,25,28–30,32).
Pooled sensitivity was 18% (95% CI, 13–23%), pooled specificity was
95% (95% CI, 93–96%), PLR was 3.54 (95% CI, 2.17–5.77), NLR was
0.89 (95% CI, 0.84–0.94), DOR was 3.6 (95% CI, 2.14–6.06) and
I2=0.0%. Parameters were homogenous and there was a
significant correlation between sensitivity and specificity,
indicating a threshold effect; therefore, a random-effects method
was used. Forest plots of all the indices of diagnostic accuracy
are shown in Fig. 4. Results were
plotted as a symmetrical sROC curve (Fig. 5). The AUC was 0.70 (SE, 0.03).
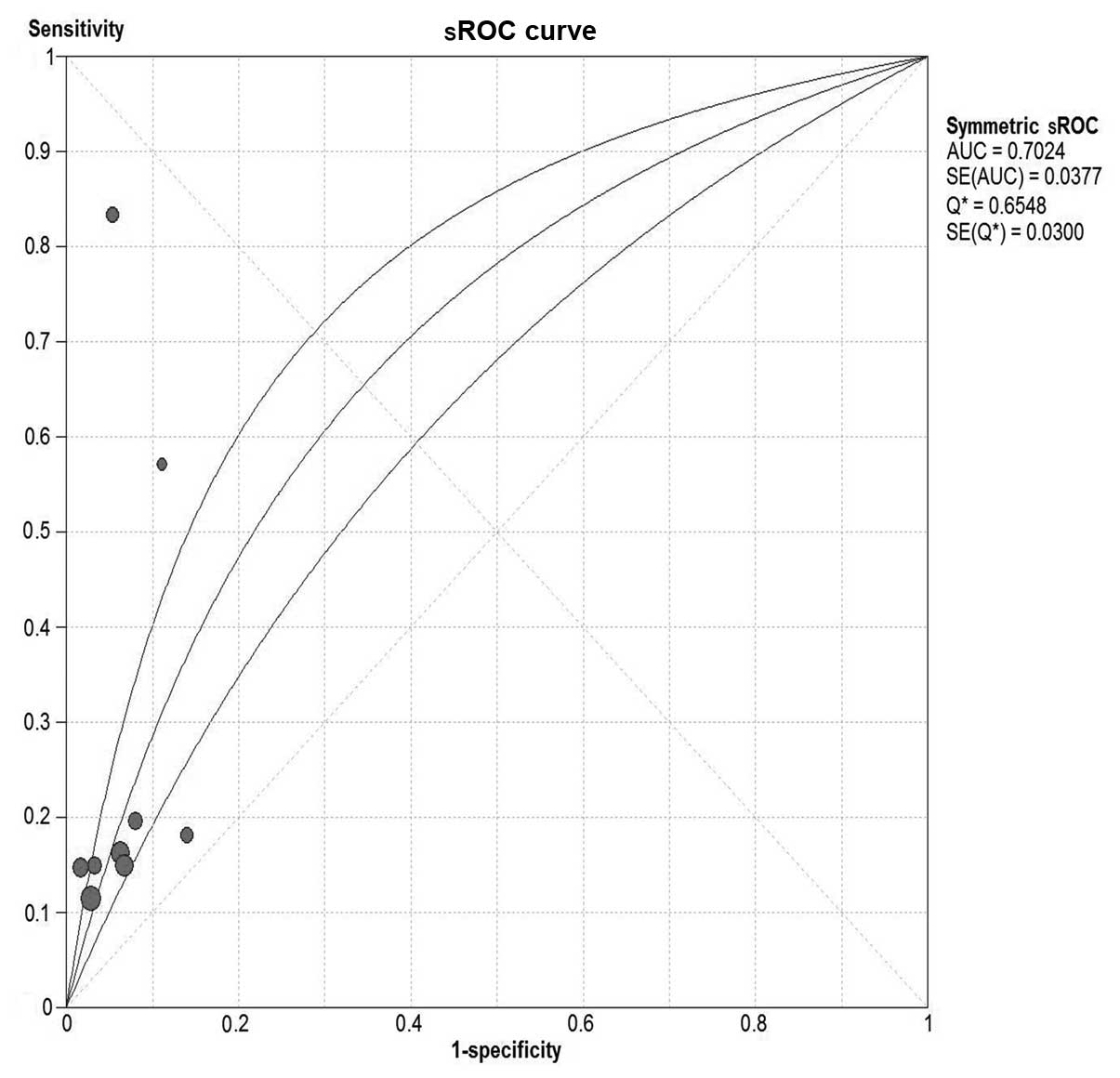
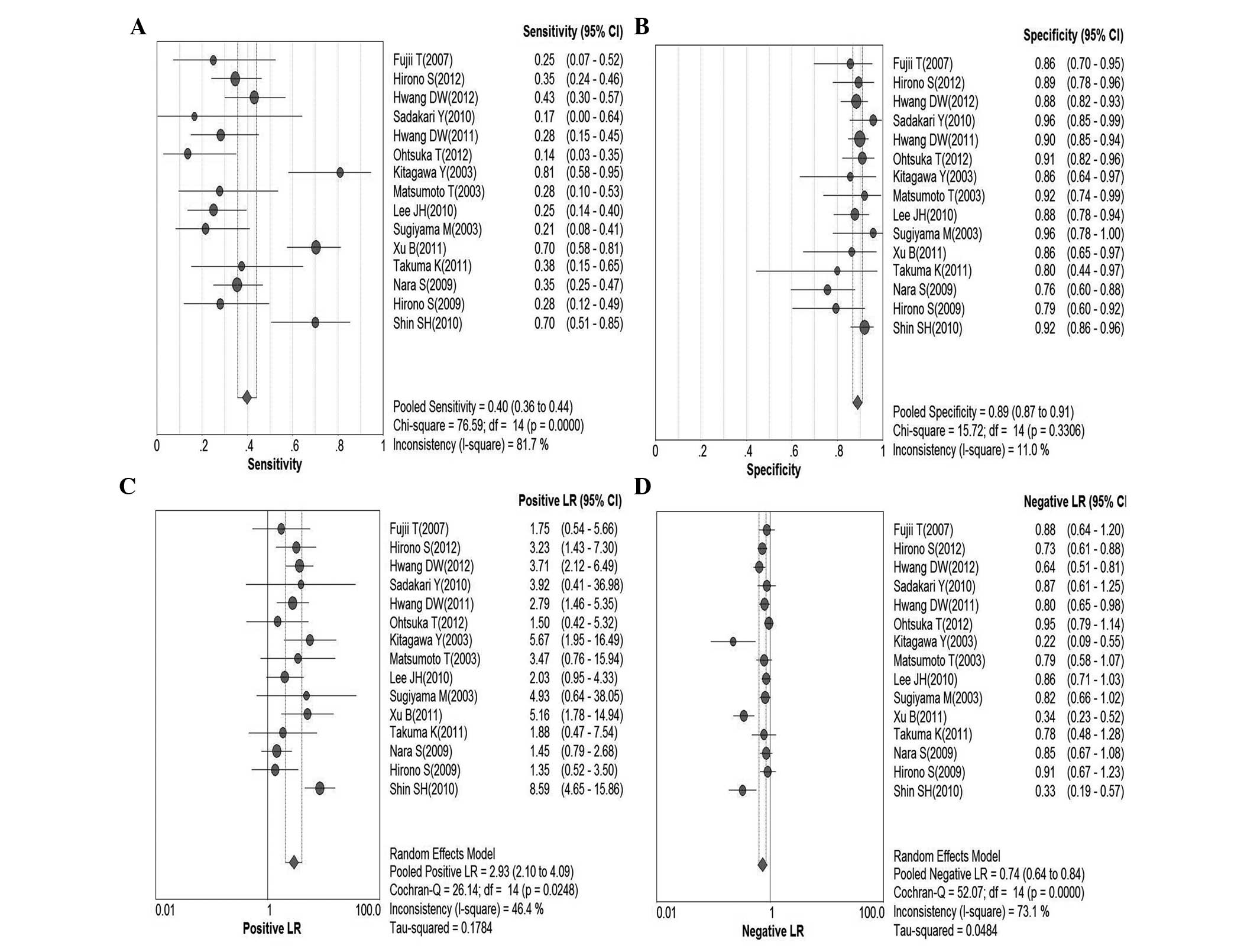
Serum CA19-9 for malignant IPMN
In total, 15 studies were included (9,19–32).
Pooled sensitivity was 40% (95% CI, 36–44%), specificity was 89%
(95% CI, 87–91%), PLR was 2.93 (95% CI, 2.10–4.09), NLR was 0.74
(95% CI, 0.64–0.84), DOR was 4.34 (95% CI, 2.65–7.10) and
I2=58.6%. These parameters were highly heterogeneous;
therefore, a random-effects method was used. Fig. 6 shows forest plots of all the
indices of diagnostic accuracy with heterogeneity denoted. Results
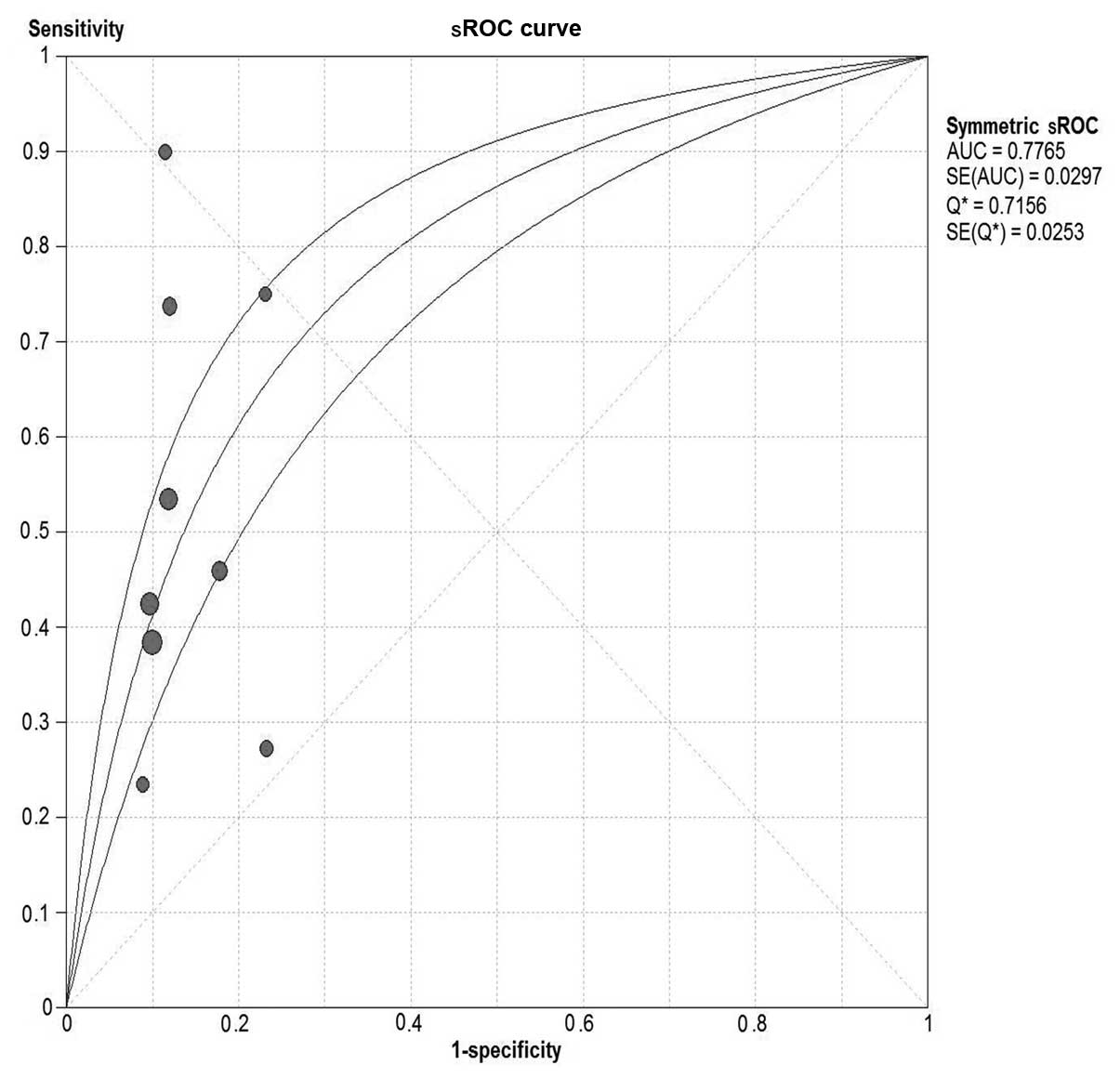
were plotted as a symmetrical sROC curve (Fig. 7) and the AUC was 0.73 (SE, 0.03).
There was no significant correlation between sensitivity and
specificity, indicating the absence of a threshold effect.
Several potential sources of heterogeneity were
pre-identified: Study location, QUADAS score and type of IPMN.
Differences in diagnostic accuracy were noted upon subgroup
analysis (Table II). These
differences were subtle and contributed to the heterogeneity. Study
quality was the main cause of heterogeneity. Meta-regression did
not identify significant differences in diagnostic accuracy among
the subgroups.
 | Table IIPredefined subgroup analysis of
indices (with 95% CIs) and subsequent meta-regression on DOR for
CA19-9. |
Table II
Predefined subgroup analysis of
indices (with 95% CIs) and subsequent meta-regression on DOR for
CA19-9.
| Subgroup | Studies, n | Sensitivity (95%
CI) | Specificity (95%
CI) | DOR (95% CI) | P-value | I2,
% |
|---|
| CA19-9 (all
studies) | 15 | | | | | |
| Study location |
|
Asia | 14 | 0.42
(0.38–0.47) | 0.85
(0.83–0.88) | 3.15
(1.84–5.42) | 0.0003 | 65.7 |
|
USA | 1 | NA | NA | NA | NA | NA |
| QUADAS score |
|
X≤12 | 5 | 0.42
(0.38–0.47) | 0.88
(0.84–0.92) | 4.80
(1.19–19.4) | 0.0001 | 83.1 |
|
X>12 | 10 | 0.43
(0.38–0.48) | 0.84
(0.81–0.87) | 3.08
(1.83–5.16) | 0.0448 | 47.8 |
| Type of IPMN |
|
Alla | 9 | 0.50
(0.45–0.55) | 0.83
(0.79–0.86) | 4.21
(1.85–9.59) | NA | 78.4 |
|
Branchb | 5 | 0.50
(0.45–0.55) | 0.83
(0.79–0.86) | 4.21
(1.85–9.59) | NA | 78.4 |
|
Mainc | 1 | NA | NA | NA | NA | NA |
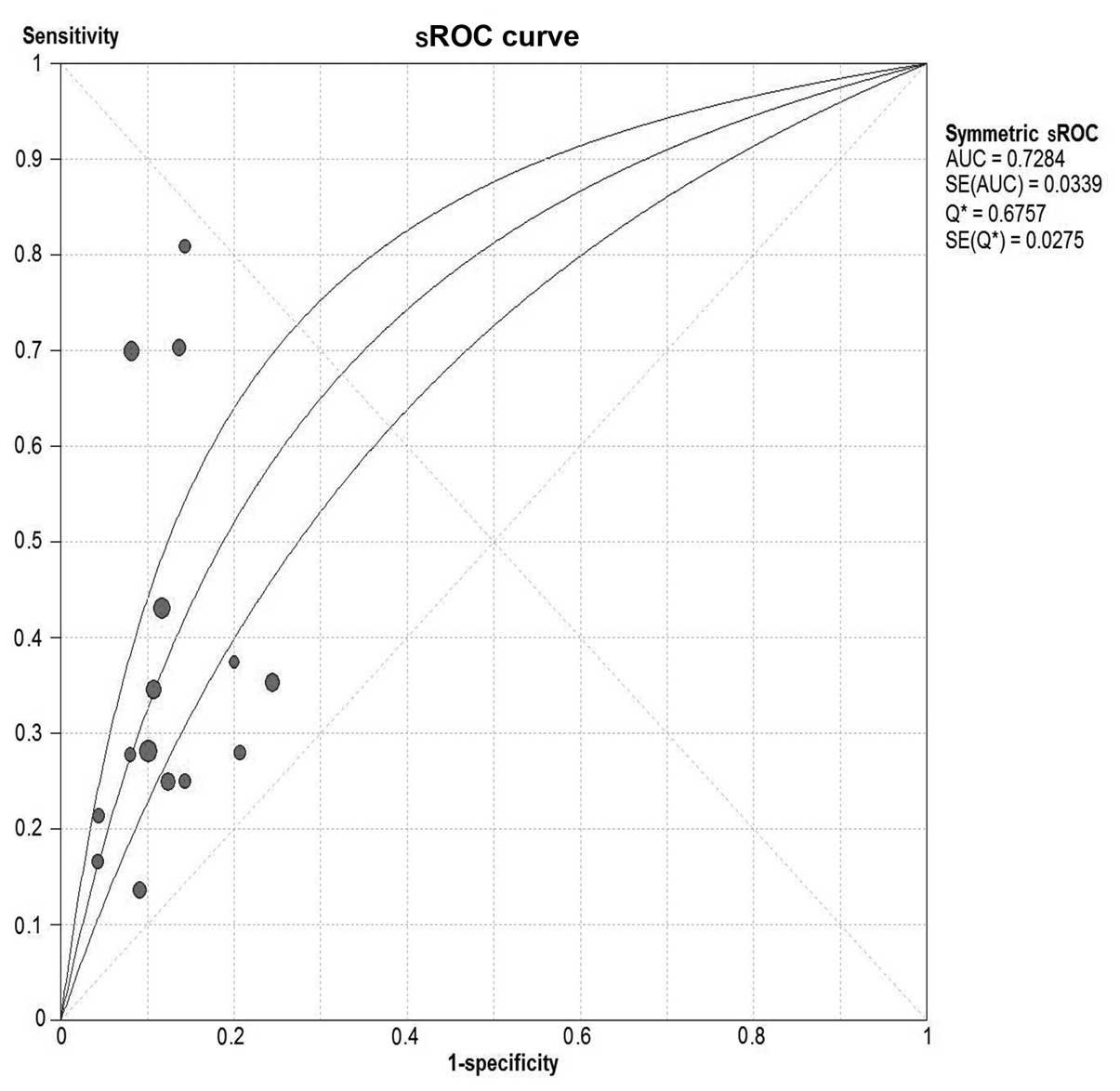
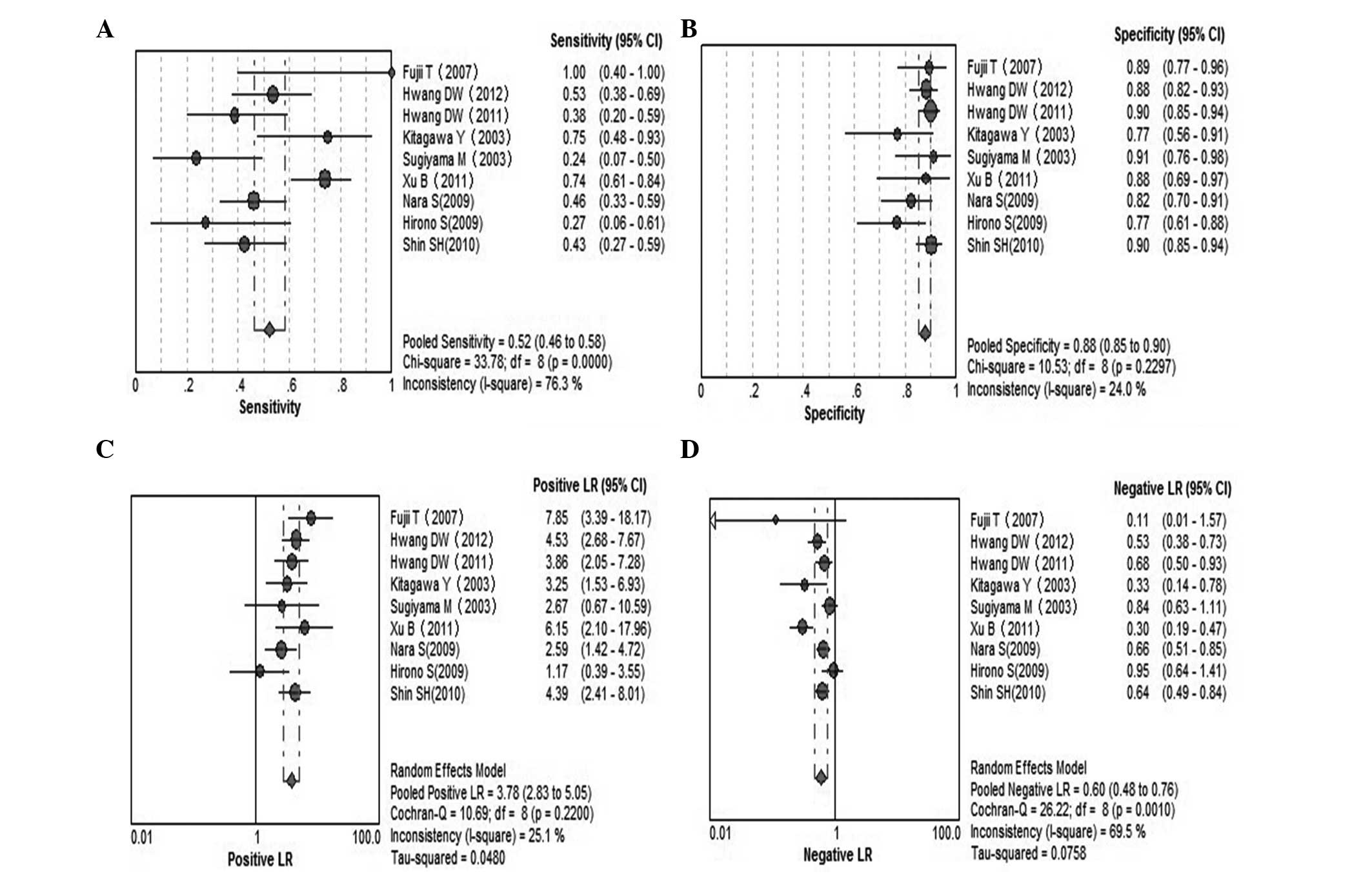
Serum CA19-9 for invasive IPMN
A total of 9 studies were included (9,20–22,24,28–30,32).
Pooled sensitivity was 52% (95% CI, 46–58%), specificity was 88%
(95% CI, 85–90%), PLR was 3.78 (95% CI, 2.83–5.05), NLR was 0.60
(95% CI, 0.48–0.76), DOR was 6.33 (95% CI, 3.89–10.30) and
I2=38.2%. Parameters were homogenous and there was a
significant correlation between sensitivity and specificity,
indicating a threshold effect; therefore, a random-effects method
was used. Forest plots of all the indices of diagnostic accuracy
are shown in Fig. 8. Results were
plotted as a symmetrical sROC curve (Fig. 9). The AUC was 0.78 (SE, 0.03).
Discussion
The natural history and prognosis of patients with
IPMN is unknown and molecular genetic events surrounding the
development of these neoplasms are under investigation. Controversy
exists regarding surgical treatment (22). The correct differential diagnosis
between benign and malignant IPMN is crucial for deciding on
appropriate management. With small IPMN, patients with questionable
malignancy on cross-sectional imaging or those with multiple
co-morbidities, and raised levels of tumor markers (CA19-9 >37
U/ml and/or CEA ≥5 µg/ml), may facilitate the decision to proceed
with surgical resection instead of conservative management. A
useful marker would guide preoperative decision-making,
postoperative follow-up and therapy (22). The CEA and CA19-9 tumor markers have
been investigated extensively in terms of the diagnosis and
prognosis of ductal adenocarcinoma (16,33–35).
The present meta-analysis showed serum CEA and CA19-9 to be
moderate predictors of malignancy and invasiveness.
CEA is a 180-kDa cell-surface glycoprotein that is
increased in >60% of patients with pancreatic ductal
adenocarcinoma (36). The pooled
sensitivities of CEA for malignant and invasive IPMN were all 18%,
indicating an FN diagnosis, and is not useful for screening
high-risk populations. Pooled specificities were 93 and 95%,
respectively, indicating the prediction of IPMN malignancy. The PLR
of 2.83 and 3.54 suggested that a CEA level >5 ng/ml is a good
predictor of malignancy and invasiveness, albeit not optimal. While
a single binary threshold facilitates clinical decision-making,
considering CEA as a spectrum may be more appropriate. Arbitrarily
raising the threshold would make CEA superior for predicting
malignancy, but a significant proportion of cases would not be
diagnosed. CEA is released from the periphery of the cancer cell
membrane and subsequently enters the systemic circulation. Analysis
of cyst fluid is useful, but in certain studies pooled estimates of
CEA in malignant cysts had a poor predictive value (14,37–41).
Therefore, the utility of CEA levels to differentiate benign from
malignant IPMN and distinguishing non-invasive from invasive IPMN
were limited. The present findings indicated that clinical
decisions, particularly those regarding surgery, should not be
based solely on an elevated serum CEA level.
CA19-9, a tumor-associated glycoprotein, increases
in 85% of patients with pancreatic ductal adenocarcinoma (42). Compared to serum CEA, serum CA19-9
is an improved predictor of malignancy. The pooled sensitivity and
specificity were 44 and 85%, respectively; the PLR was 2.36 and the
NLR was 0.73. There were several potential sources of
heterogeneity; therefore, a subgroup analysis was performed and
random-effects models were used. Subtle differences in diagnostic
accuracy were noted, although these could not account for the
heterogeneity. These findings strengthen the results of serum
CA19-9 in the study.
In addition to prediction of malignancy, increased
levels of CA19-9 in the present study correlated significantly with
invasive IPMN. Consequently, limited resections, such as
enucleation, should not be undertaken in patients with
positive-tumor markers. Lymphadenectomy should be performed as part
of an appropriate oncological resection.
As with any meta-analysis, the present study had
limitations. There was significant heterogeneity among the studies.
The study attempted to address this by employing pre-specified
subgroup analysis and meta-regression, but significant
heterogeneity persisted. Heterogeneity could be due to minor
variations in patient populations, methods of sampling, techniques
used to assay samples and the proportion of patients with malignant
disease. Studies with negative results were likely unpublished,
leading to publication bias. To the best of our knowledge,
validation of the methods used to assay serum CEA and CA19-9 levels
has not been reported.
The pooled sensitivity and specificity of CEA was
inadequate to warrant their use as a diagnostic test or to replace
conventional diagnostic imaging. The markers may serve as
complementary tools during preoperative staging investigations of
IPMN to distinguish benign and malignant tumors. Serum CA19-9 is a
useful non-invasive preoperative tool for differentiating between
invasive and benign IPMN and should be taken into account in the
decision to perform surgery.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81172315/H1617),
the Research Special Fund for Public Welfare Industry of Health and
the Translational Research of Early Diagnosis and Comprehensive
Treatment in Pancreatic Cancer (no. 201202007) and the Trends and
Prevention Strategies of Liver, Gallbladder, Pancreas Disease (no.
2012-XY-12–4).
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
DOR
|
diagnostic odds ratio
|
|
LR
|
likelihood ratio
|
|
NLR
|
negative-likelihood ratio
|
|
PLR
|
positive-likelihood ratio
|
|
QUADAS
|
quality assessment of diagnostic
accuracy studies
|
|
FN
|
false-negative
|
|
IPMN
|
intraductal papillary mucinous
neoplasms
|
|
CEA
|
carcinoembryonic antigen
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
CIS
|
carcinoma in situ
|
References
|
1
|
Kloppel G, Solcia E, Longnecker DS,
Capella C and Sobin LH: Histological Typing of Tumours of the
Exocrine Pancreas. 2nd. Springer-Verlag; Berlin-Heidelberg: pp.
1–61. 1996, View Article : Google Scholar
|
|
2
|
Hamilton SR and Aaltonen LA: Pathology and
Genetics of Tumours of the Digestive System. IARC Press; Lyon: pp.
237–241. 2000
|
|
3
|
Serikawa M, Sasaki T, Fujimoto Y, Kuwahara
K and Chayama K: Management of intraductal papillary-mucinous
neoplasm of the pancreas: treatment strategy based on morphologic
classification. J Clin Gastroenterol. 40:856–862. 2006. View Article : Google Scholar
|
|
4
|
Yamaguchi K, Nakamura M, Shirahane K, et
al: Pancreatic juice cytology in IPMN of the pancreas.
Pancreatology. 5:416–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka M, Kobayashi K, Mizumoto K and
Yamaguchi K: Clinical aspects of intraductal papillary mucinous
neoplasm of the pancreas. J Gastroenterol. 40:669–675. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimizu Y, Kanemitsu Y, Sano T, Senda Y,
Mizuno N and Yamao K: A nomogram for predicting the probability of
carcinoma in patients with intraductal papillary-mucinous neoplasm.
World J Surg. 34:2932–2938. 2010. View Article : Google Scholar
|
|
7
|
Kimura W, Sasahira N, Yoshikawa T, Muto T
and Makuuchi M: Duct-ectatic type of mucin producing tumor of the
pancreas - new concept of pancreatic neoplasia.
Hepatogastroenterology. 43:692–709. 1996.PubMed/NCBI
|
|
8
|
Sai JK, Suyama M, Kubokawa Y, et al:
Management of branch duct-type intraductal papillary mucinous tumor
of the pancreas based on magnetic resonance imaging. Abdom Imaging.
28:694–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugiyama M, Izumisato Y, Abe N, Masaki T,
Mori T and Atomi Y: Predictive factors for malignancy in
intraductal papillary-mucinous tumours of the pancreas. Br J Surg.
90:1244–1249. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmidt CM, White PB, Waters JA, et al:
Intraductal papillary mucinous neoplasms: predictors of malignant
and invasive pathology. Ann Surg. 246:644–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maire F, Voitot H, Aubert A, et al:
Intraductal papillary mucinous neoplasms of the pancreas:
performance of pancreatic fluid analysis for positive diagnosis and
the prediction of malignancy. Am J Gastroenterol. 103:2871–2877.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fritz S, Hackert T, Hinz U, Hartwig W,
Buchler MW and Werner J: Role of serum carbohydrate antigen 19-9
and carcinoembryonic antigen in distinguishing between benign and
invasive intraductal papillary mucinous neoplasm of the pancreas.
Br J Surg. 98:104–110. 2011. View
Article : Google Scholar
|
|
13
|
Hartwig W, Schneider L, Diener MK,
Bergmann F, Buchler MW and Werner J: Preoperative tissue diagnosis
for tumours of the pancreas. Br J Surg. 96:5–20. 2009. View Article : Google Scholar
|
|
14
|
Pais SA, Attasaranya S, Leblanc JK,
Sherman S, Schmidt CM and DeWitt J: Role of endoscopic ultrasound
in the diagnosis of intraductal papillary mucinous neoplasms:
correlation with surgical histopathology. Clin Gastroenterol
Hepatol. 5:489–495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrone CR, Finkelstein DM, Thayer SP,
Muzikansky A, Fernandez-delCastillo C and Warshaw AL: Perioperative
CA19-9 levels can predict stage and survival in patients with
resectable pancreatic adenocarcinoma. J Clin Oncol. 24:2897–2902.
2006. View Article : Google Scholar
|
|
16
|
Sandblom G, Granroth S and Rasmussen IC:
TPS, CA 19-9, VEGF-A, and CEA as diagnostic and prognostic factors
in patients with mass lesions in the pancreatic head. Ups J Med
Sci. 113:57–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith RA, Bosonnet L, Ghaneh P, et al:
Preoperative CA19-9 levels and lymph node ratio are independent
predictors of survival in patients with resected pancreatic ductal
adenocarcinoma. Dig Surg. 25:226–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zamora J, Abraira V, Muriel A, Khan K and
Coomarasamy A: Meta-DiSc: a software for meta-analysis of test
accuracy data. BMC Med Res Methodol. 6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohtsuka T, Kono H, Nagayoshi Y, et al: An
increase in the number of predictive factors augments the
likelihood of malignancy in branch duct intraductal papillary
mucinous neoplasm of the pancreas. Surgery. 151:76–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU
and Kim SW: Clinicopathologic analysis of surgically proven
intraductal papillary mucinous neoplasms of the pancreas in SNUH: a
15-year experience at a single academic institution. Langenbecks
Arch Surg. 397:93–102. 2012.
|
|
21
|
Hirono S, Tani M, Kawai M, et al: The
carcinoembryonic antigen level in pancreatic juice and mural nodule
size are predictors of malignancy for branch duct type intraductal
papillary mucinous neoplasms of the pancreas. Ann Surg.
255:517–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu B, Zheng WY, Jin DY, Ding WX, Lou WH
and Ramsohok L: Predictive value of serum carbohydrate antigen 19-9
in malignant intraductal papillary mucinous neoplasms. World J
Surg. 35:1103–1109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takuma K, Kamisawa T, Anjiki H, et al:
Predictors of malignancy and natural history of main-duct
intraductal papillary mucinous neoplasms of the pancreas. Pancreas.
40:371–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang DW, Jang JY, Lim CS, et al:
Determination of malignant and invasive predictors in branch duct
type intraductal papillary mucinous neoplasms of the pancreas: a
suggested scoring formula. J Korean Med Sci. 26:740–746. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin SH, Han DJ, Park KT, Kim YH, Park JB
and Kim SC: Validating a simple scoring system to predict
malignancy and invasiveness of intraductal papillary mucinous
neoplasms of the pancreas. World J Surg. 34:776–783. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sadakari Y, Ienaga J, Kobayashi K, et al:
Cyst size indicates malignant transformation in branch duct
intraductal papillary mucinous neoplasm of the pancreas without
mural nodules. Pancreas. 39:232–236. 2010. View Article : Google Scholar
|
|
27
|
Lee JH, Lee KT, Park J, et al: Predictive
factors associated with malignancy of intraductal papillary
mucinous pancreatic neoplasms. World J Gastroenterol. 16:5353–5358.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nara S, Onaya H, Hiraoka N, et al:
Preoperative evaluation of invasive and noninvasive intraductal
papillary-mucinous neoplasms of the pancreas: clinical,
radiological, and pathological analysis of 123 cases. Pancreas.
38:8–16. 2009. View Article : Google Scholar
|
|
29
|
Hirono S, Tani M, Kawai M, et al:
Treatment strategy for intraductal papillary mucinous neoplasm of
the pancreas based on malignant predictive factors. Arch Surg.
144:345–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujii T, Ishikawa T, Kanazumi N, et al:
Analysis of clinicopathological features and predictors of
malignancy in intraductal papillary mucinous neoplasms of the
pancreas. Hepatogastroenterology. 54:272–277. 2007.PubMed/NCBI
|
|
31
|
Matsumoto T, Aramaki M, Yada K, et al:
Optimal management of the branch duct type intraductal papillary
mucinous neoplasms of the pancreas. J Clin Gastroenterol.
36:261–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kitagawa Y, Unger TA, Taylor S, Kozarek RA
and Traverso LW: Mucus is a predictor of better prognosis and
survival in patients with intraductal papillary mucinous tumor of
the pancreas. J Gastrointest Surg. 7:12–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katz MH, Varadhachary GR, Fleming JB, et
al: Serum CA 19-9 as a marker of resectability and survival in
patients with potentially resectable pancreatic cancer treated with
neoadjuvant chemoradiation. Ann Surg Oncol. 17:1794–1801. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sedlack R, Affi A, Vazquez-Sequeiros E,
Norton ID, Clain JE and Wiersema MJ: Utility of EUS in the
evaluation of cystic pancreatic lesions. Gastrointest Endosc.
56:543–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tatsuta M, Iishi H, Ichii M, et al: Values
of carcinoembryonic antigen, elastase 1, and carbohydrate antigen
determinant in aspirated pancreatic cystic fluid in the diagnosis
of cysts of the pancreas. Cancer. 57:1836–1839. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Satake K, Kanazawa G, Kho I, Chung Y and
Umeyama K: Evaluation of serum pancreatic enzymes, carbohydrate
antigen 19-9, and carcinoembryonic antigen in various pancreatic
diseases. Am J Gastroenterol. 80:630–636. 1985.PubMed/NCBI
|
|
37
|
Ngamruengphong S, Bartel MJ and Raimondo
M: Cyst carcinoembryonic antigen in differentiating pancreatic
cysts: a meta-analysis. Dig Liver Dis. 45:920–926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Othman MO, Patel M, Dabizzi E, et al:
Carcino Embryonic Antigen and long-term follow-up of mucinous
pancreatic cysts including intraductal papillary mucinous neoplasm.
Dig Liver Dis. 44:844–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Correa-Gallego C, Warshaw AL and
Fernandez-del Castillo C: Fluid CEA in IPMNs: A useful test or the
flip of a coin? Am J Gastroenterol. 104:796–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park WG, Mascarenhas R, Palaez-Luna M, et
al: Diagnostic performance of cyst fluid carcinoembryonic antigen
and amylase in histologically confirmed pancreatic cysts. Pancreas.
40:42–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagula S, Kennedy T, Schattner MA, et al:
Evaluation of cyst fluid CEA analysis in the diagnosis of mucinous
cysts of the pancreas. J Gastrointest Surg. 14:1997–2003. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Safi F, Schlosser W, Falkenreck S and
Beger HG: Prognostic value of CA 19-9 serum course in pancreatic
cancer. Hepatogastroenterology. 45:253–259. 1998.PubMed/NCBI
|