Introduction
Inflammatory disease is associated with immune cell
migration, cytokine release, edema, erythema, pain, allodynia and
hyperalgesia (1), and controlling
inflammatory pain symptoms is a major clinical problem. The
interventions for the effective treatment of inflammatory pain in
patients that are refractory to available opioid and non-opioid
analgesics, or who develop serious side effects causing drug
withdrawal are currently unclear. These concerns have prompted the
identification of novel targets and experimental strategies to test
potent interventions in well-validated animal models of
inflammatory pain.
Interleukin 6 (IL-6) is a pleiotropic cytokine
produced by different types of immune cells. Its level is markedly
upregulated in various pathological conditions. IL-6 possesses
pro-inflammatory and pro-nociceptive activities (2,3). Multiple
animal studies have shown that administration of IL-6 through
intraplantar, intrathecal and intracerebroventricular routes
induces allodynia or hyperalgesia (4).
IL-6 mediates its action by binding to the receptor IL-6R, which in
turn associates with the membrane-bound protein gp130. The
IL-6/IL-6R complex initiates an intracellular cascade of
phosphorylation of several signaling proteins, including those in
the Janus-activated kinases 2 (JAK2) and signal transducer and
activator of transcription 3 (STAT3) pathway. A number of previous
studies have also shown that inhibition of IL-6 signaling is a
promising approach to treat pain (5–7).
AG490 is a synthetic derivative of
benzylidenemalononitrile. It is a specific and potent inhibitor of
JAK2 signaling (8). Although a few
studies have shown that AG490 possesses anti-hyperalgesic and
anti-allodynic effects, its role in the mediation or inhibition of
inflammatory pain, and the mechanism by which it may do so, is not
clearly understood.
The ʎ-carrageenan-induced inflammatory pain model is
widely used to study acute effects of analgesic drugs (9). The antinociceptive activity of AG490 was
evaluated using this model. Previous studies have shown that IL-6
reduces hyperalgesia by increasing spinal µ-opioid receptor during
chronic inflammation (10,11) and also releases opioid peptides from
immune cells (12,13). Therefore, whether the antinociceptive
activity of AG490 involves the opioid system at peripheral site was
also investigated.
Materials and methods
Animals
A total of 28 Male Sprague-Dawley rats (250–300 g
were used) were housed (2/cage) under standard conditions (12:12-h
light:dark cycle with ad libitum access to food and water).
The study was conducted in compliance with the Animal Welfare Act,
the implementing Animal Welfare Regulations and the principles of
the Guide for the Care and Use of Laboratory Animals.
Drugs
On the day before administering ʎ-carrageenan
(Sigma-Aldrich, St. Louis, MO, USA), it was dissolved in saline to
create a 3.5% solution and stored at 4°C. AG490 (Sigma-Aldrich) was
dissolved in 3.5% dimethylsulfoxide (DMSO) prior to the start of an
experiment on each study. Naloxone methiodide (Sigma-Aldrich) was
dissolved in water, and DMSO was diluted with water.
Induction of inflammatory pain
Unilateral hind paw inflammation in the rat was
induced by intraplantar (i.pl.) injection of 100 µl 3.5%
ʎ-carrageenan in the left hind paws (9). Inflammation was evident at 48 h
post-injection, as indicated by redness and swelling of the
affected paw.
Experimental groups, design and
treatment
The experiments were performed in rats 48 h after
ʎ-carrageenan injection. A total of 4 groups (n=6) of rats were
randomly included in the dose-response study. Group 1 was the
vehicle control, which received 100 µl i.pl. injection of 3.5% DMSO
in saline. Groups 2–4 were injected with 3 different doses of AG490
(1, 5 or 10 µg). To study the effects of naloxone on AG490-induced
antinociception, an additional group of rats (group 5; n=4) was
observed. Group 5 was co-administered with AG490 (10 µg) and
naloxone (10 µg). The drugs were administered i.pl. in a volume of
100 µl. As reported earlier, the in vivo pharmacological
effects of AG490 were observed 4 h after treatment (5,6). Thus, the
behavioral tests were performed before (baseline assessment) and 4
h after treatment. First, the rats were subjected to the thermal
hyperalgesia test; 10 min later, the paw pressure test was
performed on the same set of rats. All the experiments were
performed between 8:00 a.m. and 2:00 p.m. to reduce the confounding
influence of diurnal variations, and all the procedures were
performed in a blinded fashion.
Thermal hyperalgesia test
The thermal hyperalgesia test was performed using a
plantar analgesia instrument (IITC Life Science, Woodland Hills,
CA, USA), as described previously (6).
Briefly, rats were acclimated to the Plexiglas chambers for 30 min
prior to testing. A radiant heat source was focused at the
mid-plantar surface of the hind paw, and the paw withdrawal latency
(PWL) was recorded. Both paws were tested alternatively at 2–3 min
intervals for a total of 3 trials. A mean count and latency were
used for analysis. The percentage of maximal possible effect (MPE)
(%) was calculated according to the following formula: % MPE =
(post-drug latency - pre-drug latency)/[cut-off (20 sec) - pre-drug
latency] × 100.
Paw pressure test
The nociceptive thresholds were assessed using a
modified digital Randall-Selitto device (IITC Life Science).
Briefly, each rat was gently restrained and an increasing
mechanical pressure was exerted on the mid-plantar surface of the
hind paw until a paw withdrawal response was observed. A total of 3
trials were performed on the inflamed and contralateral paws, and
the average was taken for analysis. The paw withdrawal threshold
(PWT) was expressed in grams, and a cut-off value of 300 g was used
to prevent tissue injury (14). PWT
was calculated for each rat using ΔPWT = tested PWT - baseline PWT
(5).
Data Analysis
The data are expressed as mean ± standard error of
the mean. The difference in PWL and PWT between ipsilateral
(inflamed) and contralateral (non-inflamed) paws were compared
using a paired t-test. The results of dose-response studies and
naloxone effects were evaluated by analysis of variance followed by
Bonferroni post hoc test (P<0.05 was considered to indicate a
statistically significant difference).
Results
ʎ-carrageenan treatment induces
thermal and mechanical hyperalgesia
As reported previously (9), ʎ-carrageenan treatment to the left hind
paw produced localized inflammation, as evidenced by redness and
swelling. In comparison to the contralateral paws, the inflamed
paws showed a significant decrease in PWL and PWT to the
application of thermal and mechanical stimuli (P<0.05; data not
shown).
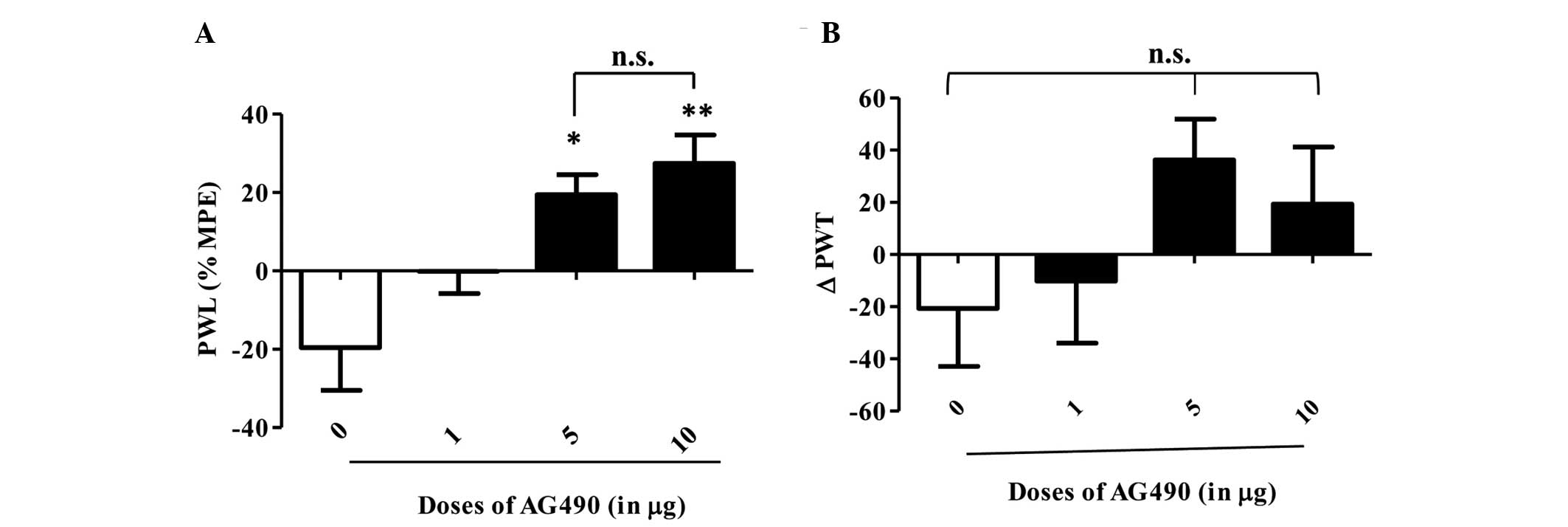
AG490 inhibits thermal hyperalgesia
and reduces mechanical hyperalgesia
An i.pl. injection of AG490 (1, 5 and 10 µg) to the
inflamed paw produced a dose-dependent, statistically significant
anti-hyperalgesic effect in the thermal test (Fig. 1A). Bonferroni post-hoc analysis showed
a significant difference between control and 5 µg (P<0.05), and
control and 10 µg of the AG490-treated groups (P<0.01). Although
the 1 µg AG490-treated group showed a trend of increased PWL
compared to the control group, it did not reach significance
(P>0.05). The most potent effect was observed for 10 µg of AG490
in comparison to the control group. AG490 treatment did not alter
the PWL of the contralateral paws (P>0.05, data not shown).
These results clearly indicate that blocking JAK2 signaling
attenuates thermal hypersensitivity in an inflamed condition. In
the mechanical hyperalgesia test, AG490 treatment resulted in a
trend towards increased PWT when compared to the control group
(Fig. 1B), but it was not of
statistical significance (P=0.0504). No difference in PWT was
observed on contralateral paws (P>0.05, data not shown).
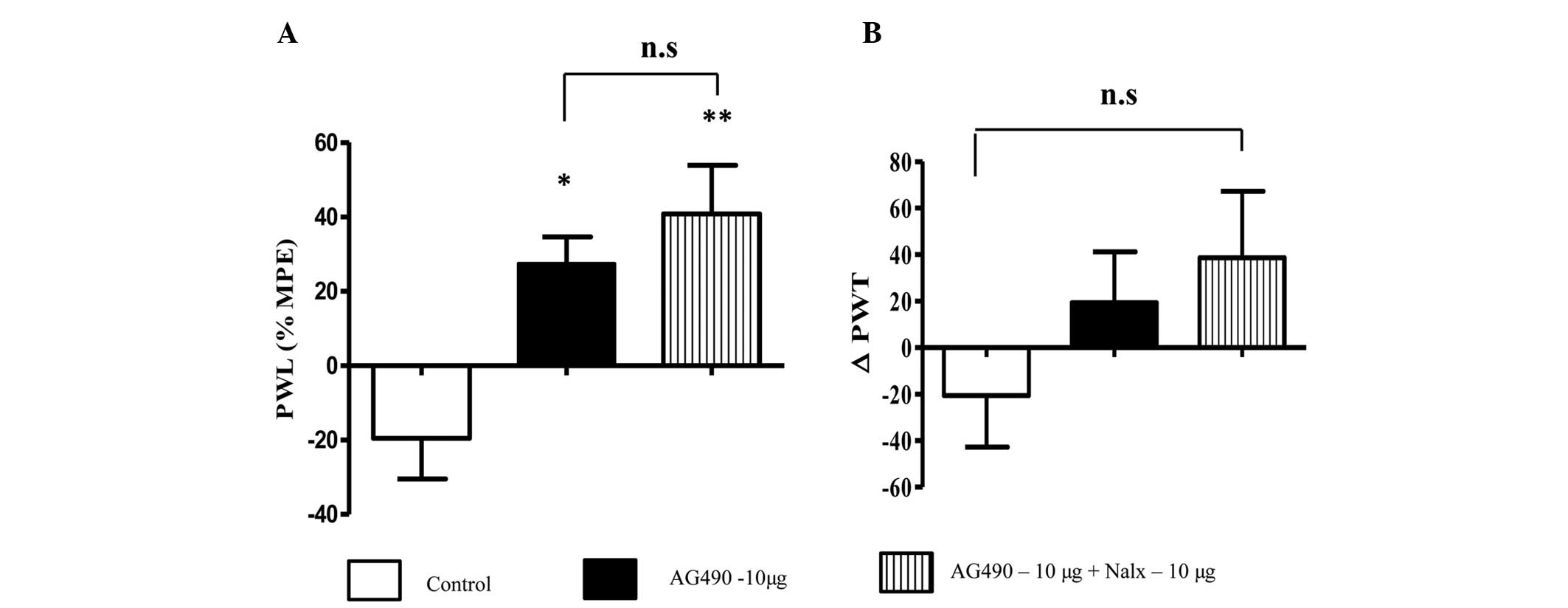
Naloxone treatment did not alter
AG490-induced thermal and mechanical hyperalgesia
Co-administration of naloxone and AG490 to the
inflamed paw increased the PWL in comparison to AG490 and control
groups (P<0.01) (Fig. 2A). This
suggests that naloxone had no effect on the antinociceptive
activity of AG490. Notably, the animals that received AG490 and
naloxone exhibited greater nociceptive effects compared to the
animals treated with AG490 alone; however, the difference between
the groups was not significant (P>0.05). A similar trend was
observed in the mechanical hyperalgesia test (Fig. 2B) without a significant difference
between groups (P>0.05). From these data, the opioid signaling
system appears to not be involved in AG490-mediated
antinociception.
Discussion
In the present study, the behavioral assays showed
that the JAK2 signaling inhibitor AG490, was effective in
attenuating thermal hyperalgesia and reducing mechanical
hyperalgesia in the ʎ-carrageenan-induced inflammatory pain model.
Furthermore, the data indicates that AG490-mediated antinociceptive
activity is independent of the opioid system.
Tyrosine kinase inhibitors, including AG490, are
effective for the treatment of various malignancies. Previous
studies have shown that blocking JAK activity with AG490 inhibits
STAT3. Coinciding with this effect was the observation of reduced
levels of cytokine production, inflammatory cell infiltration and
nitric acid production (8,15). In addition, previous studies have
demonstrated that administration of AG490 attenuates mechanical
allodynia and/or thermal hyperalgesia in painful diabetic
neuropathy, peripheral nerve injury and in the
cyclophosphamide-induced bladder pain model (5,616). The present findings are in agreement
with these studies. Acute administration of AG490 dose-dependently
attenuates thermal hyperalgesia. Additionally, in the mechanical
hyperalgesia test, AG490 treatment increased the PWT at all doses
tested; however, this effect was not statistically significant.
Therefore, we speculate that AG490 is more effective at reducing
mechanical allodynia as measured in previous studies (5,6), than for
reducing mechanical hyperalgesia, as shown in the present study. It
is also possible that differences in animal model, and route and
dose of AG490 administration influence the effects of AG490 on the
response to mechanical stimuli.
There are reports demonstrating that the cytokine
IL-6 acts at the site of inflammation on resident and circulating
immune cells to cause the release of endogenous opioids, which
subsequently activate peripheral opioid receptors to produce
analgesia (12,13). Whether the antinociceptive activity of
AG490 is mediated by release of endogenous opioid peptides was
indirectly investigated through the use of the opioid receptor
antagonist, naloxone. To the best of our knowledge, the data, for
the first time, showed that naloxone did not reverse the
AG490-mediated increase in PWL and PWT. This indicates that AG490
produced antinociceptive activity through a non-opioid mechanism.
Of note, in the thermal and mechanical sensory sensitivity tests,
AG490 and naloxone-treated animals showed increased antinociceptive
activity compared to animals receiving AG490 alone. Further studies
are required to investigate the mechanisms of this effect.
In conclusion, acute administration of AG490 is
effective in reducing ʎ-carrageenan-induced thermal hyperalgeisa,
and the effect is likely to be specifically mediated through the
JAK2 signaling pathway without involvement of the opioid system.
Further investigation is warranted to determine whether AG490 could
be a novel non-narcotic drug for the management of chronic pain
associated with inflammatory disease.
Acknowledgements
The present study was supported by the United States
Army Medical Research and Material Command Combat Causality Care
Research and the Clinical and Rehabilitative Medicine Research
programs. Dr Cheppudira was supported by the National Research
Council Senior Research Associate Fellowship.
References
|
1
|
Ji RR, Xu ZZ and Gao YJ: Emerging targets
in neuroinflammation-driven chronic pain. Nat Rev Drug Discov.
13:533–548. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oka T, Oka K, Hosoi M and Hori T:
Intracerebroventricular injection of interleukin-6 induces thermal
hyperalgesia in rats. Brain Res. 692:123–128. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeJongh RF, Vissers KC, Meert TF, Booij
LH, De Deyne CS and Heylen RJ: The role of interleukin-6 in
nociception and pain. Anesth Analg. 96:1096–1103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dominguez E, Rivat C, Pommier B, Mauborgne
A and Pohl M: JAK/STAT3 pathway is activated in spinal cord
microglia after peripheral nerve injury and contributes to
neuropathic pain development in rat. J Neurochem. 107:50–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheppudira BP, Girard BM, Malley SE,
Dattilio A, Schutz KC, May V and Vizzard MA: Involvement of
JAK-STAT signaling/function after cyclophosphamide-induced bladder
inflammation in female rats. Am J Physiol Renal Physiol.
297:F1038–F1044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guptarak J, Wanchoo S, DurhamLee J, Wu Y,
Zivadinovic D, PaulucciHolthauzen A and Nesic O: Inhibition of IL-6
signaling: A novel therapeutic approach to treating spinal cord
injury pain. Pain. 154:1115–1128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levitzki A: Tyrphostins - potential
antiproliferative agents and novel molecular tools. Biochem
Pharmacol. 40:913–918. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radhakrishnan R and Sluka KA: Spinal
muscarinic receptors are activated during low or high frequency
TENS-induced antihyperalgesia in rats. Neuropharmacology.
45:1111–1119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tekieh E, Zaringhalam J, Manaheji H,
Maghsoudi N, Alani B and Zardooz H: Increased serum IL-6 level
time-dependently regulates hyperalgesia and spinal mu opioid
receptor expression during CFA-induced arthritis. EXCLI J.
10:23–33. 2011.
|
|
11
|
Zaringhalam J, Tekieh E, Manaheji H and
Akhtari Z: Cellular events during arthritis-induced hyperalgesia
are mediated by interleukin-6 and p38 MAPK and their effects on the
expression of spinal mu-opioid receptors. Rheumatol Int.
33:2291–2299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Członkowski A, Stein C and Herz A:
Peripheral mechanisms of opioid antinociception in inflammation:
Involvement of cytokines. Eur J Pharmacol. 242:229–235. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bianchi M, Maggi R, Pimpinelli F, Rubino
T, Parolaro D, Poli V, Ciliberto G, Panerai AE and Sacerdote P:
Presence of a reduced opioid response in interleukin-6 knock out
mice. Eur J Neurosci. 11:1501–1507. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gainok J, Daniels R, Golembiowski D,
Kindred P, Post L, Strickland R and Garrett N: Investigation of the
anti-inflammatory, antinociceptive effect of ellagic acid as
measured by digital paw pressure via the Randall-Selitto meter in
male Sprague-Dawley rats. AANA J. 79 (Suppl 4):S28–S34.
2011.PubMed/NCBI
|
|
15
|
Dimitrova P and Ivanovska N: Tyrphostin
AG-490 inhibited the acute phase of zymosan-induced inflammation.
Int Immunopharmacol. 8:1567–1577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kou ZZ, Li CY, Tang J, Hu JC, Qu J, Liao
YH, Wu SX, Li H and Li YQ: Down-regulation of insulin signaling is
involved in painful diabetic neuropathy in type 2 diabetes. Pain
Physician. 16:E71–E83. 2013.PubMed/NCBI
|