Introduction
Various autoimmune rheumatic diseases (ARDs),
including rheumatoid arthritis, spondyloarthritis, vasculitis and
systemic lupus erythematosus (SLE), are associated with premature
atherosclerosis (1). Cardiovascular
disease (CVD) in ARDs is caused by traditional and non-traditional
risk factors (1). This association is
the result of the complex interaction between classic risk factors,
chronic inflammation and the production of autoantibodies. SLE is
an autoimmune inflammatory disease in which accelerated
atherosclerosis CVD and its sequelae are recognized as one of the
most frequent causes of morbidity and mortality (2).
B-type natriuretic peptide (BNP) is the gold
standard biomarker in determining the diagnosis and prognosis of
heart failure (HF) and studies on natriuretic peptide-guided HF
management appear to be promising (3).
BNP may be of use in excluding myocardial infarction and to assist
in determining prognosis in acute coronary syndrome (3). Patients with HF often present with signs
and symptoms that are non-specific and with a wide differential
diagnosis, making diagnosis by clinical presentation alone
challenging (4).
Although lipids are a major risk factor for CVD and
are routinely measured for CVD risk stratification, the association
between dyslipidemia and BNP in SLE patients remains unclear.
Establishing the association between levels of lipids and BNP in
SLE patients is critical for understanding the role of lipids in
the CVD risk among SLE patients. No previous study has investigated
the correlation between BNP and lipid profiles in active SLE
patients with HF. Accordingly, the present study was designed to
evaluate the presence of dyslipidemia and the plasma concentrations
of BNP in active SLE patients.
Patients and methods
Study population
A total of 46 patients of Northern Han Chinese
descent with active SLE, diagnosed according to the criteria of the
American College of Rheumatology (5),
were enrolled. Patients for the study were selected from
individuals attending the in-patient Department of Internal
Medicine at Qingdao Municipal Hospital (Qingdao, China). At the
same time, 40 healthy controls were recruited, who were ethnically,
gender- and age-matched with the patients. All the patients
enrolled in the present study were non-smokers, non-alcoholics and
had no association with any other autoimmune disease. In the SLE
patients, 26 cases were diagnosed with HF and 20 without HF. All
the blood samples from the patients and healthy controls were used
with informed consent and approval from the Ethics Committee of
Qingdao Municipal Hospital.
Patient characteristics and clinical
assessment
Systemic Lupus Erythematosus Disease Activity Index
(SLEDAI) is a global score reflecting all aspects of disease
activity and a validated model for the assessment of disease
activity in SLE (6). Disease activity
was determined using the SLEDAI score. For all the patients and
controls, blood pressure was assessed.
BNP level was defined as a plasma level ≤100 pg/ml.
Hypercholesterolemia was defined as a total cholesterol (TC) level
of >6.5 mmol/l or low-density lipoprotein cholesterol (LDL-C)
level of >3.3 mmol/l. Hypertriglyceridemia was defined as a
triglyceride (TG) level of >1.8 mmol/l and
hypoalphalipoproteinemia was defined as a high-density lipoprotein
cholesterol (HDL-C) level of <0.78 mmol/l. The reference
interval was obtained according to the National Guide to Clinical
Laboratory Procedures of China (3rd edition) (7).
Laboratory assessment
Peripheral blood was sampled from the controls and
all the patients. Fasting blood samples were obtained from an
antecubital vein after subjects had been seated for 30 min. A
tourniquet was used but was released prior to withdrawal of blood
into the vacuum tubes (Weihai Hongyu Medical Devices Co., Ltd,
Weihai, China). Blood samples were divided into 3 parts: One part
of the blood was anticoagulated with ethylene diamine tetraacetic
acid for the BNP assay, the second part was used to prepare serum
by centrifugation at 3,000 × g for 15 min for other laboratory
assessments, and the remaining part was anticoagulated with sodium
citrate and collected for erythrocyte sedimentation rate (ESR). BNP
was measured by an immunofluorescence assay in fresh plasma
(Triage® MeterPlus Analyzer; Biosite Incorp., San Diego, CA, USA)
and the lipid level was measured using serum. TC and TG were
measured by a colorimetric method (NingBo RuiYuan Biotechnology
Co., Ltd., Zhejiang, China). HDL-C and LDL-C were quantified by the
GPO-PAP method (Beckman Coulter, Miami, FL, USA). Apolipoprotein
(Apo) B and ApoA-I were measured by an enzymatic method (Beckman
Coulter). Lipoprotein(a) was analyzed by the latex-enhanced
immunoturbidimetric method (NingBo RuiYuan Biotechnology Co.,
Ltd.).
All the biochemical parameters were immediately
analyzed using an automatic biochemistry analyzer (Beckman
Coulter). The hematological indexes of the blood, including red
blood cells (RBC), white blood cells and platelets (PLT), were
analyzed by an automatic hematological analyzer (Sysmex XS-800i;
Sysmex Corporation, Kobe, Japan). The immunological parameters,
including immunoglobulin G (IgG), IgM, IgA, IgE, C3, C4 and
high-sensitivity C-reactive protein (CRP), were quantified by
immunoturbidimetry using an automatic nephelometric immunoassay
analyzer (BN ProSpec; Siemens, Munich, Germany). Autoantibodies,
such as antinuclear antibody (ANA), anti-dsDNA and anti-Sm
antibodies, were detected using immunoblotting according to the
manufacturer's instructions (Euroimmun AG, Lübeck, Germany). For
all the subjects, ESR was analyzed using an automatic analyzer
(Monitor-J+ analyzer; Electa-Lab s.r.l., Forli, Italy). Accuracy,
precision and quality control in the laboratory were under internal
performance verification, internal quality control and external
quality assessment for laboratory medicine by the National Center
for Clinical Laboratories (Beijing, China).
Statistical analysis
Statistical analysis was performed using the SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. The difference between subject
groups was analyzed using the Student's t-test independently.
Correlation analysis was performed using Spearman's Rho rank test.
P<0.05 was considered to indicate a statistically significant
difference. All the figures were generated with the GraphPad Prism
software, version 5.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
The demographic characteristics, clinical features,
laboratory measurements and physiological characteristics of the
patients with SLE and the controls are shown in Table I. Of note, the positive results of ANA,
anti-dsDNA and anti-Sm autoantibodies in the SLE patients were
found in 46, 28 and 11 patients, respectively. The mean value of
ESR for the patients was 56 mm/h with the range from 13 to 140
mm/h. The mean value of CRP for the patients was 21.2 mg/l with the
range from 3 to 64 mg/l. The mean value of SLEDAI was 15.4 with the
range from 11 to 30. Compared with the control subjects, the median
level of ESR in patients with SLE was 56 (range, 13–140), the
median RBC counting was 3.1 (range, 1.8–5.1) and the median PLT
counting was 151.5 (range, 51–250).
 | Table I.Demographic characteristics, clinical
features, physiological characteristics and laboratory measurements
of the studied subjects. |
Table I.
Demographic characteristics, clinical
features, physiological characteristics and laboratory measurements
of the studied subjects.
| Characteristics | SLE patients,
n=46 | Healthy controls,
n=40 | P-value |
|---|
| Demographic
characteristics |
|
|
|
|
Female | 40 (87) | 33 (83) | NS |
| Male | 6 (13) | 7 (18) | NS |
| Age, years | 41.7 (24–66) | 40.6 (22–65) | NS |
| SLEDAI | 15.5 (10–30) | – | – |
| Laboratory
measurements |
|
|
|
| C3,
g/l | 0.63 (0.15–1.24) | – | – |
| C4,
g/l | 0.21 (0.06–0.6) | – | – |
| IgG,
g/l | 12.34 (3.9–20.3) | – | – |
| IgA,
g/l | 2.6 (0.3–6.2) | – | – |
| IgM,
g/l | 1.37 (0.4–8.3) | – | – |
| IgE,
g/l | 391.7 (17–3,280) | – | – |
| ANA | 46 (100) | – | – |
|
Anti-dsDNA | 28 (61) | – | – |
|
Anti-Sm | 11 (24) | – | – |
| CRP,
mg/l | 21.2 (3–64) | – | – |
| ESR,
mm/h | 56 (13–140) | 9 (4–20) | <0.01a |
| TC,
mmol/l | 5.40±0.40 | 4.30±0.52 |
0.615 |
| LDL-C,
mmol/l | 2.82±0.26 | 2.31±0.13 |
0.973 |
| HDL-C,
mmol/l | 1.07±0.07 | 1.28±0.22 |
<0.001b |
| TG,
mmol/l | 4.39±0.55 | 1.65±0.42 |
<0.001b |
| ApoA-I,
g/l | 1.04±0.08 | 1.52±0.25 |
<0.001b |
| ApoB,
g/l | 1.1±0.15 | 0.93±0.13 |
0.526 |
| Lp(a),
mg/dl | 21.05±0.07 | 19.85±0.11 |
0.573 |
Changes in the lipid profile
As shown in Table I,
HDL-C and ApoA-I levels were decreased in SLE patients (HDL-C,
1.07±0.07 mmol/l; ApoA-I, 1.04±0.08 mmol/l) compared to the healthy
controls (HDL-C, 1.28±0.22 mmol/l; ApoA-I, 1.52±0.25 mmol/l)
(P<0.001). The TG level was increased markedly in SLE patients
(4.39±1.34 mmol/l) when matched with the healthy controls
(1.65±0.42 mmol/l) (P<0.001).
In the analysis of the studied variables, when
patients were subclassified according to HF, those with HF showed a
markedly increased BNP level (1,112.6±170.4 pg/ml) than those
without HF (33.5±4.80 pg/ml) (P<0.0001). The average
concentration of HDL-C in the SLE group with HF was 0.95±0.05
mmol/l (range, 0.72–1.69), which significantly declined compared to
those patients without HF (1.32±0.05; range, 1.10–1.56)
(P<0.0001) (Table II). The level
of ApoA-I markedly decreased (0.96±0.10; range, 0.73–1.54)
(P<0.0001) in SLE patients with HF compared to those without HF
(1.20±0.04; range, 1.00–1.42) (Table
II). No statistical significances were found between other
lipid parameters in SLE patients with or without HF.
 | Table II.Lipid profile and BNP of SLE patients
with or without HF. |
Table II.
Lipid profile and BNP of SLE patients
with or without HF.
| Variables | SLE patients with
HF | SLE patients without
HF | P-value |
|---|
| TC, mmol/l | 5.26±0.51 | 5.68±0.67 | 0.632 |
| LDL-C, mmol/l | 2.83±0.38 | 2.82±0.30 | 0.980 |
| HDL-C, mmol/l | 0.95±0.05 | 1.32±0.05 |
<0.0001a |
| TG, mmol/l | 4.97±1.93 | 3.23±1.26 | 0.558 |
| ApoA-I, g/l | 0.96±0.10 | 1.20±0.04 |
<0.0001a |
| ApoB, g/l | 1.17±0.21 | 0.95±0.15 | 0.509 |
| Lp(a), mg/dl | 19.43±5.94 | 24.02±4.29 | 0.601 |
| BNP, pg/ml | 1,112.6±170.4 | 33.50±4.80 | – |
Associations of lipid profiles and BNP
with SLE patients
The associations of lipid profiles, SLEDAI with BNP
and SLEDAI with lipid profiles in SLE patients were detected by
Spearman's correlation analysis. The results showed that the level
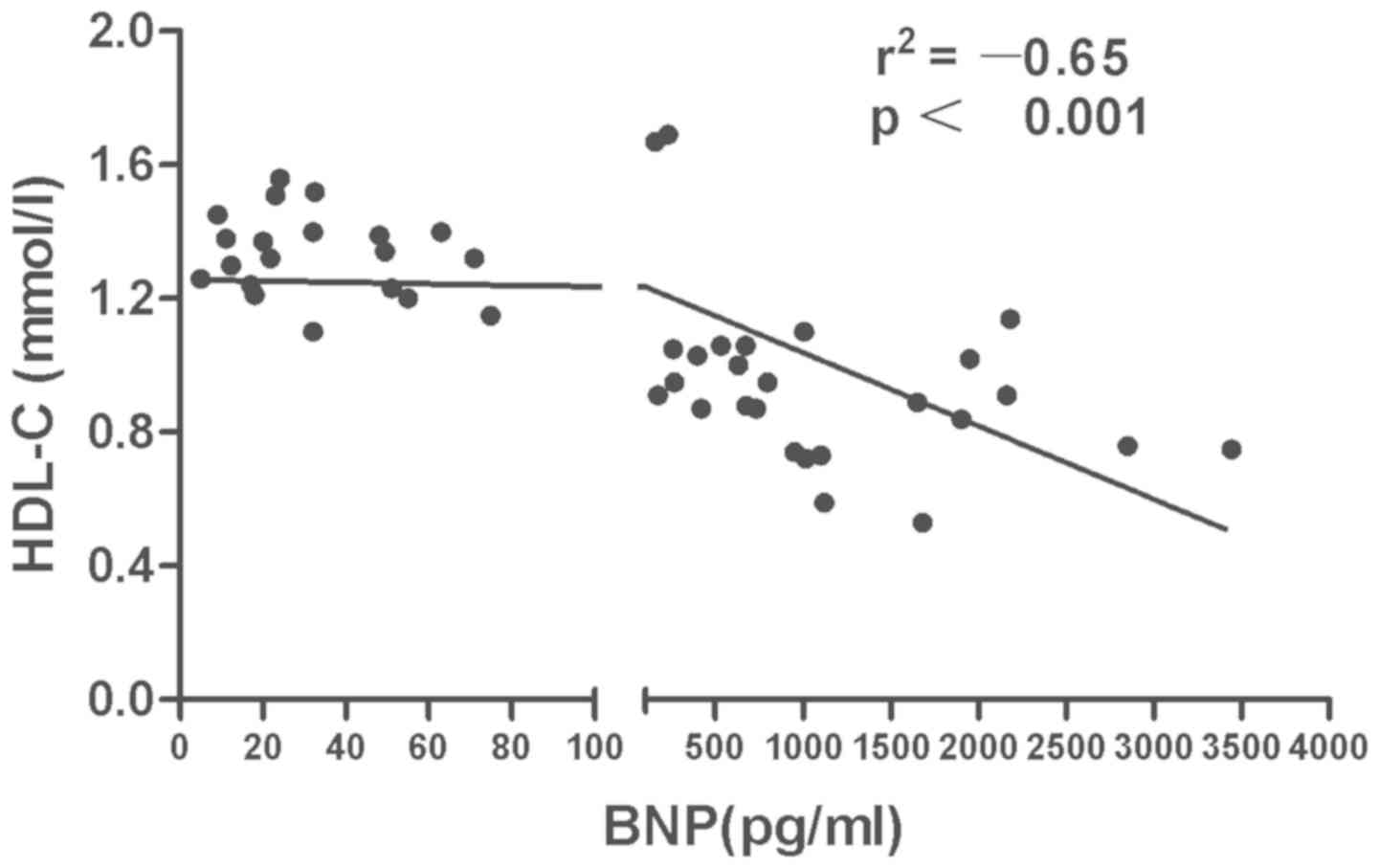
of HDL-C in SLE patients was negatively correlated with BNP
(r=–0.65, P<0.0001) (Fig. 1). A
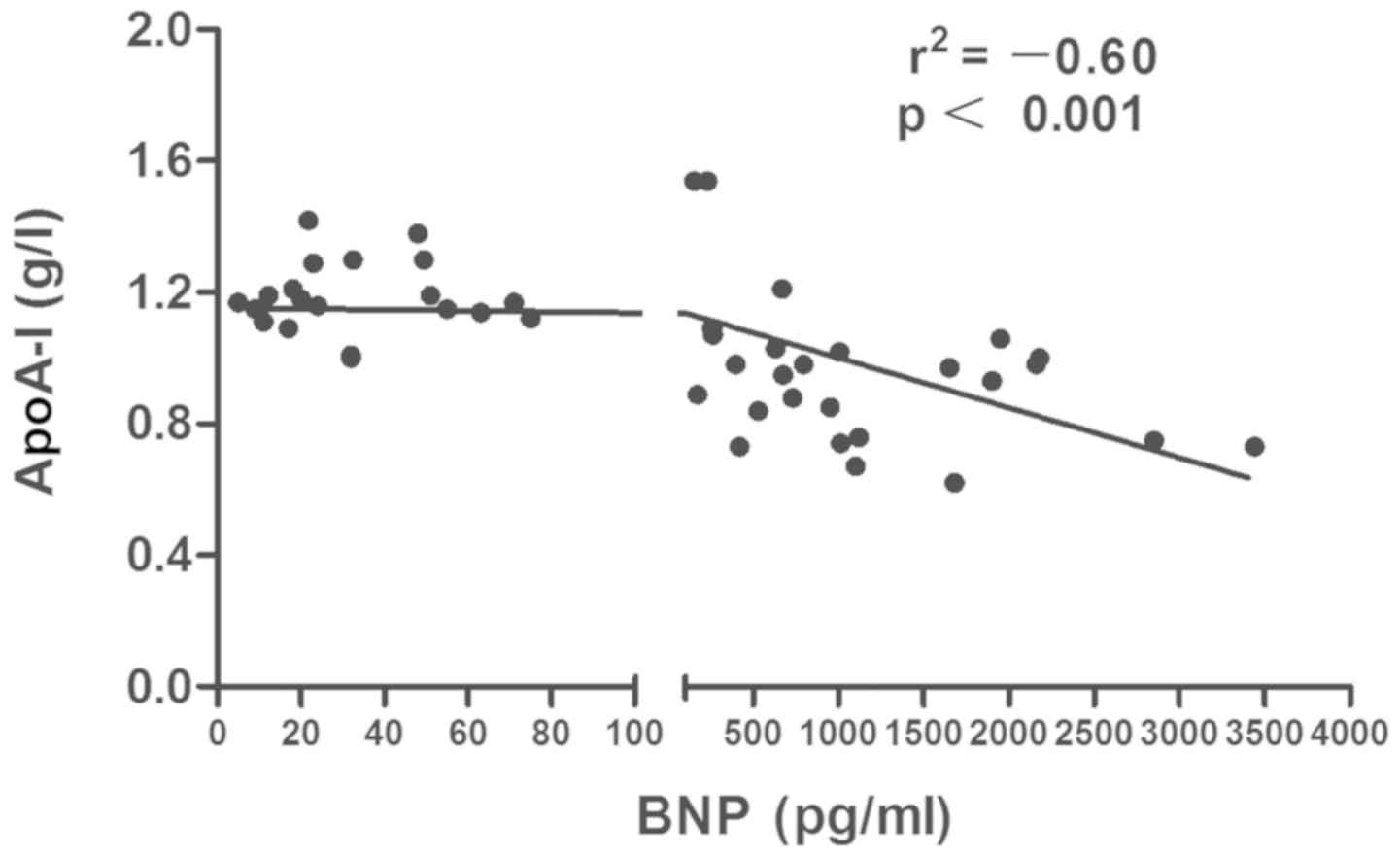
significant inverse association was observed between ApoA-I and BNP
level (r=–0.60, P<0.0001) (Fig. 2).
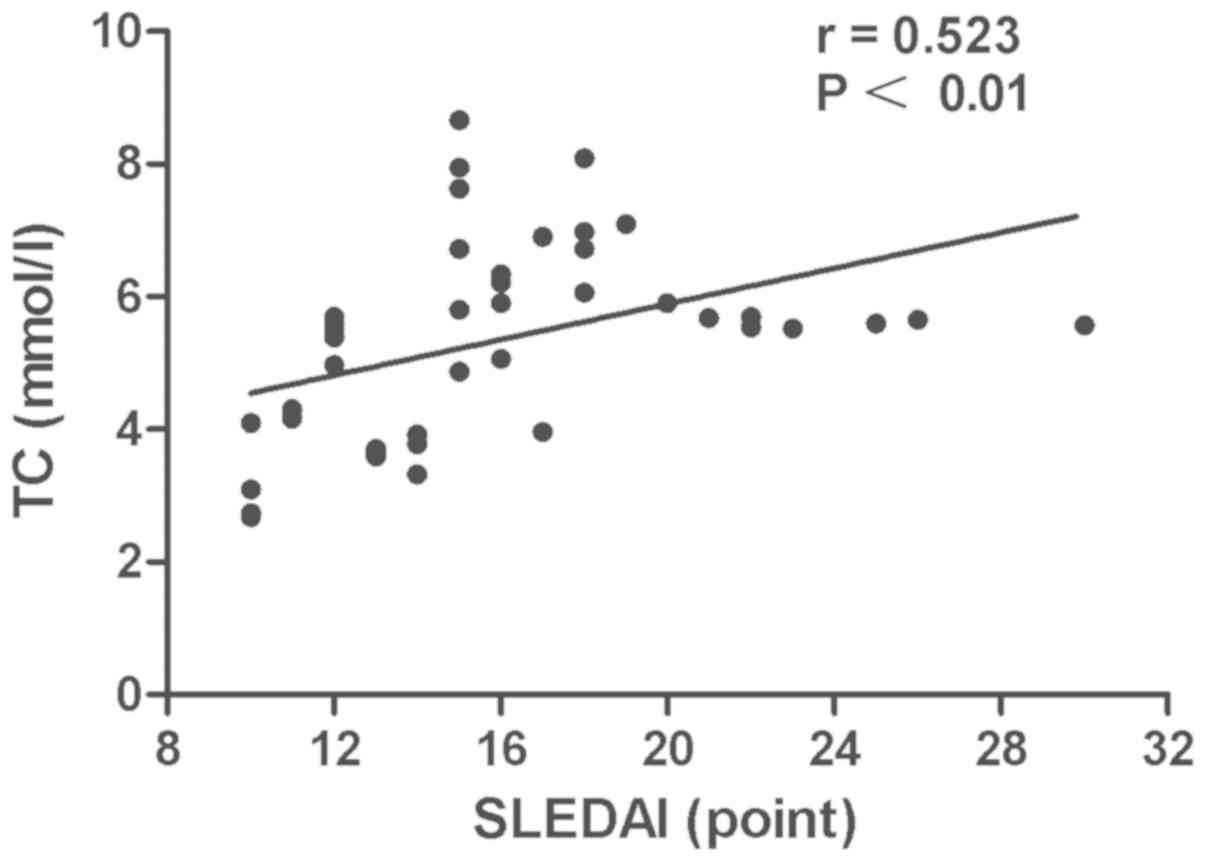
Disease activity was positively associated with the TC (r=0.523,
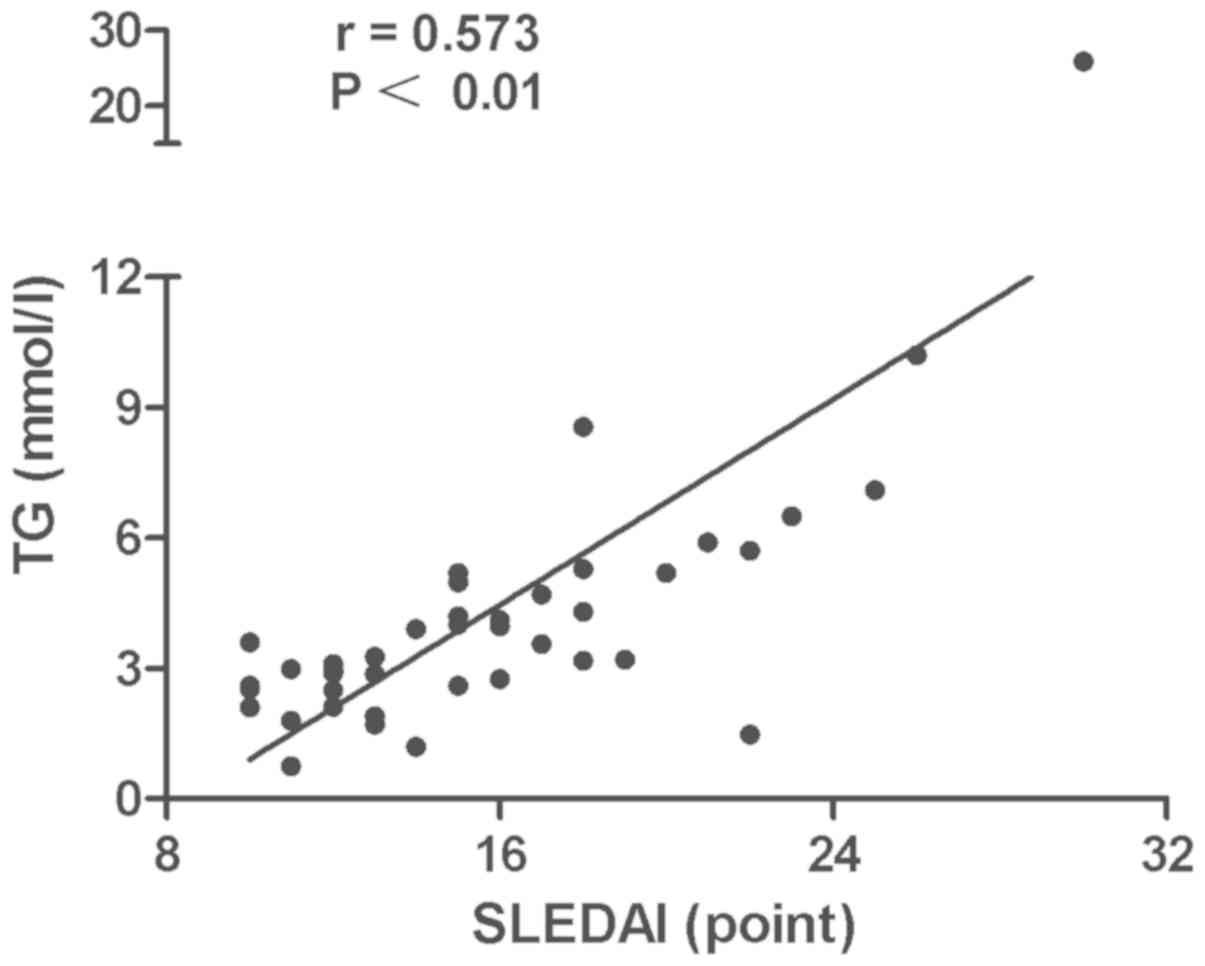
P<0.01) (Fig. 3) and TG levels
(r=0.573, P<0.01) (Fig. 4). No
statistically significant associations were identified between the
BNP level and other characteristics, clinical manifestations or
laboratory parameters in the patients with SLE.
Discussion
Myocardial infarction, cerebrovascular events and
subclinical atherosclerosis are increasingly recognized as serious
complications of SLE (8,9). Patients with SLE have a higher prevalence
of subclinical atherosclerosis and a higher risk of CV events
compared to the general population (10). The estimated prevalence of CVD in the
SLE population is between 6 and 10%, with an annual incidence of
1.3–1.5% (9,11,12).
Systemic inflammation and autoimmune reactions lead
to monocyte and lymphocyte recruitment and activation. Increased
lipid deposition and augmented inflammation in vascular intima have
been suggested to underlie accelerated atherosclerosis in ARDs
(13). In particular, the association
between dyslipidaemia and CV risk in AIRD appears to be more
complex compared to the general population (14).
Dyslipoproteinemia observed in patients with SLE has
a multifactorial origin, and elucidating which factors are clearly
involved in the pathophysiology of this lipid disorder is complex.
The present study reported dyslipidemia in Northern Han Chinese
patients with active SLE. The data clearly showed that TG increased
and HDL-C and ApoA-I were reduced significantly in active SLE
patients.
The natriuretic peptides represent the gold standard
for biomarkers in HF and the understanding regarding their biology
and their clinical use have grown exponentially (4). BNP production in normal healthy
individuals is minimal, with a level of ~10 pg/ml (15). In the conditions of myocardial stretch,
the induction of the BNP gene results in the production and
secretion. Elevated BNP levels are also predictors of future HF or
other CV events in asymptomatic patients without evident HF
(4). The present study identified
significantly higher concentrations of BNP in the active SLE
patients with HF compared with those without HF. The novel findings
of the investigation were the significantly reduced level of HDL-C
and ApoA-I in active SLE patients with HF. These findings indicated
a negative association of the levels of BNP with HDL-C and ApoA-I
in the patients with active SLE. Therefore, it is possible that
HDL-C and ApoA-I may play an extremely important role for the
evaluation of HF in active SLE patients.
The association between disease activity, which was
evaluated by the SLEDAI, and changes in the lipid profile has
commonly been reported in cross-sectional studies in the literature
(16–20). The dyslipoproteinemia (elevated TC,
LDL-C and TG) that has been reported in the SLE population has been
associated with disease activity (21)
and future CV events (22).
Borba et al (21) confirmed that untreated SLE patients
have dyslipoproteinemia that is aggravated by disease activity. The
biochemical mechanism that may explain the lipoprotein
abnormalities in untreated SLE patients includes decreased
lipoprotein lipase and Apo C-II activity (23), which could be correlated with
autoimmune phenomena (24). In the
patients of the present study, who in the majority of cases were on
corticosteroid therapy, a significant independent positive
association was identified between SLEDAI and TG or TC levels in
these active SLE patients.
The results of the present study support the
previous finding that dyslipoproteinemia is common in SLE patients,
with a pattern that is characterized by an increase in TG and a
decrease of HDL-C and ApoA-I. The data indicated the presence of a
CV risk in active SLE with high disease activity, which was
demonstrated by the high frequency of dyslipidemia and higher BNP
concentrations. Therefore, dyslipoproteinemia may underlie some of
the increased risk for CVD and HF in patients with SLE.
References
|
1
|
Hollan I, Meroni PL, Ahearn JM, Cohen
Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M,
Shields K, et al: Cardiovascular disease in autoimmune rheumatic
diseases. Autoimmun Rev. 12:1004–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nikpour M, Urowitz MB, Ibañez D and
Gladman DD: Frequency and determinants of flare and persistently
active disease in systemic lupus erythematosus. Arthritis Rheum.
61:1152–1158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaggin HK and Januzzi JL Jr: Natriuretic
peptides in heart failure and acute coronary syndrome. Clin Lab
Med. 34:43–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaggin HK and Januzzi JL Jr: Biomarkers
and diagnostics in heart failure. Biochim Biophys Acta.
1832:2442–2450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bombardier C, Gladman DD, Urowitz MB,
Caron D, Chang CH, Austin A, Bell A, Bloch DA, Corey PN, Decker JL,
et al: The Committee on Prognosis Studies in SLE: Derivation of the
SLEDAI. A disease activity index for lupus patients. Arthritis
Rheum. 35:630–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye Y, Wang Y and Shen Z: National Guide to
Clinical Laboratory Procedures of China. (3rd). Medical
Administration Department of Ministry of Public Health (China).
2006.
|
|
8
|
Aranow C and Ginzler EM: Epidemiology of
cardiovascular disease in systemic lupus erythematosus. Lupus.
9:166–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manzi S, Selzer F, Sutton-Tyrrell K,
Fitzgerald SG, Rairie JE, Tracy RP and Kuller LH: Prevalence and
risk factors of carotid plaque in women with systemic lupus
erythematosus. Arthritis Rheum. 42:51–60. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ammirati E, Moroni F, Pedrotti P, Scotti
I, Magnoni M, Bozzolo EP, Rimoldi OE and Camici PG: Non-invasive
imaging of vascular inflammation. Front Immunol. 5:3992014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bruce IN, Gladman DD and Urowitz MB:
Premature atherosclerosis in systemic lupus erythematosus. Rheum
Dis Clin North Am. 26:257–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esdaile JM, Abrahamowicz M, Grodzicky T,
Li Y, Panaritis C, du Berger R, Côte R, Grover SA, Fortin PR,
Clarke AE, et al: Traditional Framingham risk factors fail to fully
account for accelerated atherosclerosis in systemic lupus
erythematosus. Arthritis Rheum. 44:2331–2337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ronda N, Favari E, Borghi MO, Ingegnoli F,
Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F and Meroni
PL: Impaired serum cholesterol efflux capacity in rheumatoid
arthritis and systemic lupus erythematosus. Ann Rheum Dis.
73:609–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Symmons DP and Gabriel SE: Epidemiology of
CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev
Rheumatol. 7:399–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hunt PJ, Richards AM, Nicholls MG, Yandle
TG, Doughty RN and Espiner EA: Immunoreactive amino-terminal
pro-brain natriuretic peptide (NT-PROBNP): A new marker of cardiac
impairment. Clin Endocrinol (Oxf). 47:287–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ardoin SP, Schanberg LE, Sandborg C, Yow
E, Barnhart HX, Mieszkalski K, Ilowite NT, von Scheven E, Eberhard
A, Levy DM, et al: APPLE investigators: Laboratory markers of
cardiovascular risk in pediatric SLE: The APPLE baseline cohort.
Lupus. 19:1315–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soep JB, Mietus-Snyder M, Malloy MJ,
Witztum JL and von Scheven E: Assessment of atherosclerotic risk
factors and endothelial function in children and young adults with
pediatric-onset systemic lupus erythematosus. Arthritis Rheum.
51:451–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Posadas-Romero C, Torres-Tamayo M,
Zamora-González J, Aguilar-Herrera BE, Posadas-Sánchez R,
Cardoso-Saldaña G, de Guevara Ladrón G, Solis-Vallejo E and El
Hafidi M: High insulin levels and increased low-density lipoprotein
oxidizability in pediatric patients with systemic lupus
erythematosus. Arthritis Rheum. 50:160–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lilleby V, Haugen M, Mørkrid L, Frey
Frøslie K, Holven KB and Førre O: Body composition, lipid and
lipoprotein levels in childhood-onset systemic lupus erythematosus.
Scand J Rheumatol. 36:40–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tyrrell PN, Beyene J, Benseler SM,
Sarkissian T and Silverman ED: Predictors of lipid abnormalities in
children with new-onset systemic lupus erythematosus. J Rheumatol.
34:2112–2119. 2007.PubMed/NCBI
|
|
21
|
Borba EF and Bonfá E: Dyslipoproteinemias
in systemic lupus erythematosus: Influence of disease, activity,
and anticardiolipin antibodies. Lupus. 6:533–539. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stampfer MJ, Krauss RM, Ma J, Blanche PJ,
Holl LG, Sacks FM and Hennekens CH: A prospective study of
triglyceride level, low-density lipoprotein particle diameter, and
risk of myocardial infarction. JAMA. 276:882–888. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ilowite NT, Samuel P, Ginzler E and
Jacobson MS: Dyslipoproteinemia in pediatric systemic lupus
erythematosus. Arthritis Rheum. 31:859–863. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beaumont JL and Beaumont V: Autoimmune
hyperlipidemia. Atherosclerosis. 26:405–418. 1977. View Article : Google Scholar : PubMed/NCBI
|