Introduction
Stroke is the second cause of fatality and the
leading cause of disability worldwide. Currently, in developing
countries its prevalence is also increasing rapidly. In China,
stroke is a major cause of fatality (1). Presently, ischemic stroke is the most
common type of stroke in China (2).
Numerous independent and major risk factors, such as hypertension,
diabetes mellitus, hypercholesterolemia, smoking, alcohol
consumption, family history, lack of exercise, diet and lifestyle,
are known causes of ischemic stroke (3).
Previous studies have proved that the heritable
elements can influence the pathogenesis of ischemic stroke. A
significant number of genomic alleles and variants have been
discovered to increase the risk of ischemic stroke (4); however, these only explain a minor part
of the assumed heritability. Exploring the genetic polymorphisms
contributing to the susceptibility of ischemic stroke is important.
Recent studies have shown that inflammation is one of the key risk
factors and has an important role in the development of ischemic
stroke (5). Therefore, genes involved
in inflammatory responses are under investigation to identify the
variants predisposing to ischemic stroke.
Platelet-activating factor acetylhydrolase (PAF-AH),
also known as lipoprotein-associated phospholipase A2 (Lp-PLA2), is
a newly identified inflammatory enzyme involved in lipoprotein
metabolism and inflammatory pathways (6). PAF-AH may have an important role in the
pathophysiology of inflammation. Clinical and epidemiological
studies have indicated that elevated PAF-AH concentrations are
associated with incident and recurrent stroke events, as this
enzyme exhibits proinflammatory and oxidative activities (7). Several other studies have examined the
association of PAF-AH gene polymorphisms with coronary artery
disease. V279F in exon 9 of the PAF-AH gene is associated with
coronary artery disease and carotid atherosclerosis in the Japanese
population. R92H in exon 4 is associated with coronary artery
disease in the USA (8). According to
this information, we hypothesized that the genetic variants in
PAF-AH may have a critical role in the susceptibility to ischemic
stroke. To confirm this hypothesis, a case-control study was
conducted to investigate the association between two PAF-AH
polymorphisms (V279F and R92H) with ischemic stroke in an eastern
Chinese population.
Materials and methods
Study population
The present study was a hospital-based, case-control
study. A total of 375 patients with ischemic stroke and 370 healthy
controls were diagnosed at Weifang People's Hospital (Weifang,
Shandong, China) were recruited between January 2011 and February
2015. The ischemic stroke diagnoses were carried out according to
the World Health Organization guidelines (9) and the reported procedures. According to
the TOAST classification, ischemic stroke can be divided into five
subtypes: i) Large-artery atherosclerosis (LAA), ii) small-vessel
occlusion (SVO), iii) cardioembolism (CE), iv) stroke of other
determined etiology, and v) stroke of undetermined etiology
(10). Patients with LAA and SVO, two
of the most common subtypes of ischemic stroke, were included,
while the other subtypes were excluded. All the subjects in the
control group were free of clinical or radiological evidence of
stroke and other neurological diseases. Those having mental or
significant physical diseases, as well as familial or
self-psychiatric history, were excluded.
The completion of questionnaires for each subject
was performed by trained interviewers, and their clinical assay
markers were measured using standard laboratory procedures. The
study was approved by the Ethics Committee of the Weifang People's
Hospital and written informed consent was obtained from every
recruited subject. This study was conducted in accordance with the
Declaration of Helsinki.
DNA extraction and genotyping
Genomic DNA extraction was executed using commercial
kits designed for extracting blood DNA (Qiagen, Valencia, CA, USA)
and following the manufacturer's protocol. TE buffer was used to
dissolve the extracted DNA. The resulted DNA solution was stored at
−20°C until further use, and was also used as a template for the
following polymerase chain reaction (PCR). The sequences of the
primers for PCR are shown in Table I.
PCR amplification was performed in a total volume of 10 µl that
contained 1X GC buffer I (Takara, Otsu, Japan), 3.0 mM
Mg2+ (Takara), 0.3 mM deoxyribonucleotide triphosphate
(dNTP) (Generay Biotech, Shanghai, China), 1 unit HotStarTaq
polymerase (Qiagen, Hilden, Germany), 1 µl each primer (Sangon,
Shanghai, China) and 1 µl genomic DNA. The PCR cycling program was
set at 95°C for 2 min, followed by 11 cycles of 94°C for 20 sec,
65°C (decreased 0.5°C per cycle) for 40 sec, 72°C for 1.5 min, and
subsequently 24 cycles of 94°C for 20 sec, 59°C for 30 sec, and
72°C for 1.5 min, and a final extension at 72°C for 2 min.
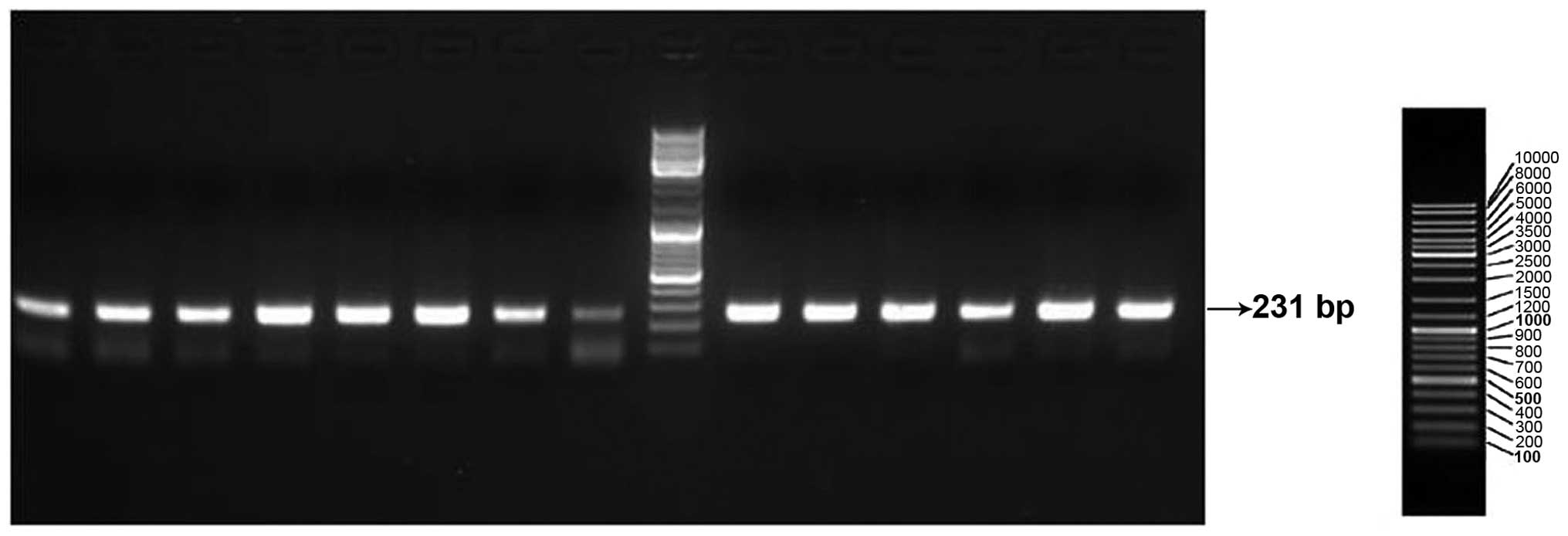
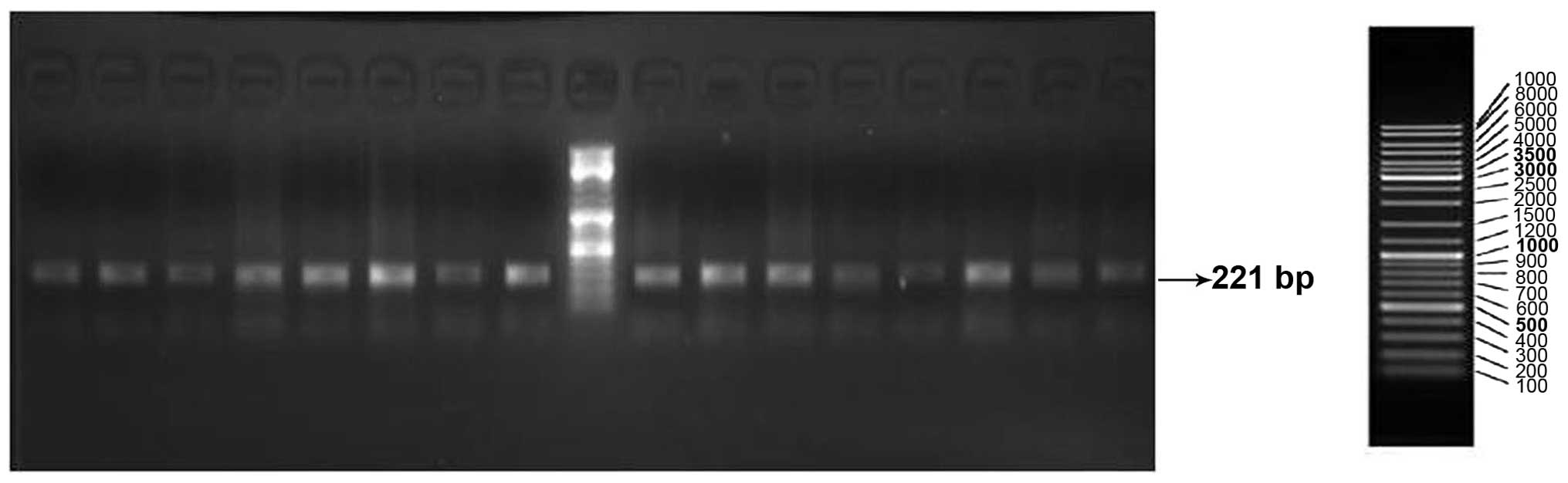
Multiplex PCR products were checked for quality and yield by
running 5 µl in 2% agarose-TBE gels. PCR products (15 µl) were
treated with 5 units of shrimp alkaline phosphatase and 2 units of
exonuclease I to remove excess dNTPs and primers, respectively. The
results of electrophoresis are shown in Figs. 1 and 2.
 | Table I.Primer sequences of V279F and
R92H. |
Table I.
Primer sequences of V279F and
R92H.
| SNPs | Primer sequences |
|---|
| R92H | Forward: 5′
CAATCACCACAGCAGCCTAA3′ |
|
| Reverse: 5′
TCCCATCCAACTCAGAATGG3′ |
| V279F | Forward: 5′
TTTATGGGGGCAAAAGAATAGCC3′ |
|
| Reverse: 5′
AACCATCCCCATGAAATSAACAAT3′ |
Statistical analysis
Continuous variables are presented as mean ±
standard deviation and categorical variables as percentages.
Continuous variables were compared using student's t-test.
Categorical variables were compared by χ2 test.
Differences of the distributions of alleles and genotypes between
cases and controls were analyzed using χ2 test. All the
genotype frequencies were checked for Hardy-Weinberg analysis in
two groups with the χ2 test.
The association of the PAF-AH gene polymorphisms
with ischemic stroke was evaluated by computing the odds ratios
(OR) and 95% confidence intervals (CI) from logistic regression
analyses following adjustment for confounding risk factors.
P<0.05 was considered to indicate a statistically significant
difference for all statistical analyses. All the statistical
analyses were carried out with Stata 12.0 software (StataCorp,
College Station, TX, USA).
Results
Characteristics
The clinical characteristics of the control subjects
and ischemic stroke patients are shown in Table II. There were no significant
differences in age, gender or body mass index. The genotype and
allele frequencies of the two PAF-AH polymorphisms in patients and
control subjects are shown in Tables
III and IV. All the genotype
distributions in the patients and controls were in the
Hardy-Weinberg equilibrium. As shown in Tables III and IV, there was no significant difference in
the distributions of genotypes and alleles of V279F between
ischemic stroke patients and controls. By contrast, the significant
association was observed for R92H in a dominant model. A higher
frequency of the HH+RH genotype for R92H was observed in all the
participants with ischemic stroke (OR=1.44; 95% CI, 1.06–2.01;
P=0.02). The RH genotype (28.8%) of R92H was represented at an
increased frequency in the group of patients (OR=1.42; 95% CI,
1.02–1.97; P=0.03). The frequency of R92H H allele was
significantly higher in patients with ischemic stroke compared to
the control group (OR=1.41; 95% CI, 1.06–1.73; P=0.02).
 | Table II.Clinical characteristics of the study
subjects. |
Table II.
Clinical characteristics of the study
subjects.
| Characteristics | Cases | Control | P-value |
|---|
| Agea, years | 63.4±4.73 | 60.1±8.09 | 0.81 |
| Males, n (%) | 254 (67.7) | 243 (65.7) | 0.74 |
| BMIa, kg/m2 | 27.2±2.91 | 26.8±2.03 | 0.23 |
| Hypertension, n
(%) | 263 (70.1) | 218 (58.9) | <0.01 |
| Diabetes, n (%) | 131 (34.9) | 69
(18.6) | <0.01 |
| Hyperlipidemia, n
(%) | 167 (44.5) | 129 (34.9) | <0.01 |
| Smoking, n (%) | 131 (34.9) | 67
(18.1) | <0.01 |
 | Table III.Genotype and allele distributions of
R92H in patients with ischemic stroke and the controls. |
Table III.
Genotype and allele distributions of
R92H in patients with ischemic stroke and the controls.
| R92H | Cases, n (%) | Control, n (%) | OR (95% CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Genotype |
|
|
|
|
|
|
| RR | 259 (69.1) | 287 (77.6) | Reference | Reference |
|
|
| RH | 104 (27.7) | 79
(21.4) | 1.42 (1.02–1.97) | 0.03 | 1.41 (1.01–2.08) | 0.04 |
| HH | 12 (3.2) | 4
(1.1) | 2.12 (0.62–7.41) | 0.19 | 1.72 (0.48–6.15) | 0.40 |
|
(HH+RH)/RR | 116/259 | 83/287 | 1.44 (1.06–2.01) | 0.02 | 1.42 (1.02–2.00) | 0.04 |
|
HH/(RH+RR) | 12/363 | 4/386 | 2.11 (0.54–6.39) | 0.25 | 1.55 (0.44–5.59) | 0.48 |
| Allele |
|
|
|
|
|
|
| R | 622 (82.9) | 653 (87.8) | Reference |
|
|
|
| H | 128 (17.1) | 87
(12.2) | 1.41 (1.06–1.73) | 0.02 |
|
|
 | Table IV.Genotype and allele distributions of
V279F in patients with ischemic stroke and the controls. |
Table IV.
Genotype and allele distributions of
V279F in patients with ischemic stroke and the controls.
| V279F | Cases, n (%) | Control, n (%) | OR (95% CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Genotype |
|
|
|
|
|
|
| VV | 338 (90.1) | 334 (90.2) | Reference | Reference |
|
|
| VF | 35 (9.3) | 33 (8.9) | 0.93 (0.57–1.46) | 0.77 | 1.02 (0.63–1.62) | 0.88 |
| FF | 2
(0.5) | 3
(0.8) | 0.23 (0.02–2.12) | 0.35 | 0.21 (0.02–2.01) | 0.17 |
|
(FF+VF)/VV | 37/338 | 36/334 | 0.86 (0.54–1.27) | 0.54 | 0.92 (0.61–1.51) | 0.83 |
|
FF/(VF+VV) | 2/373 | 3/367 | 0.24 (0.02–2.22) | 0.36 | 0.21 (0.02–2.10) | 0.18 |
| Allele |
|
|
|
|
|
|
| V | 711 (94.8) | 701 (94.7) | Reference |
|
|
|
| F | 39 (5.2) | 39 (5.3) | 0.82 (0.44–1.24) | 0.35 |
|
|
Association between polymorphisms and
ischemic stroke
The association between the polymorphisms and the
disease was assessed using univariate or multivariate logistic
regression analyses. Logistic regression analyses revealed that
following adjustment for the confounding factors (hypertension,
diabetes mellitus, hyperlipidemia and smoking), the presence of the
RH+HH genotype and the RH genotype of single nucleotide
polymorphism R92H was associated with a higher risk of ischemic
stroke (OR=1.42; 95% CI, 1.02–2.00; P=0.04; and OR=1.41; 95% CI,
1.01–2.08; P=0.04, respectively).
The statistical differences between the PAF-AH gene
and different subtypes of ischemic stroke were analyzed. The HH+RH
genotype and the RH genotype of the R92H gene were significantly
associated with large-artery atherosclerotic stroke, even after
adjusting for the confounding factors (OR=1.73; 95% CI, 1.18–2.55;
P<0.01; and OR=1.67; 95% CI, 1.13–2.48; P=0.01, respectively).
However, the same differences were not observed between the
remaining small vessel occlusive stroke subgroup and all genetic
models (P>0.05), as shown in Table
V.
 | Table V.Genotype and allele distributions of
R92H and its associations with the stroke subtypes. |
Table V.
Genotype and allele distributions of
R92H and its associations with the stroke subtypes.
| R92H | Cases, n (%) | Control, n (%) | OR (95% CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| LAA subgroup |
|
|
|
|
|
|
| RR | 133 (64.4) | 151 (73.7) | Reference |
|
|
|
| RH | 70 (32.4) | 42 (20.5) | 1.73
(1.19–2.52) | <0.01 | 1.67
(1.13–2.48) |
0.01 |
| HH | 6 (3.2) | 12 (5.9) | 3.67
(1.06–12.76) |
0.06 | 3.02
(0.81–11.27) |
0.10 |
| Dominant model |
|
|
|
|
|
|
| RR | 133 (64.4) | 151 (73.7) | Reference |
|
|
|
|
HH+RH | 76 (36.4) | 54 (26.3) | 1.82
(1.27–2.62) | <0.01 | 1.73
(1.18–2.55) | <0.01 |
| Recessive
model |
|
|
|
|
|
|
|
RR+RH | 203 (97.1) | 193 (94.1) | Reference |
|
|
|
| HH | 6 (2.9) | 12 (11.4) | 0.75
(0.21–1.90) |
0.11 | 0.63
(0.14–1.72) |
0.15 |
| Allele |
|
|
|
|
|
|
| R | 336 (80.4) | 344 (83.9) | Reference |
|
|
|
| H | 82 (19.6) | 66 (16.1) | 1.74
(1.26–2.39) |
0.23 |
|
|
| SVO subgroup |
|
|
|
|
|
|
| RR | 126 (75.9) | 106 (64.2) | Reference |
|
|
|
| RH | 39 (23.5) | 56 (33.9) | 1.09
(0.71–1.68) |
0.69 | 1.11
(0.71–1.73) |
0.65 |
| HH | 1 (0.6) | 3 (1.8) | 0.59
(0.07–5.31) |
1.00 | 0.33
(0.03–3.36) |
0.35 |
| Dominant model |
|
|
|
|
|
|
| RR | 126 (75.9) | 106 (64.2) | Reference |
|
|
|
|
HH+RH | 40 (24.1) | 59 (35.8) | 1.07
(0.70–1.64) |
0.75 | 1.07
(0.69–1.66) |
0.78 |
| Recessive
model |
|
|
|
|
|
|
|
RR+RH | 165 (99.4) | 162 (98.2) | Reference |
|
|
|
| HH | 1 (0.6) | 3 (1.8) | 0.58
(0.06–5.19) |
0.99 | 0.32
(0.03–3.23) |
0.33 |
| Allele |
|
|
|
|
|
|
| R | 291 (87.7) | 268 (81.2) | Reference |
|
|
|
| H | 41 (12.3) | 62 (18.7) | 1.04
(0.70–1.53) |
0.85 |
|
|
Discussion
The present study investigated the association
between the PAF-AH genotypes and the risk of ischemic stroke in the
eastern Chinese Han population. The major finding of this study is
that in the Chinese Han population the frequencies of the minor
alleles of the R92H polymorphisms of the PAF-AH gene were
significantly higher in ischemic stroke patients. This difference
remained following all the factor-related adjustments. However, no
association of V279F with the risk of ischemic stroke was
identified.
Several previous studies have explored the
association between the PAF-AH polymorphism and cardiovascular
disease. However, the results of these studies were inconsistent.
Li et al (11) reported that
the V279F variant was associated with coronary artery disease in
the Chinese Han population. However, the association between the
V279F variant and cardiovascular disease was not found in the South
Korean population (12). With regards
to ischemic stroke, Hiramoto et al (13) identified an association with the FF+VF
genotype and the F allele. In the present study, this association
has not been replicated in the population of eastern China. Two
points should be considered with regards to these findings. Genetic
heterogeneity may be an important factor. Firstly, genotype
frequencies of the V279F in the control subjects (88% VV, 11% VF,
and 1% FF) differ from those in the Japanese populations (75% VV,
22% VF, and 3% FF). Secondly, the study by Hiramoto et al
(13) was conducted with a relatively
small sample size and there was a low frequency of homozygosity of
the minor allele. It may be more likely to produce false positive
results.
The R92H is a polymorphism, with a non-synonymous
change located within the coding region of the Lp-pla2 gene in exon
4, which should result in an arginine-to-histidine substitution at
position 92. The association of R92H with cardiovascular disease is
also contradictory. Sutton et al (8) reported that the R92H polymorphism was
significantly associated with the risk of CHD, with the R92H
variant allele observed more frequently in the experiment group
compared to the control group. Zheng et al (14) identified the significant association of
the R92H variant with premature myocardial infarction in the
Chinese population. In the present study, the focus was on the
association between R92H in the PAF-AH gene and ischemic stroke.
The HH+RH genotype, the RH genotype and the H allele of R92H were
significantly associated with the increased risk of ischemic stroke
in the Chinese Han population.
The association between R92H in the PAF-AH gene and
ischemic stroke in the Chinese population can possibly be explained
as follows: i) Several previous studies have investigated the
association between the PAF-AH polymorphism and the risk of
ischemic stroke. A study of older individuals by Rosso et al
(15) found that PAF-AH was associated
with ischemic and hemorrhagic strokes. In the Bruneck study
(16), PAF-AH activity was identified
as a top hit associated with ischemic stroke. ii) A few studies
have investigated the association between the R92H polymorphism and
plasma PAF-AH activity. A meta-analysis including a total of 14
studies showed that the R92H variant shows the strongest
association with PAF-AH activity among the variants in PAF-AH gene
(17).
When evaluating the association of R92H with stroke
subtypes, a significant association was found for this polymorphism
with the LAA subgroup, but not the SVO subgroup. This can be
explained by the role of PAF-AH in the process of atherosclerosis.
Lyso-PC, the important bioproduct of PAF-AH, is involved in the
inflammatory cytokine production and in the induction of the
expression of adhesion molecules and cytokines. It has a
chemoattractant property for macrophages, and it induces vascular
smooth muscle migration (18).
Additionally, Lyso-PC can upregulate PAF-AH activity, resulting in
a vicious cycle in which pro-inflammatory mediators are
upregulated, contributing to plaque progression and destabilization
(19). In addition, oxNEFAs, another
detrimental substrate of PAF-AH, can promote atherosclerosis by
increasing oxidative stress and the presence of oxidized LDL and
other lipoproteins in the plasma and arterial walls, thereby
initiating fatty streak formation (20).
There are several limitations in the present study
that require discussion, such as the relatively small sample size
and the lack of PAF-AH activity measurements. Therefore, further
studies including functional evaluations are warranted to elucidate
the potential mechanism of these polymorphisms in ischemic
stroke.
In conclusion, the present study demonstrated that
the PAF-AH gene polymorphism R92H may modify the risk of ischemic
stroke in the eastern Chinese Han population. Further studies
involving functional evaluations are warranted to clarify the
potential mechanism of these polymorphisms in ischemic stroke.
References
|
1
|
Liu L, Wang D, Wong KS and Wang Y: Stroke
and stroke care in China, Huge burden, significant workload and a
national priority. Stroke. 42:3651–3654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thrift AG, Dewey HM, Macdonell RA, McNeil
JJ and Donnan GA: Incidence of the major stroke subtypes, Initial
findings from the North East Melbourne stroke incidence study
(NEMESIS). Stroke. 32:1732–1738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flossmann E, Schulz UG and Rothwell PM:
Systematic review of methods and results of studies of the genetic
epidemiology of ischemic stroke. Stroke. 35:212–227. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schunkert H, König IR, Kathiresan S,
Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M,
Gieger C, et al: Large-scaleassociation analyses identifies 13 new
susceptibility loci for coronary artery disease. Nat Genet.
43:333–338. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dada N, Kim NW and Wolfert RL: Lp-PLA2: An
emerging biomarker of coronary heart disease. Expert Rev Mol Diagn.
2:17–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y, Zhang P, Zhang L, Osman H, Mohler
ER III, Macphee C, Zalewski A, Postle A and Wilensky : Role of
lipoprotein-associated phospholipase A2 in leukocyte activation and
inflammatory responses. Atherosclerosis. 191:54–62. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sutton BS, Crosslin DR, Shah SH, Nelson
SC, Bassil A, Hale AB, Haynes C, Goldschmidt-Clermont PJ, Vance JM,
Seo D, et al: Comprehensive genetic analysis of the platelet
activating factor acetylhydrolase (PLA2G7) gene and cardiovascular
disease in case-control and family datasets. Hum Mol Genet.
17:1318–1328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ragoschke-Schumm A, Yilmaz U, Kostopoulos
P, et al: 'Stroke room': Diagnosis and treatment as a single
location for rapid intraarterial stroke treatment. Cerebrovasc Dis.
40:251–257. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams HP Jr, Bendixen BH, Kappelle LJ,
Biller J, Love BB, Gordon DL and Marsh EE III: Classification of
subtype of acute ischemic stroke. Definitions for use in a
multicenter clinical trial. TOAST. Trial of Org 10172 in acute
stroke treatment. Stroke. 24:35–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Qi L, Lv N, Gao Q, Cheng Y, Wei Y,
Ye J, Yan X and Dang A: Association between lipoprotein-associated
phospholipase A2 gene polymorphism and coronary artery disease in
the Chinese Han population. Ann Hum Genet. 75:605–611. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang Y, Kim OY, Koh SJ, Chae JS, Ko YG,
Kim JY, Cho H, Jeong TS, Lee WS, Ordovas JM and Lee JH: The
Val279Phe variant of the lipoprotein-associated phospholipase A2
gene is associated with catalytic activities and cardiovascular
disease in Koreanmen. J Clin Endocrinol Metab. 91:3521–3527. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiramoto M, Yoshida H, Imaizumi T,
Yoshimizu N and Satoh K: A mutation in plasma platelet-activating
factor acetylhydrolase (Val279->Phe) is a genetic risk factor
for stroke. Stroke. 28:2417–2420. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng GH, Xiong SQ, Chen HY, Mei LJ and
Wang T: Associations of platelet-activating factor acetylhydrolase
(PAFAH) gene polymorphisms with circulating PAF-AH levels and risk
of coronary heart diseaseor blood stasis syndrome in the Chinese
Han population. Mol Biol Rep. 41:7141–7151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosso C, Rosenbaum D, Pires C, Cherfils C,
Koujah N, Mestari F, Gillet E, Crozier S, Sahli-Amor M, Samson Y,
et al: Lipoprotein-associated phospholipase A2 during the
hyperacute stage of ischemic and hemorrhagic strokes. J Stroke
Cerebrovasc Dis. 23:277–282. 2014. View Article : Google Scholar
|
|
16
|
Tsimikas S, Willeit J, Knoflach M, Mayr M,
Egger G, Notdurfter M, Witztum JL, Wiedermann CJ, Xu Q and Kiechl
S: Lipoprotein-associated phospholipase A2 activity, ferritin
levels, metabolic syndrome and 10-year cardiovascular and
non-cardiovascular mortality, Results from the Bruneck study. Eur
Heart J. 30:107–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Hao Y, Mo X, Wang L, Lu X, Huang
J, Cao J, Li H and Gu D: PLA2G7 gene polymorphisms and coronary
heart disease risk, A meta-analysis. Thromb Res. 126:498–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zalewski A and Macphee C: Role of
lipoprotein-associated phospholipase A2 in atherosclerosis: Biology
epidemiology and possible therapeutic target. Arterioscler Thromb
Vasc Biol. 25:923–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Macphee CH, Nelson JJ and Zalewski A:
Lipoprotein-associated phospholipase A2 as a target of therapy.
Curr Opin Lipidol. 16:442–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vittos O, Toana B, Vittos A and Moldoveanu
E: Lipoprotein-associated phospholipase A2 (Lp-PLA2): A review of
its role and significance as a cardiovascular biomarker.
Biomarkers. 17:289–302. 2012. View Article : Google Scholar : PubMed/NCBI
|