Introduction
Pi Han Yao is a traditional Chinese herbal medicine,
derived from the Gueldenstaedtia delavayi Franch plant
(1). It was first termed ‘Pi Han Yao’
in the early 1970s in the Xichang Chinese Herbal Medicine book
(2). As a Panzhihua-Xichang region
folk medicine, Pi Han Yao is mainly used to treat exogenous
diseases and has been found to be safe and effective in treating
fevers, headaches, dizziness, sore throat, gasping syndrome and
cough. The whole dry plant is used, and is usually consumed in a
decoction daily. Although there have been studies on the
fat-soluble components of the Gueldenstaedtia delavayi
Franch plant, the pharmacologically active mechanisms for the
effectiveness of Pi Han Yao in treating exogenous diseases remain
to be elucidated. In addition, the full chemical constituents of Pi
Han Yao have yet to be elucidated and there have been no studies on
the quantitative determination of the formononetin and maackiain
content, which are essentially the active components of the plant.
Therefore, the present study investigated the material basis and
mechanisms of the Chinese medicine Pi Han Yao by determining the
chemical constituents of the plant decoction. Seven flavanone
compounds were isolated from the Pi Han Yao decoction. Based on
spectrum analysis, 5 of the 7 were identified. The 5 identified
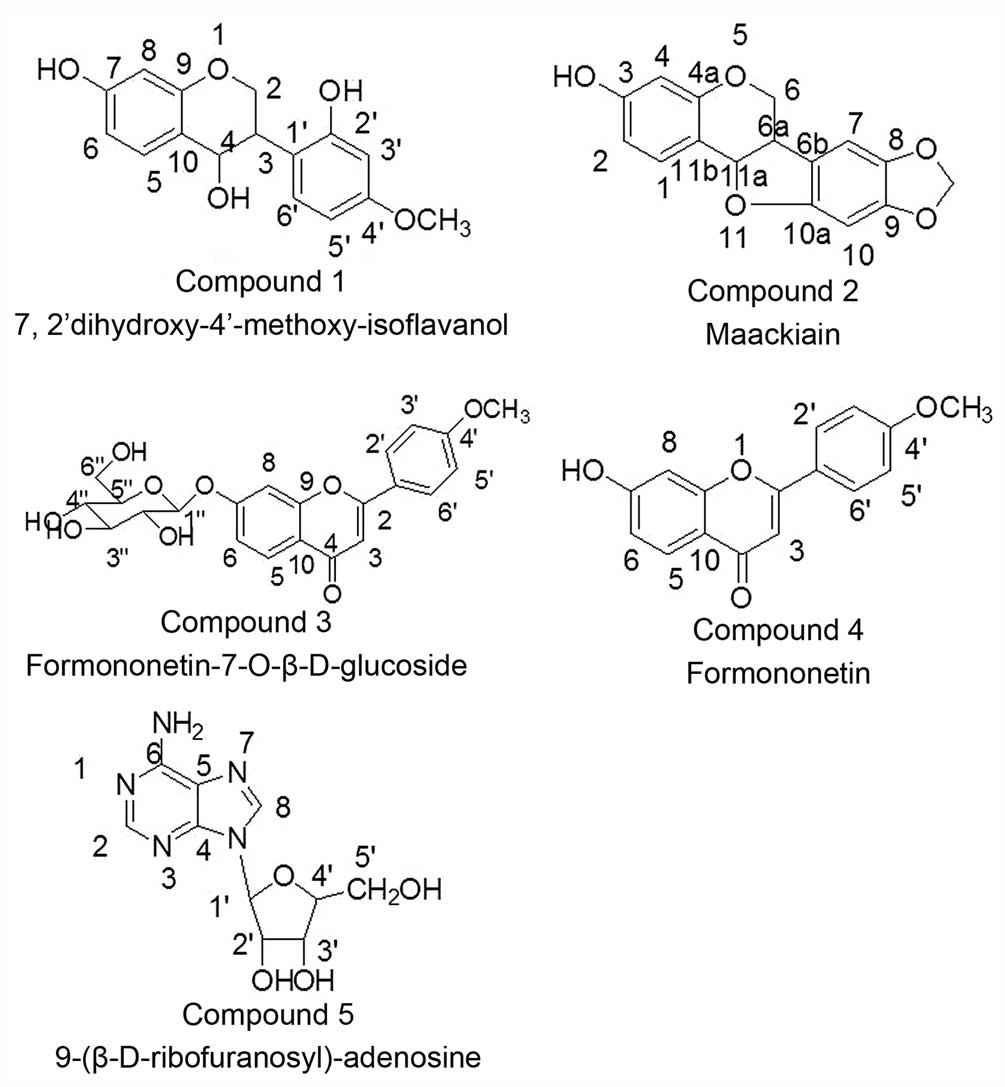
chemical structures were 1, 7,2′-dihydroxy-4′-methoxy-isoflavanol;
2, maackiain; 3, formononetin-7-O-β-D-glucoside; 4,
formononetin; and 5, 9-(β-D-ribofuranosyl)-adenosine. To the best
of our knowledge, this is the first time that these compounds have
been isolated from Pi Han Yao. Further study is required to
identify the other 2 compounds and establish their structures.
Formononetin, 1 of the 7 compounds isolated from the Pi Han Yao
water solution, has been shown to have estrogen-like effects
(3,4),
antioxidation effects (5), and it can
stimulate the specific adaptive immune system (5,6). This
compound has also been reported to have beneficial effects for
certain cancers, as well as antiatheroscloresis, and diuresis
effects (7,8). Maackiain also has a strong diuresis
effect, and therefore, formononetin and maackiain were chosen as
the index components in the quantitative measurement of Pi Han Yao
(9).
Materials and methods
Plant materials
The plant material was identified as Gueldenstaedtia
delavayi Franch by Professor Guihua Jiang.
Chemicals
The formononetin standard was purchased from Chengdu
at Staples Biological Technology Co., Ltd. (Chendgu, China; batch
no. 111703–201012). The maackiain standard was prepared by us to a
purity of 99.5%, as previously described (10).
The chromogenic agent, 10% sulfuric acid ethanol and
iodine steam, was purchased from Chengdu Kelon (Chengdu, China).
Methanol and acetonitrile with chromatographic purification were
from Fisher Scientific (Schwerte, Germany). Distilled water was
used and all the reagents were of analytical grade.
Instrumentation and conditions
The following instruments were used in the study:
Bruker Avance 600 probe: 13C-1H DUL TE: 300K
(Bruker, Zurich, Switzerland); Zabspec (Micromass, Waters Corp.,
Manchester, UK); Rotary Evaporators RE-52 (Shanghai Yarong
Biochemistry Instrument Plant, Shanghai, China); Ultraviolet
Instrument ZF-2 (Shanghai Anting Electron Instrument Plant,
Shanghai, China); high performance liquid chromatography (HPLC;
Agilent 1200 HPLC; Agilent Technologies, Inc., Santa Clara, CA,
USA); Dikma C18 chromatography column (5 µm, 4.6×250 mm; Dikma,
Beijing, China); and a X24 Digital display micro-melting point
tester (Beijing Tech Instrument Co., Ltd., Beijing, China).
Silica gel column chromatography (200–300 mesh;
Qingdao Oceanic Chemical Plant, Qingdao, China); high-performance
thin-layer chromatography (HPTLC; Qingdao Oceanic Chemical Plant);
thin-layer chromatography (TLC) silica gel G and silica gel GF254
(Qingdao Oceanic Chemical Plant); and Sephadex LH20 (Pharmacia,
Uppsala, Sweden) were used.
Extraction and isolation
The whole dry plants of Pi Han Yao (12 kg) were
extracted with 120 liters of water at 90°C three times, 30 min each
time, and subsequently concentrated under vacuum to the 30 liters.
Following this, the water extract was extracted with ethyl acetate
(EtOAc) and n-butanol (n-BuOH), successively. The EtOAc and n-BuOH
layers were concentrated to 64.1 and 141.7 g of residues,
respectively, and were subsequently separated by repeated
chromatography yielding 7 flavanone compounds.
Results
Investigating the chemical constituent
of Pi Han Yao Structural determination
Compound 1
White needles (methanol); melting point (mp),
195–197°C; [α]D20+210° (c 0.10, methanol),
exhibited a positive reaction to the ferric chloride-ferricyanatum
kalium test. 1H-nuclear magnetic resonance (NMR): δ:3.56
(1H, m, H-3), 3.63 (3H, s, OCH3–4′), 3.78 (1H, t, J=10.26 Hz,
H-2a), 4.29 (1H, dd, J=10.98, 5.16 Hz, H-2a), 5.62 (1H, d, J=7.02
Hz, H-4), 6.37 (1H, d, J=2.4 Hz, H-8), 6.56 (1H, dd, J=2.22, 8.04
Hz, H-5′), 6.67 (1H, d, J=2.22 Hz, H-3′), 6.86 (1H, d, J=2.22 Hz,
H-8), 6.94 (1H, dd, J=8.10, 2.58 Hz, H-6), 7.20 (1H, d, J=8.40 Hz,
H-6′), 7.58 (1H, d, J=8.40 Hz, H-5). 13C-NMR: δ162.6
(C-4′), δ162.4 (C-2′), δ161.5 (C-7), δ158.4 (C-9), δ133.8 (C-5),
δ126.3 (C-, 6′), δ121.0 (C-1′), δ112.9 (C-10), δ111.9 (C-6), δ107.5
(C-5′), δ105.2 (C-8), δ98.2 (C-3′), δ80.2 (C-4), δ67.7 (C-2), δ41.0
(C-3) and δ56.3 (C-OCH3). Compound 1 was identified as
7,2′-dihydroxy-4′-methoxy-isoflavanol by comparison of
Heteronuclear Single Quantum Correlation, H-H-Correlation
Spectroscopy and Distortionless Enhancement by Polarisation
Transfer data (11,12).
Compound 2
Colorless needles (methanol, pyridine) reacted
positively to FeCl3. The compound was purple-red when
exposed to the UV lamp (254 nm), mp180–181°C (12); 1H-NMR:δ7.53 (1H, d, J=8.82
Hz, H-1), 6.90 (1H, dd, J=8.46, 2.16 Hz, H-2), 6.84 (1H, s, H-7);
δ6.83 (1H, d, J=2.22 Hz, H-4), 6.64 (1H, s, H-10), 5.91 (1H, d,
J=1.14 Hz, −OCH2O-aH); 5.88 (1H, d, J=1.14 Hz,
−OCH2O-bH), 5.57 (1H, d, J=7.32 Hz, H-11 a, 4.25 (1H,
dd, J=11.04, 4.74 Hz, H-6β), 3.77 (1H, t, J=10.62 Hz, H-6α), 3.48
(1H, m, H-6a). 13C-NMR: δ40.5 (C-6a), 66.5 (C-6), 79.0
(C-11a), 93.7 (C-10), 101.5 (−OCH2-O), 104.0 (C-4), 105.3 (C-7),
110.7 (C-2), 111.8 (C-11b), 118.7 (C-6b), 132.5 (C-1), 141.9 (C-8),
148.3 (C-9), 154.7 (C-10a), 157.3 (C-4a), 160.4 (C-3). Mass
spectrometry (MS): m/z: 307 [M+Na], m/z: 285 [M+H]. Compound 2 was
identified as maackiain by comparison (1H-NMR and
13C-NMR) with previous data (13,14).
Compound 3
White needles (methanol) that were easily soluble in
methanol, mp217–219°C. 1H-NMR: δ:8.42 (1H, s, H-2), 8.04 (1H, d,
J=8.76 Hz, H-5), 7.52 (2H, d, J=8.40 Hz, H-2′, 6′), 7.23 (1H, s,
H-8), 7.13 (1H, d, J=8.82 Hz, H-6), 6.98 (2H, d, J=8.40 Hz, H-3′,
5′), 5.09 (1H, d, J=6.96 Hz, glc H-1′), 3.30 (3H, s,
OCH3). δ4.5–5.5: Glycosidic bond −OH, δ3–4 glycosidic
bond −H. 13C-NMR: δ175.1 (C-4), δ161.9 (C-7), δ159.5
(C-4′), δ157.5 (C-9), δ154.1 (C-2), δ130.5 (C-2′, 6′), δ127.4
(C-5), δ124.5 (C-1′), δ123.9 (C-3), δ119.0 (C-10), δ116.1 (C-6),
δ114.1 (C-3′5′), δ103.9 (C-8), δ100.5 (C-1′), δ77.7 (C-3′), δ77.0
(C-5′), δ73.6 (C-2′), δ70.1 (C-4′), δ61.1 (C-6′), δ55.6
(C-OCH3). Compound 3 was identified as
formononetin-7-O-β-D-glucoside by comparison
(1H-NMR and 13C-NMR) with previous data
(15).
Compound 4
White needles (methanol), mp257–258°C; showed a
positive reaction to the ferric chloride-ferricyanatum kalium test.
1H-NMR: δ:8.30 (1H, s, H-2), 7.95 (1H, dd, J=8.76,2.22
Hz, H-5), 7.49 (2H, d, J=8.40 Hz, H-2′, 6′), 6.96 (1H, d, J=8.76
Hz, H-3′, 5′), 6.92 (1H, dd, J=8.76, 2.22 Hz, H-6), 6.85 (2H, d,
J=2.22 Hz, H-8), 3.77 (3H, s, OCH3). 13C-NMR: δ175.1
(C-4), δ163.0 (C-4′), δ159.4 (C-7), δ157.9 (C-9), δ153.8 (C-3),
δ130.5 (C-2′, 6′), δ127.8 (C-5), δ124.7 (C-1′), δ123.6 (C-2),
δ117.1 (C-10), δ115.6 (C-6), δ114.1 (C-3′5′), δ102.6 (C-8). MS:
m/z: 291 [M+Na], m/z: 269 [M+H]. Compound 4 was identified as
formononetin by comparison with previous data (11,16).
Compound 5
White needles (methanol), soluble in dimethyl
sulfoxide; 1H-NMR: δ:8.32 (1H, s, H-8), 8.12 (1H, s,
H-2), 7.30 (2H, s, H-NH2), 5.86 (1H, d, J=6.24 Hz, H-1′), 5.00–5.50
(3H, OH-H), 4.59 (1H, m, H-2′), 4.12 (1H, m, H-3′), 3.94 (1H, m,
H-4′), 3.65 (1H, m, H-5′), 3.53 (1H, m, H-5′). 13C-NMR:
δ156.6 (C-6), δ152.8 (C-2), δ149.6 (C-4), δ140.4 (C-8), δ119.8
(C-5), δ88.4 (C-1′), δ86.4 (C-4′), δ73.9 (C-2′), δ71.1 (C-3′),
δ62.2 (C-5′). MS: m/z: 255 [M+Na], m/z: 237 [M+H]. Compound 5 was
identified as 9-(β-D-ribofuranosyl)-adenosine by comparison
(1H-NMR and 13C-NMR) with literature data
(17,18).
The structures of compounds 1–5 are shown in
Fig. 1. The structures of compounds 6
and 7 were complex and thus remain to be identified.
Content tests of formononetin and maackiain in Pi
Han Yao Chromatographic conditions
The detection of the compounds was performed at 310
nm. Satisfactory separation was obtained with a reverse-phase
analytical column (250×4.6 mm, 5 µl, serial no. 8132964) and eluted
with a methanol:water solution (70:30, v/v) at a flow rate of 1.0
ml/min. The column temperature was 25°C and the injection volume
was 10 µl. Theoretical plate numbers of formononetin and maackiain
were >4,000.
Standard solutions
i) Preparation of stock standard solutions:
Formononetin and maackiain stock solution were prepared to a final
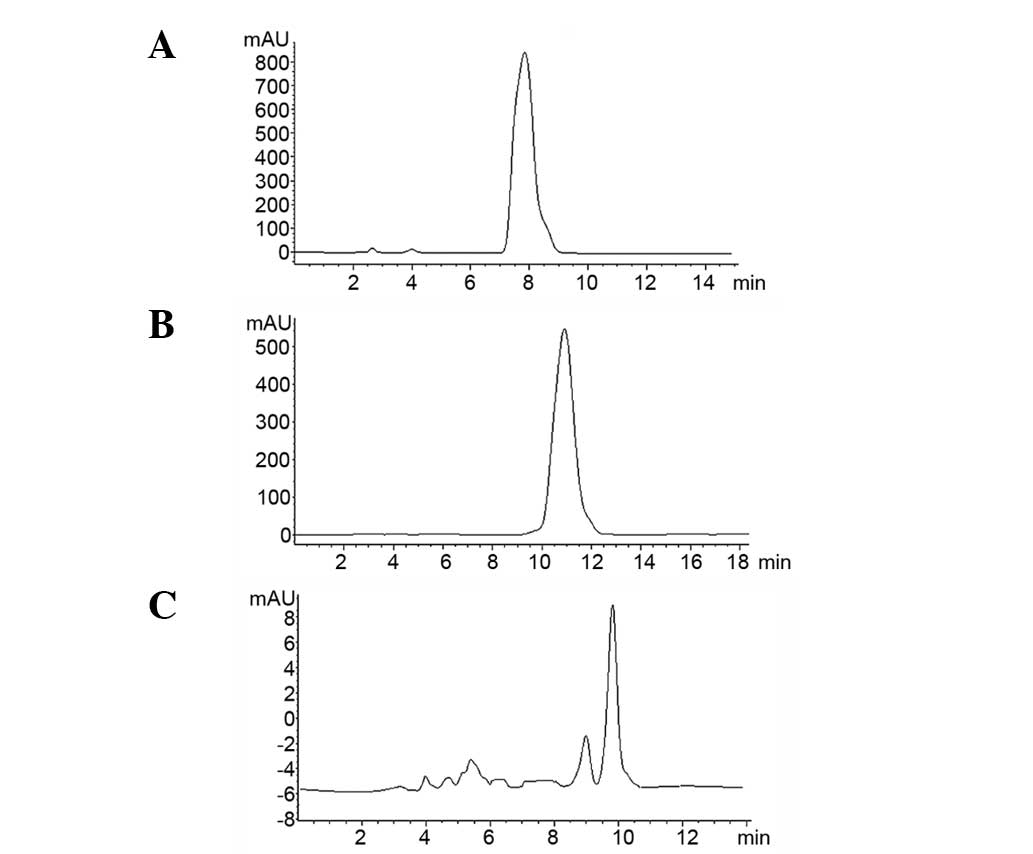
concentration of 0.73 mg/ml in methanol. Chromatograms are shown in
Fig. 2A and B.
ii) Sample preparation. The Pi Han Yao powder (3 g)
was processed with ultrasonic extraction (power, 300 W; rate, 50
Hz) for 1 h in EtOAc, and subsequently recovered EtOAc in
vacuo to yield the extract. The extract was dissolved with
methanol in a 10 ml volumetric flask and filtered through a 0.45-µm
filter prior to injection. The chromatogram is shown in Fig. 2C.
Calibration
Calibration curves were constructed in the range of
2–20 µl for formononetin and maackiain. The regression equation
curves are listed in Table I.
 | Table I.Calibration graphs for the Pi Han Yao
constituents. |
Table I.
Calibration graphs for the Pi Han Yao
constituents.
| Constituent | Regression equation
(R2) | Linear range, µg |
|---|
| Formononetin | y=4535.1×+18.587
(0.9995) |
0.03992–0.3992 |
| Maackiain | y=4258.3×+774.11
(0.9995) | 0.0292–0.292 |
The data showed a linear association between peak
areas and concentration over the listed range for formononetin and
maackiain. The detection limit was 0.03992–0.3992 µg for
formononetin and 0.0292–0.292 µg for maackiain.
Precision and accuracy
The precision of injection was evaluated by
performing replicate injections of the sample solution, and the
relative standard deviations of formononetin and maackiain were
0.26 and 0.55%, respectively. These results demonstrated that the
method was highly precise.
Stability
To confirm that the standards remained stable over
time, the concentrations of the two components were determined at
0, 2, 4, 8, 12 and 24 h, respectively, and the solution remained
relatively stable. The RSD values of formononetin and maackiain
were maintained at 1.59 and 2.57%, respectively. The stability of
the sample solutions was also evaluated in the same manner by
determining the concentrations of the two components at 0, 2, 4, 8
and 12 h, respectively. The test solution remained relatively
stable. The RSD values of formononetin and maackiain were
maintained at 2.18 and 5.98%, respectively.
Recovery study
The percentage of recovery was determined by adding
a known concentration of the formononetin and maackiain standards
to selected samples during the extraction process. The amounts
added were ~50% of the actual concentration of the samples. The
concentration of the formononetin and the maackiain standards in
the mixture were subsequently determined using the same methods
described for the sample analysis. Without exception, recovery
rates of 100.31% were achieved for the compound analyzed.
Quantitative determination of samples
The formononetin and maackiain contents of Pi Han
Yao from plants collected from 10 different sources were determined
in the same way as the sample analysis. The results are shown in
Table II.
 | Table II.Results of the quantitative
determination of the Pi Han Yao samples (n=10). |
Table II.
Results of the quantitative
determination of the Pi Han Yao samples (n=10).
|
|
|
| Formononetin | Maackiain |
|---|
|
|
|
|
|
|
|---|
| Sample | Producing area in
China | Growth | Peak area | Content, µg/g | Peak area | Content, µg/g |
|---|
| 1 | Te Erguo, Pu Ge | Wild | 750.9 | 0.651 | 351.3 | 0.0211 |
| 2 | Happy, Man Shuiwan,
Mian Ning | Wild | 853.9 | 0.741 | 339.6 | 0.0204 |
| 3 | Dawn, Sha Ba, Mian
Ning | Cultivated | 660.4 | 0.573 | 206.8 | 0.0124 |
| 4 | Victory, Sha Ba, Mian
Ning | Wild | 867.7 | 0.753 | 368.5 | 0.0212 |
| 5 | Li Zheng, Hong Mo,
Mian Ning | Wild | 879.0 | 0.762 | 476.3 | 0.0286 |
| 6 | Long Feng, Sha Ba,
Mian Ning | Wild | 711.8 | 0.617 | 481.7 | 0.0289 |
| 7 | Shi an, Da Xing | Cultivated | 664.3 | 0.576 | 382.1 | 0.0229 |
| 8 | Min Yun, Xi Ning | Wild | 719.6 | 0.624 | 421.8 | 0.0253 |
| 9 | Lang Huan, Lang
Huan | Cultivated | 728.0 | 0.631 | 318.3 | 0.0191 |
| 10 | A Yue, De Chang | Cultivated | 709.8 | 0.616 | 369.0 | 0.0222 |
Analysis of the different Pi Han Yao plants from the
10 locations showed that the contents of formononetin and maackiain
varied depending on the source. The content of formononetin ranged
from 0.576–0.762 µg/g with an average value of 0.6544 µg/g. The
content of maackiain ranged from 0.0124–0.0289 µg/g, with an
average value of 0.02221 µg/g. Overall the content of the two
chemicals in wild Pi Han Yao was generally higher compared to
cultivated plants. However, the difference was not significant.
Furthermore, the content of formononetin and maackiain in Pi Han
Yao was lower compared to the plant itself.
Discussion
To the best of our knowledge, the chemical
constituents of the Chinese medicine Pi Han Yao decoction were
investigated for the first time in this study. Five flavanone
compounds were identified as follows: 1,
7,2′-dihydroxy-4′-methoxy-isoflavanol; 2, maackiain; 3,
formononetin-7-O-β-D-glucoside; 4, formononetin; and 5,
9-(β-D-ribofuranosyl)-adenosine. These compounds were obtained from
Pi Han Yao for the first time, in accordance with previous studies
(19–24).
The result of the formononetin and maackiain
contents of Pi Han Yao from 10 different areas is shown in Table II. A method to determine formononetin
and maackiain in the Pi Han Yao by HPLC has been reported in the
present study, and this method is convenient, efficient, accurate,
reliable and provides good replications, and thus, can be used for
the quality control of Pi Han Yao.
The formononetin and maackiain contents were scanned
from 200 to 400 nm by UV-1100. The maximum absorption wavelength of
formononetin was 250 nm, and the maximum absorption wavelengths of
maackiain were 310 and 215 nm. In order to determine the
formononetin and maackiain content at the same conditions, the
detection at 310 nm exhibited high sensitivity and little
interference. Therefore, formononetin and maackiain were detected
at 310 nm.
In conclusion, the present study examined the
extraction efficiency in solvents, the extraction method and the
extraction time. The results showed that ethyl acetate ultrasound
extraction had the highest extraction efficiency for constituents
of Pi Han Yao.
References
|
1
|
Editorial Committee of the Flora of China
of Chinese Academy of Science. Beijing: Flora of China. Science
Press. 2004.
|
|
2
|
Province XChbiSC: Xi Chang Chinese Herbal
Medicine. 1:Xi Chang: Xi Chang People's Enterprise. 1972.
|
|
3
|
Wang WQ, Han ZK, Chen WH and Chen J: The
enhancing effect of formononetin on the development of mammary
gland in mice and exploration of its mechanism. Journal of Nanjing
Agricultural University. 16:191993.
|
|
4
|
Xin D, Liu Z, Xue C, et al: Study on
estrogen-like effects of formononetin and its correlation with
blood lipids. Chinese Journal of Gerontology. 29:23402009.
|
|
5
|
Fu N, Liu C, Zhang R, et al: Study on the
antioxidation about the flavonoids and triterpene compounds of
glycyrrhiza. Pharmacology and Clinics of Chinese Materia
Medica. 5:261994.
|
|
6
|
Zhang R and Zhengkang H: Explore the
effect of flavonoids on immunologic function in mice and
β-endorphin level in the blood of mice. Chinese Journal of
Immunology. 10:1771994.
|
|
7
|
Qu X and Jingdao L: Summarized the recent
developments about the flavonoids of Maackia amurensis Rupr. et.
Maxim. Journal of Medical Science Yan Bian University.
16:2311993.
|
|
8
|
Roberts DW, Doerge DR, Churchwell MI, da
Gamboa Costa G, Marques MM and Tolleson WH: Inhibition of
extrahepatic human cytochromes P450 1A1 and 1B1 by metabolism of
isoflavones found in Trifolium pratense (red clover). J
Agric Food Chem. 52:6623–6632. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miao K, Zhang J, Dong Y, et al: The
advances in the study on chemical components and pharmacological
action of Sophora flavescens. Natural Product Research and
Development. 13:69–73. 2001.
|
|
10
|
Wang CH, Wang Y, Wang GC, et al: Chemical
constituents from roots of Millettia speciosa. Chinese
Traditional and Herbal Drugs. 39:972–975. 2008.(In Chinese).
|
|
11
|
Li HN, Yan H, Pang XY, et al: Study on
chemical constituents of flavonoids of Sophora tonkinensis.
China Journal of Chinese Materia Medica. 349:282–285. 2009.
|
|
12
|
Gomotsang B, Cornelius CWW, Runner RTR, et
al: Two new isoflavanoids from Bolusanthus speciosus.
Bulletin of the Chemical Society of Ethiopia. 15:1312001.
|
|
13
|
Gao D and Ruyi Z: Study on chemical
constituents of glycyrhiza yunnanensis from Yunnan province.
Chinese Herbal Medicines. 25:507–508. 1994.
|
|
14
|
Li Wu Jun, Toshio Miyase, Akira Ueno, et
al: Studies on the constituents of Sophora flavesens
AITON.II. Chemical and Pharmaceutical Bulletin. 33:3231–3236. 1985.
View Article : Google Scholar
|
|
15
|
Cui BL, Nakamura M, Kinjo J, et al:
Chemical Constituents of Astragali Semen. Chemical and
Pharmaceutical Bulletin. 41:178–182. 1993. View Article : Google Scholar
|
|
16
|
Song C, Zheng ZLD, et al: Study on the
isoflavone compounds of Astragalus membranaceus (Fisch.)
Bunge. Chinese Bulletin of Botany. 39:7641997.
|
|
17
|
Basílio Janke EM, Limbach HH and Weisz K:
Binding of an acetic acid ligand to adenosine: A low-temperature
NMR study. J Am Chem Soc. 126:2135–2141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chenon MT, Pugmire RJ, Grant DM, Panzica
RP and Townsend LB: Carbon-13 magnetic resonance. XXV. A basic set
of parameters for the investigation of tautomerism im purines.
Established from carbon-13 magnetic resonance studies using certain
purines and pyrrolo [2,3-d]pyrimidines. J Am Chem Soc.
97:4627–4636. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han Y, Gao L and Taibao W: Study on
chemical components of essential oil of gueldenstaedtia
multiflora bge. Journal of Shanxi College of TCM. 11:18–21.
2010.
|
|
20
|
Han Y and Liming G: Study on chemical
constituents of gueldenstaedtia multiflora bge. Journal of
Shanxi College of TCM. 10:83–84. 2009.
|
|
21
|
Li K, Li XM and Wang BG: Chemical from
herd of constituents of Gueldenstaedtia stenophylla.
Zhongguo Zhong Yao Za Zhi. 33:1711–1713. 2008.(In Chinese).
PubMed/NCBI
|
|
22
|
Wang J and Rong Z: Study on the chemical
constituents of Gueldenstaedtia multiflora Bge. Acta
Botanica Boreali-Occidentalia Sinica. 9:127–130. 1989.
|
|
23
|
Wei YX, Chen L and Wang JX: Studies on the
chemical constituents of Gueldenstaedtia stenophylla. Zhong
Yao Cai. 30:954–956. 2007.(In Chinese). PubMed/NCBI
|
|
24
|
Zhu Rong ZD and Xu RS: Study on the
chemical constituents of Gueldenstaedtia multiflora Bge.
Chinese Traditional and Herbal Drugs. 15:1–3. 1984.
|