Introduction
Hepatitis B virus (HBV) infection remains one of the
most serious global health problems. Conservative estimates of the
global prevalence of HBV infection approximate that the number of
affected individuals is >2 billion worldwide, among which the
infection may develop into chronic hepatitis B (CHB) in ~240
million people. As a non-cytopathic virus, HBV infection may induce
the host immune responses, producing substantial liver damage and
resulting in chronic hepatitis, cirrhosis and hepatocellular
carcinoma (HCC) (1). Covalently closed
circular DNA (cccDNA), generated from the partially double-stranded
genomic DNA (relaxed circular DNA) in the nucleus of infected
hepatocytes, represents the transcriptional template for HBV RNA
production and has a significant role in the persistence of HBV
infection, the infection of liver transplant cases (2) and the pathogenesis of HCC (3). To date, the treatment with nucleoside
analogues, such as lamivudine, adefovir (ADV) and entecavir, either
as a monotherapy or with interferon-α (INF-α), has been the major
intervention capable of eradicating HBV from infected cells
(4). However, long-term nucleoside
analogue monotherapy may not eradicate cccDNA in the nucleus
directly and completely, and may result in HBV infection relapse
due to the continuous emergence of drug-resistant mutation. The
efficacy of INF-α is limited to a small percentage of highly
selected patients and is often associated with adverse effects,
such as flu-like symptoms, fatigue, leucopenia, depression,
anorexia and hair loss (5).
In China, numerous herbs or their derivatives have
also been widely used in the treatment of HBV infection.
Cimicifuga foetida L. (C. foetida), which mainly
consists of C. foetida, Kudzuvine root, Chinese herbaceous
peony and liquorice, has been used as a medical plant for
anti-pyretic and detoxificative purposes in ancient China for
thousands of years, and has been proved to have anti-bacterial,
anti-inflammatory, anti-pyretic and anticancer activities (6). C. foetida has also shown a
promising anti-HBV effect in CHB patients, and has been approved
for the treatment of HBV infection by the State Food and Drug
Administration of China (7). Thus far,
however, the anti-HBV effect of C. foetida has not yet been
characterized in vivo and in vitro. In the present
study, the inhibition of HBV replication, particularly at the
cccDNA level during the combination therapy of ADV and C.
foetida, was compared to the monotherapy of ADV, and
demonstrated that C. foetida may stimulate the release of
the inflammatory cytokines, such as IFN-γ, and possibly inhibit the
function of the immunosuppressive cytokines in the therapy, such as
transforming growth factor-β (TGF-β).
Patients and methods
Patients and samples
A total of 60 randomly selected patients with
treatment-naive, new diagnosed CHB, from the Department of
Infectious Diseases, Renmin Hospital, Hubei University of Medicine
(Shiyan, China), were recruited and divided into 2 groups: Group I
comprised of 30 patients and group II comprised of 30 patients,
respectively. CHB was documented by the presence of HBV DNA in the
serum for >6 months and a serum alanine aminotransferase (ALT)
level greater than twice the normal range (4). Patients who were coinfected with
hepatitis D, hepatitis C or human immunodeficiency virus, or those
with Wilson's disease or primary biliary cirrhosis were excluded
from the study. While the patients in group I received a
monotherapy of ADV (10 or 30 mg daily) for >48 weeks, the
patients in group II received a combination therapy of ADV and
C. foetida. Briefly, 10 g of C. foetida, 10 g of
Chinese herbaceous peony and 10 g of liquorice, and 15 g of
Kudzuvine root were shade-dried and decocted together for 1 h with
1 liter of boiling reverse-osmotic water twice. The decoctions were
mixed, filtered, concentrated and lyophilized, and the dose of
which (100 ml) was taken three times a day (8). Each patient signed an informed consent
document approved by the Ethics Committee of Shiyan People's
Hospital (Shiyan, China). Liver biopsy specimens were collected
from each patient at baseline and week 48, were frozen in liquid
nitrogen and were stored at −70°C until experimental analysis.
Serum samples were collected simultaneously and stored at −70°C
until used for the measurement of hepatitis B surface antigen
(HBsAg), hepatitis B e antigen (HBeAg) and ALT levels.
Intrahepatic (IH) HBV cccDNA
quantification
DNA was extracted from biopsy specimens using the
QIAamp® DNA Mini kit (Qiagen, Hilden, Germany). Determination of IH
HBV cccDNA levels, prior and subsequent to the treatment, was
carried out by quantitative polymerase chain reaction, as described
previously with a slight modification (9). Variation in the amounts of liver tissue
was normalized by quantifying β-globin in each sample with the
Roche DNA control kit to allow standardization of the extracted DNA
and expression of HBV cccDNA as copies per cell (copies/cell).
Assays for serum HBV DNA, HBsAg, HBeAg
and ALT
DNA was extracted from 200 µl serum using the
QIAamp® DNA Blood Mini kit (Qiagen). Serum HBV DNA levels were
measured using the Cobas® TaqMan® test as described previously
(Roche Diagnostics, Indianapolis, IN, USA) (10). Serum HBsAg and HBeAg levels were
quantified through enzyme immunoassays (ARCHITECT platform; Abbott
Laboratories, Abbott Park, IL, USA) according to the manufacturer's
protocol. The lower limits of the detection were 15 IU/ml for HBV
DNA, 0.05 IU/ml for HBsAg and 1 IU/ml for HBV DNA, respectively.
During the study, the serum HBV DNA and HBsAg levels were detected
every 3 weeks. Serum ALT was measured prior and subsequent to the
treatment using the ALAT (GPT) FS kit (DiaSys Diagnostic Systems
GmbH, Holzheim, Germany) according to the manufacturer's
protocol.
Serum IFN-γ and TGF-β
quantification
Serum IFN-γ and TGF-β levels were quantified using
ELISA kits (R&D Systems Inc., Minneapolis, MN, USA) prior and
subsequent to the treatment. The A450 nm was determined with the
ELISA reader (Multiskan EX; Thermo Labsystems, Helsinki,
Finland).
Statistical analysis
Continuous variables from the treatment groups are
expressed as the mean (range) and were analyzed using a non-paired
Student's t-test using statistical software SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). HBV DNA (IU/ml) and HBsAg (IU/ml) were
logarithmically transformed for analysis. The Kaplan-Meier method
(using a log-rank test) was applied for the cumulative rates of
sustained virological response (SVR) (11) and HBsAg seroclearance. Differences were
two-sided and P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline characteristics of
patients
The baseline characteristics of the patients prior
to treatment are shown in Table I. All
the patients were positive for HBsAg and HBeAg ≥6 months, with a
serum ALT level greater than twice the normal range.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristics | Group I (n=30) | Group II (n=30) | P-value |
|---|
| Age, years | 43 (41–56) | 45 (35–55) | 0.2 |
| Male | 24 | 25 | 1.0 |
| Serum ALT, IU/l | 220 (120–390) | 225 (118–450) | 0.6 |
| Serum HBV DNA, log
copies/ml | 8.2
(6.3–8.9) | 6.8
(5.8–8.1) | 0.1 |
IH HBV cccDNA, serum HBsAg, IFN-γ and
TGF-β quantification
The lower limits of detection for IH cccDNA were
2.4×10−4 copies/cell. IH cccDNA levels were detectable
in all patients prior to the treatment, and in 27 patients in group
I and 25 in group II following the treatment. The median IH cccDNA
level was 3.82 copies/cell in group I and 3.75 copies/cell in group
II, respectively, prior to the treatment (P=0.68). Following the
treatment, a significant reduction of the median IH cccDNA level
was found in group II (0.15 copies/cell, P=0.017) but not in group
I (0.76 copies/cell, P=0.05), which was significantly lower in
group II (P=0.01). Prior to the treatment, log10 HBsAg
was 3.52 in group I and 3.71 in group II, respectively (P=0.26).
Following the treatment, 2 patients in group I, and 4 patients in
group II had achieved HBsAg seroclearance, and a significant
reduction of log10 HBsAg could be found both in group I
(2.78, P=0.012) and in group II (2.27, P<0.0001), and there was
no significant difference identified between the 2 groups (P=0.20).
Prior to the treatment, the median serum IFN-γ level was 14.8 pg/ml
in group I and 15.3 pg/ml in group II, respectively (P=0.29).
Following the treatment, a significant increase of the median serum
IFN-γ level was identified in group II (20.9 pg/ml, P=0.0005) but
not in group I (17.1 pg/ml, P=0.06), which was significantly higher
in group II (P=0.004). The median serum TGF-β level was 672.9 pg/ml
in group I and 630.5 pg/ml in group II prior to the treatment
(P=0.15). Following the treatment, a significant reduction of the
median TGF-β level was identified in groups I (338.9 pg/ml,
P<0.0001) and II (286.0 pg/ml, P<0.0001), which was
significantly lower in group II (P=0.002) (Table II).
 | Table II.Virological and serological responses
following 48 weeks of anti-viral therapy. |
Table II.
Virological and serological responses
following 48 weeks of anti-viral therapy.
| Assays | Group I | Group II | P-value |
|---|
| Mean IH cccDNA,
copies/cell (range) |
|
|
|
|
Prior to
treatment | 3.82 (0.035 to
42.2) | 3.75 (0.049 to
37.6) | 0.682 |
|
Subsequent to
treatment | 0.76 (neg to
4.42) | 0.15 (neg to
2.08) | 0.013 |
|
P-value | 0.052 | 0.017 |
| Mean serum IFN-γ,
pg/ml (range) |
|
|
|
|
Prior to
treatment | 14.8 (8.6 to
24.4) | 15.3 (9.2 to
26.4) | 0.291 |
|
Subsequent to
treatment | 17.1 (9.1 to
27.7) | 20.9 (9.3 to
36.9) | 0.004 |
|
P-value | 0.063 | 0.0005 |
| Mean serum TGF-β,
pg/ml (range) |
|
|
|
|
Prior to
treatment | 672.9 (495.5 to
831.8) | 630.5 (428.7 to
921.9) | 0.151 |
|
Subsequent to
treatment | 338.9 (253.4 to
450.1) | 286.0 (135.9 to
519.7) | 0.002 |
|
P-value | <0.0001 | <0.0001 |
| Mean serum HBsAg, log
IU/ml (range) |
|
|
|
|
Prior to
treatment | 3.52 (2.14 to
4.90) | 3.71 (2.27 to
4.69) | 0.263 |
|
Subsequent to
treatment | 2.78 (−2.04 to
3.48) | 2.27 (−2.02 to
3.41) | 0.201 |
|
P-value | 0.012 | <0.0001 |
Cumulative rate analysis
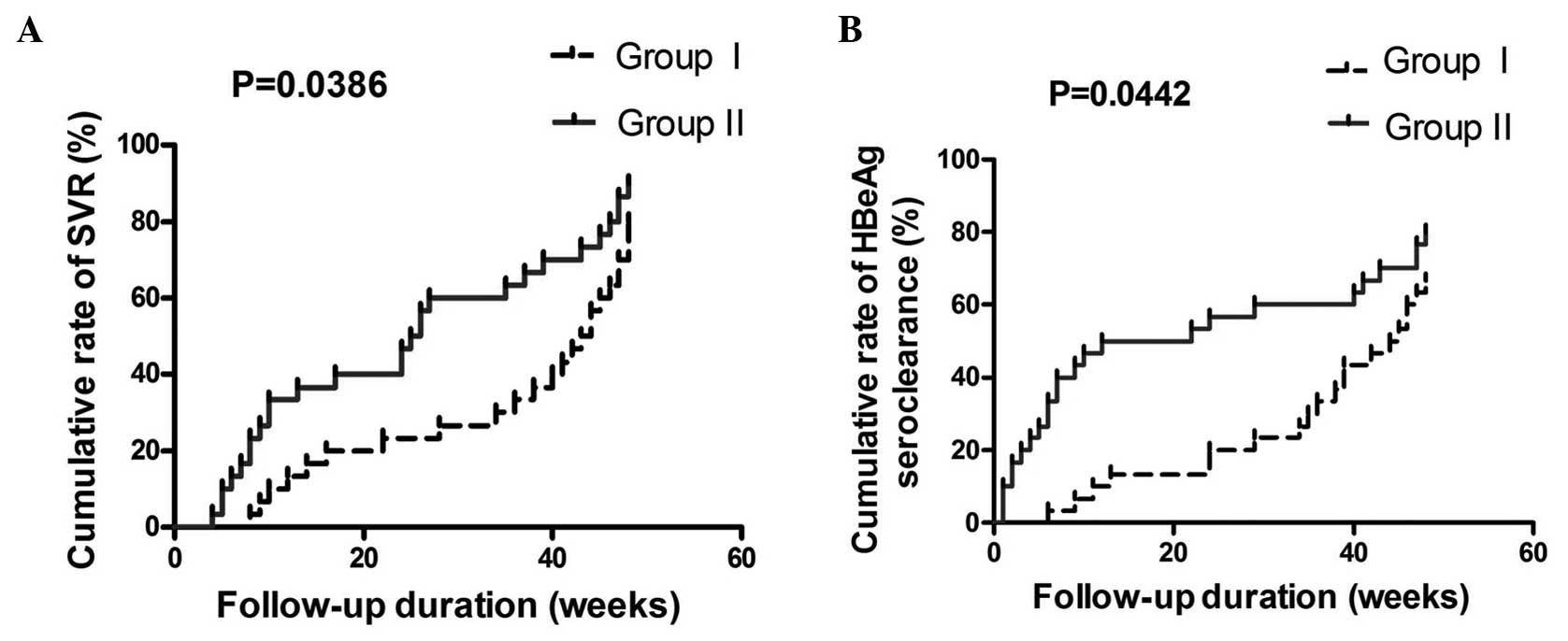
According to serum ALT detection, all the patients
recruited exhibited a achieved biochemical response (4) after 48 weeks of therapy. A total of 24
patients in group I, and 27 patients in group II achieved SVR
(11) during the treatment. SVR
cumulative percentage rates at 6, 12, 24 and 48 weeks were 3.3, 20,
36.7 and 80% in group I and 23.3, 36.7, 66.7 and 90% in group II
(P=0.0386). A total of 20 patients in group I, and 24 patients in
group II achieved HBeAg seroclearance. HBeAg seroclearance
cumulative percentage rates at 6, 12, 24 and 48 weeks were 3.3, 10,
20 and 66.7% in group I and 33.3, 50, 56.7 and 80% in group II
(P=0.0442) (Fig. 1).
Discussion
Infection of hepatocytes by HBV appears to be
non-cytopathic and the histopathology is a consequence of the
adaptive immune reaction to infection. During transient infections,
which are generally <6 months in duration, Th1-type cytokines,
including IFN-γ and TNF-β, potentially control HBV replication by
activating virus-specific cytotoxic T cells, cluster of
differentiation 8+ (CD8+) T-cell, natural
killer (NK) cell and macrophage responses, consequently stimulating
a cascade of inflammatory cytokines and degrading HBV RNA directly
(12). In CHB patients, complete
clearance of cccDNA cannot be spontaneously achieved due to
inefficiencies in the innate and adaptive immune responses in these
patients, in which high levels of Th2-type cytokines, such as
TGF-β, may suppress innate antiviral immunity by blocking the cell
cycle in G1, inhibiting the secretion of IFN-γ and TNF-α from
HBV-specific T cells, impairing NK cell function by reducing NK
cell receptor 2B4/SLAM-associated protein expression, and
activating NK group 2 member D/DNAX protein 10 (13).
While nucleotide analogue monotherapy or with INF-α
may not eliminate cccDNA directly, and treatment withdrawal is
associated with reactivation of HBV replication, a number of other
strategies utilizing host functions for HBV therapeutics are under
way. For example, RNA interference, a major development in gene
therapy leading to gene silencing, may be used to inhibit cccDNA
amplification by targeting HBV nuclear localization signal,
recruiting small interfering RNAs that are ~21 nucleotides in
length and that hybridize to a homologous mRNA target, resulting in
degradation of mRNA (14).
Furthermore, by binding to the viral RNA substrate through its
N-terminal zinc finger motifs, and in turn recruiting a host RNA
processing complex, specifically the exosome, zinc finger proteins
can markedly degrade HBV mRNA. An adoptive T cell therapy may also
be a promising approach to ultimately eliminate cccDNA (15). While HBV-infected cells continuously
produce HBsAg from the cccDNA template and a high number of
hepatocytes (5–30%) remained positive for HBV S protein even
following a long-term antiviral therapy, one modified cytolytic T
cell carrying a chimeric T cell receptor may be designed to target
S antigen-positive cells, and therefore license cytolytic T cells
to eliminate these cells (16).
In general, current antiviral agents can control but
not directly eradicate IH cccDNA. However, one previous study
supports a direct deamination role of APOBEC3A (A3A) or APOBEC3B
(A3B) on cccDNA, through interaction with the HBV core while
sparing the cellular genome, and IFN-α and LT-β R agonists can
result in extensive guanine to adenine (A) hypermutation and
subsequent degradation of cccDNA mediating by A3A or A3B (17). Consequently, in combination with other
antiviral strategies, the use of IFN-α and LT-β R agonists may be a
potential to directly remove cccDNA from the nuclei of infected
hepatocytes. Further studies are required to clarify the underlying
mechanisms of this therapeutic strategy.
In China, Cimicifuga was firstly recorded to
cure diseases in Shennong's Herbal Classic 2,000 years ago. Several
types of evidence support the notion that the C. foetida
extract is able to inhibit HBV replication, although the mechanism
by which it does this remains to be established. However, two
possible mechanisms can be postulated: i) C. foetida may
interfere directly with the process of a HBV-DNA synthesisor; and
ii) C. foetida may potentially induce cytokines or cell
factors that enhance the degradation of HBV (18). While combination therapy has emerged as
a new approach to the treatment of chronic HBV infection with the
objective to decrease the occurrence of adverse effects relapse,
the eradication of IH cccDNA during the combination therapy of ADV
and C. Foetida was analyzed, and a significant reduction of
the median IH cccDNA level was identified in group II but not in
group I. The SVR and HBeAg seroclearance were significantly higher
in group II compared to group I, indicating that combination
therapy of C. foetida and ADV may be more superior to
monotherapy of ADV in HBV transcription inhibition.
Of the specific inflammatory cytokines that are
known to suppress HBV replication, IFN-γ, mainly produced by
CD4+ Th1 cells, CD8+ T cells, NK cells, NKT
cells (5), has a critical role in the
suppression of HBV replication by stimulation of inducible nitric
oxide (NO) synthase and production of NO, activating the nuclear
factor-κB signaling pathway that destabilizes the integrity of HBV
capsids, and degrades HBV mRNA by the formation of the inhibitor of
κB kinase-α (IKK-α) and IKK-β (12,19). By
contrast, TGF-β1, which was mainly released by CD4+
CD25+ cells, Th17 cells, Treg cells and
Foxp3+, may be the predominant immunosuppressive
cytokines in HBV infection persistence. First, TGF-β1 may impair NK
functions by reducing NKG2D/DAP10 and 2B4/SAP expression on NK
cells. Second, TGF-β1 may suppress innate antiviral immunity by
blocking the cell cycle in the G1 phase (20). In the present study, following the
treatment there was a significant increase of the median serum
IFN-γ level in group II but not in group I, and the median serum
TGF-β level was significantly lower in group II compared to group I
even when there was a significant reduction identified in the two
groups. Therefore, we assume that C. foetida may be useful
in the therapeutic management of HBV infection by stimulating IFN-γ
production as well as inhibiting TGF-β secretion as a result.
In conclusion, this novel treatment suggests a new
strategy for treating HBV infection. C. foetida may reduce
the course of antiviral therapy, minimize the emergence of
drug-resistant mutants, and reduce the financial burden of patients
consequently.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of Hubei Province (grant no.
2015CFB290).
References
|
1
|
European Association For The Study Of The
Liver: EASL Clinical Practice Guidelines: Management of chronic
hepatitis B. J Hepatol. 50:227–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaiteerakij R, Komolmit P, Sa-nguanmoo P
and Poovorawan Y: Intrahepatic HBV DNA and covalently closed
circular DNA (cccDNA) levels in patients positive for anti-HBc and
negative for HBsAg. Southeast Asian J Trop Med Public Health.
41:867–875. 2010.PubMed/NCBI
|
|
3
|
Wong DK, Huang FY, Lai CL, Poon RT, Seto
WK, Fung J, Hung IF and Yuen MF: Occult hepatitis B infection and
HBV replicative activity in patients with cryptogenic cause of
hepatocellular carcinoma. Hepatology. 54:829–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chinese Society of Hepatology and Chinese
Society of Infectious Diseases, Chinese Medical Association: The
guideline of prevention and treatment for chronic hepatitis B (2010
version). Zhonghua Gan Zang Bing Za Zhi. 19:13–24. 2011.(In
Chinese). PubMed/NCBI
|
|
5
|
Seeger C and Mason WS: Molecular biology
of hepatitis B virus infection. Virology. 479(480): 672–686. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian Z, Pan R, Chang Q, Si J, Xiao P and
Wu E: Cimicifuga foetida extract inhibits proliferation of
hepatocellular cells via induction of cell cycle arrest and
apoptosis. J Ethnopharmacol. 114:227–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang RH: The effect of Cimicifuga
foetida on chronic hepatitis therapy used by Pro. Chi-Zhi
Zhang. Forum on Traditional Chinese Medicine. 27:122012.(In
Chinese).
|
|
8
|
Zheng TP, Sun AJ, Xue W, Wang YP, Jiang Y,
Zhang Y and Lang JH: Efficacy and safety of Cimicifuga
foetida extract on menopausal syndrome in Chinese women. Chin
Med J (Engl). 126:2034–2038. 2013.PubMed/NCBI
|
|
9
|
Bowden S, Jackson K, Littlejohn M and
Locarnini S: Quantification of HBV covalently closed circular DNA
from liver tissue by real-time PCR. Methods Mol Med. 95:41–50.
2004.PubMed/NCBI
|
|
10
|
Weinberger KM, Wiedenmann E, Böhm S and
Jilg W: Sensitive and accurate quantitation of hepatitis B virus
DNA using a kinetic fluorescence detection system (TaqMan PCR). J
Virol Methods. 85:75–82. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marcellin P, Lau GK, Bonino F, Farci P,
Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin
C, et al: Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B
Study Group: Peginterferon alfa-2a alone, lamivudine alone, and the
two in combination in patients with HBeAg-negative chronic
hepatitis B. N Engl J Med. 351:1206–1217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramezani A, Banifazl M, Mamishi S, Sofian
M, Eslamifar A and Aghakhani A: The influence of human leukocyte
antigen and IL-10 gene polymorphisms on hepatitis B virus outcome.
Hepat Mon. 12:320–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z
and Wei H: TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP
expression on human NK cells contributes to HBV persistence. PLoS
Pathog. 8:e10025942012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panjaworayan N, Payungporn S, Poovorawan Y
and Brown CM: Identification of an effective siRNA target site and
functional regulatory elements, within the hepatitis B virus
posttranscriptional regulatory element. Virol J. 7:2162010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao R, Nie H, Cai D, Zhang J, Liu H, Yan
R, Cuconati A, Block TM, Guo JT and Guo H: Inhibition of hepatitis
B virus replication by the host zinc finger antiviral protein. PLoS
Pathog. 9:e10034942013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krebs K, Böttinger N, Huang LR,
Chmielewski M, Arzberger S, Gasteiger G, Jäger C, Schmitt E, Bohne
F, Aichler M, et al: T cells expressing a chimeric antigen receptor
that binds hepatitis B virus envelope proteins control virus
replication in mice. Gastroenterology. 145:456–465. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding S and Robek MD: Cytidine deamination
and cccDNA degradation: A new approach for curing HBV? Hepatology.
60:2118–2121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soler MC, Molina JL, Díaz HA, Pinto VC,
Barrios YL, He K, Roller M and Weinstein-Oppenheimer CR: Effect of
the standardized Cimicifuga foetida extract on Hsp 27
expression in the MCF-7 cell line. Biol Res. 44:243–249. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang JJ and Lewin SR: Immunopathogenesis
of hepatitis B virus infection. Immunol Cell Biol. 85:16–23. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SO, Kumar M and Gupta S: TGF-β and
iron differently alter HBV replication in human hepatocytes through
TGF-β/BMP signaling and cellular microRNA expression. PLoS One.
7:e392762012. View Article : Google Scholar : PubMed/NCBI
|