Introduction
Warfarin remains the most commonly prescribed oral
anticoagulant drug (1,2), and has long been applied in the
prevention and treatment of various venous and arterial
thromboembolic disorders, such as deep venous thromboembolism,
pulmonary embolism, prosthetic heart valves and atrial fibrillation
(AF) (3). Due to inter-individual
variation of warfarin dosing, inadequate or excessive warfarin may
result in thromboembolism or bleeding. Therefore, the warfarin dose
should be monitored and controlled within a narrow therapeutic
window in clinical application. In fact, in patients undergoing
warfarin therapy, the prothrombin time (PT), expressed as the
international normalized ratio (INR), was maintained within the
therapeutic range, thereby providing effectiveness and safety of
warfarin (4). A number of studies have
shown that patients with an INR above the upper limit of the
therapeutic range have an increased risk of bleeding (5,6), and
patients with an INR below the lower limit have an increased risk
of thromboembolic events (7,8).
The inter-individual variation of warfarin dose
response is caused by various factors, such as patient
demographics, environment, clinical background, and particularly,
genetic polymorphism (9,10). Therefore, the Food and Drug
Administration proposed the genetic testing for estimating initial
warfarin dose in 2007 (11). From then
on, numerous studies have reported a variety of genetic
polymorphisms in genes associated with the warfarin
pharmacodynamics and pharmacokinetics. Compared with subjects with
wild-type genes, subjects with these polymorphisms require lower or
higher warfarin doses to achieve adequate anticoagulant effect
(12). Among these genes associated
with the warfarin dose responses, cytochrome P450 2C9 (CYP2C9) and
vitamin K epoxide reductase complex 1 (VKORC1) are two of the most
prominent genes and their genetic polymorphisms could account for
30 to 40% of the warfarin dose variation (13,14).
During the initiation period of warfarin therapy,
patients are at greatest risk of overanticoagulation and bleeding
complications, particularly in those carrying mutant CYP2C9 alleles
(15). These early complications are
mainly caused by inter-individual variation in response to the
loading dose warfarin. Therefore, numerous warfarin-dosing
algorithms have been developed to predict the warfarin maintenance
dose and improve the effect and safety of warfarin therapy
(16,17). Furthermore, previous studies have
mainly focused on developing a warfarin-dosing algorithm, but these
algorithms also require verification and evaluation for its
clinical value. In addition, polymorphism frequencies are different
among ethnic groups, and compared with a Caucasian population,
Chinese patients are more sensitive to warfarin (3). Therefore, it is necessary to develop a
warfarin-dosing algorithm based on detected genetic polymorphisms
and evaluate its clinical application in patients of the Han
nationality undergoing warfarin therapy.
The present study aimed to develop a novel
individualized dosing regimen based on genetic polymorphisms in
CYP2C9 and VKORC1 in AF patients of Han nationality. The clinical
application of this warfarin-dosing algorithm was further evaluated
to predict the warfarin maintenance dose in a second unrelated
population of AF patients in a randomized and controlled trial.
Patients and methods
Subjects
A total of 202 AF patients of Han nationality (111
male and 91 female, with an average age of 42.3±7.1 years) were
enrolled in the study between January 2014 and June 2015. The
inclusion criteria of the study were as follows: i) Patients had
received continuous warfarin therapy for ≥3 month; ii) all the
cases maintained a balanced diet without smoking and drinking; iii)
no medications were taken that may interfere with the
pharmacokinetics or pharmacodynamics of warfarin; iv) there was no
hepatic or renal impairments in patients by laboratory tests; and
v) no hemorrhage or thrombosis complications occurred during the
warfarin therapy. Demographics of age, gender, height, body weight
and maintenance doses of warfarin were collected from each AF
patients. All the AF patients received chest X-ray film,
electrocardiogram and echocardiography. On arrival at the hospital,
plasma and blood samples were obtained. All 202 AF patients were
divided into cohorts 1 (122 cases) and 2 (60 cases). Cohort 1 was
used to produce the model for estimating warfarin dose, and cohort
2 was used to evaluate the feasibility of clinical application of
warfarin-dosing algorithm in AF patients. The study was approved by
the Ethics Committee of Shanghai Seventh People's Hospital and
conducted in accordance with the Declaration of Helsinki. Written
informed consent was obtained from each patient.
Determination PT and INR
Venous blood was collected from 202 enrolled AF
patients in tubes containing anticoagulant 3.2% sodium citrate (the
blood to anticoagulant ratio was 9:1). Centrifugation was performed
at 2,000 × g for 10 min to separate plasma. The PT was measured
using a Thromborel S reagent kit (Siemens Healthcare Diagnostic,
Erlangen, Germany) on a Sysmex CA7000 analyzer (Sysmex Corp., Kobe,
Japan). INR was calculated based on PT.
Genotyping of CYP2C9 and VKORC1
Peripheral venous blood was obtained from AF
patients, and leukocyte genomic DNA was extracted by a DNA
extraction kit (Qiagen, Crawley, UK), following the manufacturer's
protocols. Subsequently, the DNA was frozen at −20°C until
analysis. The polymorphisms of CYP2C9 (rs1057910) and VKORC1
(rs9923231) were determined by the polymerase chain
reaction-restriction fragment length polymorphism (PCR-RFLP)
method, and the protocols were in accordance with the techniques
described previously, with certain modifications (18). DNA samples were amplified by PCR in a
final volume of 20 µl, containing 0.25 µM primer, 0.1 mM
deoxynucleoside triphosphate (dNTP), 0.625 units Taq polymerase
(Molzyme, Bremen, Germany) and 0.5 µg genomic DNA in 10 mM Tris
[tris(hydroxymethyl)aminomethane]-HCl (pH 9.0) solution (1.5 mM
MgCl2, 50 mM KCl and 0.1% Triton X-100). The PCR primers
were designed and synthesized by Biomedical Engineering Co., Ltd.
(Shanghai, China). Sequences for the forward and reverse primers of
CYP2C9 were 5′-CACGAGGTCCAGAGATACA-3′ and
5′-GGAATGAGATAGTTTCTGAATTTAAT-3′, respectively. Sequences for the
forward and reverse primers of VKORC1 were
5′-GCCAGCAGGAGAGGGAAATA-3′ and 5′-AGTTTGGACTACAGGTGCCT-3′,
respectively. Cycling conditions for CYP2C9 were as follows:
Initial denaturation at 94°C for 5 min, followed by 30 cycles of
denaturation at 94°C for 40 sec, annealing at 56°C for 30 sec, and
extension at 72°C for 30 sec, with a final extension step at 72°C
for 10 min. Cycling conditions for VKORC1 were as follows: Initial
denaturation at 94°C for 5 min, followed by 25 cycles of
denaturation at 94°C for 1 min, annealing at 59°C from 1 min, and
extension at 72°C for 1 min, with a final extension step at 72°C
for 10 min. The amplified PCR products were incubated with
restriction enzymes (Fermentas; Thermo Fischer Scientific, Waltham,
MA, USA) KpnI (CYP2C9) and MspI (VKORC1) in a final
volume of 30 µl at 37°C overnight to ensure complete digestion.
Finally the DNA fragments were separated by 3% agarose gel
electrophoresis, and bands were stained with ethidium bromide and
visualized with a UV transilluminator.
Clinical application of the
warfarin-dosing algorithm
The 60 patients of cohort 2 were randomly divided
into control group (30 cases) and experimental group (30 cases). In
the experimental group, the genotypes of CYP2C9 and VKORC1 were
determined to calculate the warfarin dose (predicted dose).
Patients were administered oral warfarin based on the ‘predicted
dose’ for 7 days, and subsequently the warfarin doses were adjusted
according to the measured INR values. In the control group,
warfarin was initiated at 2.5 mg/day (standard current initiation
dose of warfarin in China), and it was adjusted based on the INR
values. Follow-up was carried out after initiation of warfarin
therapy for 50 days, and the INR was measured daily during
hospitalization. When the measured INR values of patients were
within the therapeutic range of 1.8–3.0 for ≥7 days, a stable
warfarin maintenance dose was reached (19). The time to reach a stable warfarin
maintenance dose was recorded and compared between the control and
experimental groups.
Statistical analysis
All the quantitative data are expressed as mean ±
standard deviation. The commercially available software SPSS 19.0
(IBM, Corp., Armonk, NY, USA) was used to carry out statistical
analysis. In the homogeneity test for variance, the unpaired
Student's t-test was used to compare the quantitative data and
Fisher's exact test was used to compare the enumeration data. The
Kaplan-Meier method was performed to analyze the time interval
between initiation of warfarin therapy and warfarin maintenance
dose, and their difference between the control and experimental
groups was compared with the log-rank test. Pearsons correlation
test was performed to analyze the linear correlation between
predicted dose and actual dose of warfarin. The associations
between warfarin dose and other variables were analyzed by
multivariate linear regression and to develop a novel
warfarin-dosing algorithm. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
There was a total of 202 AF patients of Han
nationality who participated in the study. Among them, 122 patients
with stable control of anticoagulation were recruited to develop
warfarin-dosing algorithm based on the CYP2C9 and VKORC1 genotypes
(cohort 1), and 60 patients were recruited to evaluate the clinical
application of warfarin-dosing algorithm (cohort 2). The patient
demographics of age, gender, height, weight, PT, INR, left atrial
size, ejection fraction, warfarin dose requirements and genotype
are shown in Table I.
 | Table I.Characteristics of the study
populations. |
Table I.
Characteristics of the study
populations.
| Characteristics | Cohort 1 n=122 | Cohort 2 n=60 |
|---|
| Age, years | 58.7 | 56.5 |
| Male/female, n | 76/46 | 35/25 |
| Height, cm | 165.6 | 165.1 |
| Weight, kg | 62.1 | 63.3 |
| PT, sec | 24.3 | 23.7 |
| INR |
2.4 |
2.5 |
| Left atrial size,
mm | 45.7 | 49.7 |
| Ejection fraction,
% | 53.2 | 55.2 |
| Daily warfarin dose,
mg |
2.8 |
2.8 |
| CYP2C9 genotype,
n |
|
|
*1/*1 | 113 |
54 |
|
*1/*3 |
9 |
6 |
| VKORC1 genotype,
n |
|
| AA | 105 |
49 |
| AG |
15 |
10 |
| GG |
2 |
1 |
CYP2C9 and VKORC1 genotyping
All 122 samples were genotyped for the CYP2C9
(rs1057910) and VKORC1 (rs9923231), and these two polymorphisms
previously have been reported to affect warfarin-dose requirement.
There were 113 subjects with homozygous CYP2C9*1/*1 and 9 subjects
with heterozygous CYP2C9*1/*3. For VKORC1 genotyping, there were
105 subjects with homozygous AA genotype, 15 subjects with
heterozygous AG genotype and 2 subjects with homozygous GG
genotype. The observed genotype frequency of CYP2C9 and VKORC1
showed no deviation from Hardy-Weinberg equilibrium
(P>0.05).
Associations of warfarin dose with
CYP2C9 and VKORC1
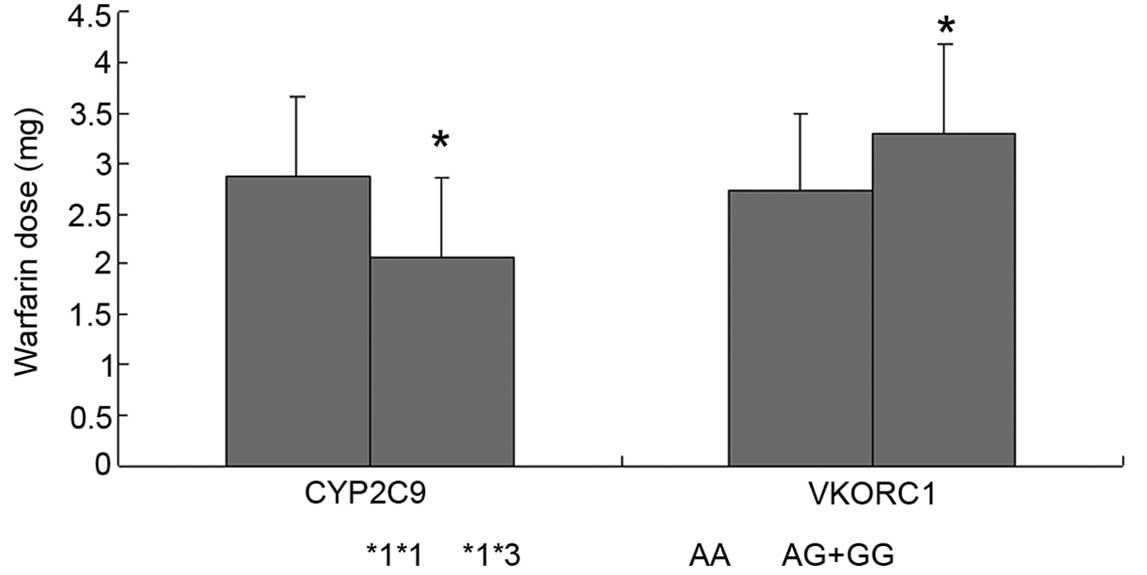
The mean warfarin daily dose requirement was
2.06±0.79 mg in heterozygous CYP2C9*1/*3 patients, which was
significantly lower than that in homozygous wild-type CYP2C9*1/*1
patients (2.88±0.77 mg, P<0.05). The mean warfarin daily dose
requirement was 3.30±0.87 mg in patients with the VKORC1 AG and GG
genotype, which was significantly higher than that in patients with
AG genotype (2.74±0.76 mg, P<0.05) (Fig. 1). The multivariate regression analysis
was performed to detect variables influencing warfarin dose. The
results showed that age, height, and CYP2C9 and VKORC1 genotypes
can develop models for estimating warfarin dose, and the best
warfarin-dosing algorithm has the largest R2 value (Table II).
 | Table II.Regression equation for modeling
warfarin daily dose requirements based on age, height and
genotype. |
Table II.
Regression equation for modeling
warfarin daily dose requirements based on age, height and
genotype.
| Model, X
variables | Regression
equation | P-value | R2 for
model, % |
|---|
| Age, years | y=2.81–0.0132
(age) | <0.01 | 15.8 |
| Height | y=2.17+0.0227
(height) | <0.01 | 14.2 |
| CYP2C9 genotype | y=2.01–0.371
(CYP*3) | <0.01 | 18.7 |
| VKORC1 genotype | y=1.43+0.274
(VKORC1) | <0.01 | 17.1 |
| Age, height, and
CYP2C9 and VKORC1 genotypes | Dose=0.438–0.0143
(age)+0.0149 (height)-0.324 (CYP*3)+0.209 (VKOR) | <0.01 | 56.4 |
|
Clinical application of
warfarin-dosing algorithm in AF patients
The warfarin-dosing algorithm was further evaluated
in a second unrelated population of AF patients, who were treated
with oral warfarin and with stable control of anticoagulation
(cohort 2). The patient characteristics and demographics of cohort
2 are shown in Table I. There were no
significant differences in age, gender, height, weight, PT, INR,
left atrial size, ejection fraction and warfarin dose requirements
between cohorts 1 and 2. No significant differences were identified
in the frequency of the CYP2C9 or VKORC1 genotypes between the two
cohorts. The 60 patients of cohort 2 were randomly divided into
control group (30 cases received 2.5 mg/day of oral warfarin for
the first 7 days) and experimental group (30 cases received oral
warfarin based on the ‘predicted dose’ from warfarin-dosing
algorithm for the first 7 days), and subsequently warfarin doses
were adjusted according to INR values. The two groups showed
homogeneity in age, gender, height, weight, PT, INR, left atrial
size and ejection fraction (Table
III). During the 50-day follow-up, 19 patients (63.3%) in the
control group and 26 patients (86.7%) in the experimental group
received the warfarin maintenance dose, and patients with the
predicted warfarin dose showed more cases acquiring warfarin
maintenance dose (P<0.05). Among all 45 patients who reached the
stable warfarin maintenance dose, patients in the experimental
group had a shorter time elapse from initiation until stable
warfarin maintenance dose (25.8±1.7 days) than that in the control
group (33.1±1.9 days) (P<0.05) (Table
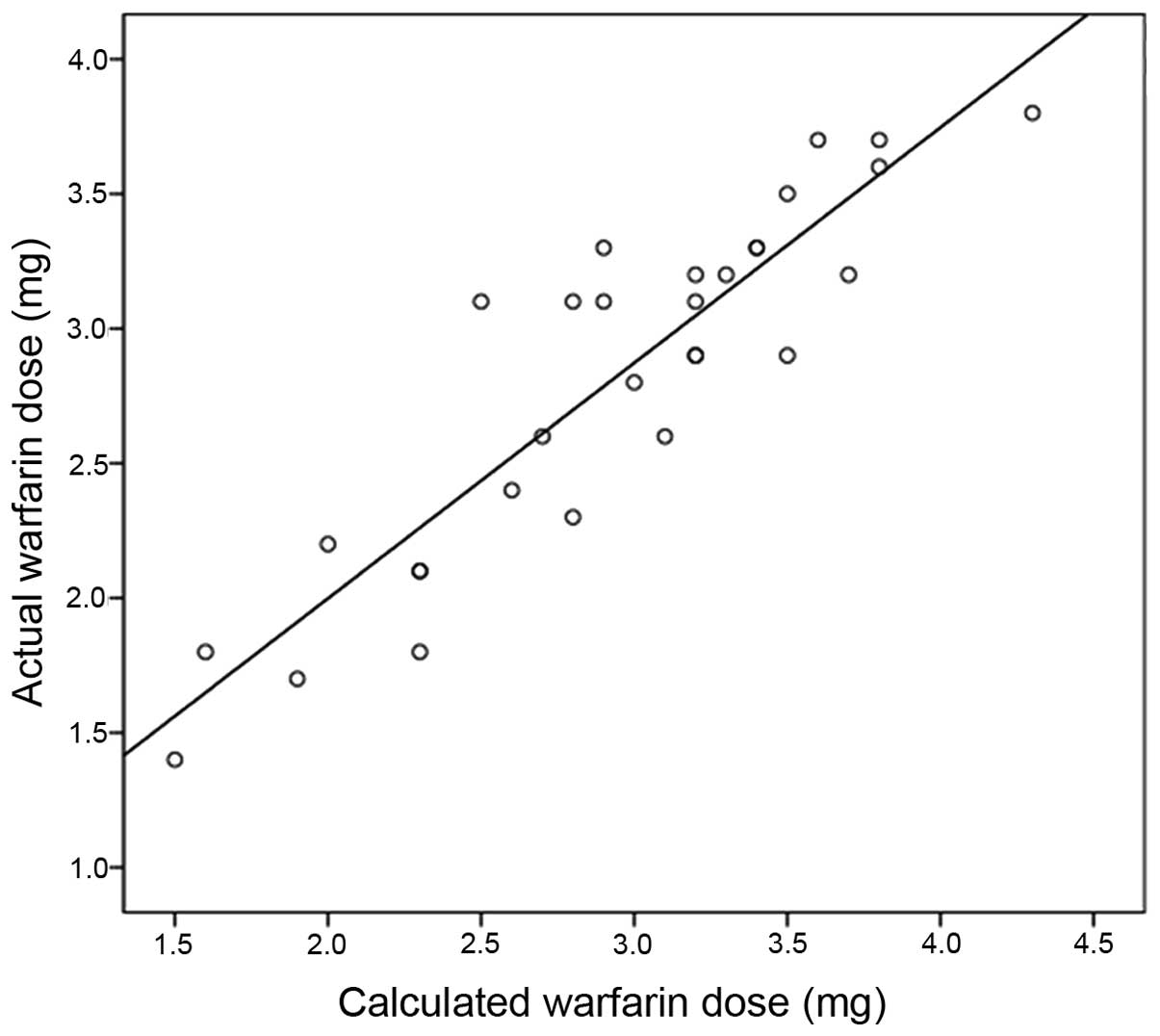
III). In these 45 patients, Pearsons correlation analysis
showed a significant correlation between the calculated warfarin
dose using the warfarin-dosing algorithm and actual dose (r=0.822;
P<0.01) (Fig. 2).
 | Table III.Characteristics of the study
populations in cohort 2. |
Table III.
Characteristics of the study
populations in cohort 2.
| Characteristics | Control group,
n=30 | Experimental group,
n=30 |
|---|
| Age, years | 57.0 | 56.1 |
| Male/female, n | 76/46 | 35/25 |
| Height, cm | 165.0 | 165.3 |
| Weight, kg | 62.1 | 63.3 |
| PT, sec | 23.4 | 24.0 |
| INR | 2.6 | 2.4 |
| Left atrial size,
mm | 49.2 | 50.2 |
| Ejection fraction,
% | 54.5 | 55.8 |
| CYP2C9 genotype,
n |
|
*1/*1 | 27 | 27 |
|
*1/*3 | 3 | 3 |
| VKORC1 genotype,
n |
| AA | 26 | 25 |
| AG | 5 | 5 |
| GG | 1 | 0 |
| Time for obtaining
stable | 33.1±1.9 |
25.8±1.7a |
| warfarin
maintenance dose (day) | (n=19) | (n=26) |
Discussion
AF is the one common cardiac arrhythmia in the
clinical setting with substantial morbidity and mortality. AF
patients have an increased risk of stroke and systemic
thromboembolism (20). The risk of
complications in AF can be reduced by anticoagulant therapy, such
as warfarin (21). However, during the
initiation period of warfarin therapy, patients have a higher
incidence of overanticoagulation and resultant bleeding. Due to the
inter-individual variability in response to warfarin, it is
difficult to makes accurate dose predication. A number of factors,
such as patient demographics, environment, clinical background and
genetic polymorphism, contribute to the inter-individual variation
of the warfarin dose response (9,10). Among
them, genetic polymorphisms in genes are associated with the
warfarin pharmacodynamics, and pharmacokinetics have increasingly
important roles in predicting warfarin dose.
In the present study, the genotypes of CYP2C9 and
VKORC1 were determined in 122 AF patients undergoing warfarin
therapy. The results showed that warfarin dose could be estimated
by variables, such as age, height, and the CYP2C9 and VKORC1
genotypes. A new warfarin-dosing algorithm was also developed and
its validity was confirmed in a second cohort of AF patients.
Compared with the control group, patients with a predicted dose
from the warfarin-dosing algorithm showed more patients acquiring
the warfarin maintenance dose during the 50-day follow-up, a
significantly shorter time elapse from initiation until warfarin
maintenance dose. Among all the patients acquiring the warfarin
maintenance dose, the predicted warfarin dose was significantly
correlated with the actual dose.
CYP2C9 is one isoform of the cytochrome P450 complex
and participates in the metabolism of ~15% of the clinically used
drugs, including non-steroidal anti-inflammatory drugs, angiotensin
II blockers and oral anticoagulants (22). CYP2C9 is located on chromosome 10q24.2,
and its alleles have been identified. The wild-type allele CYP2C9*1
shows normal enzyme activity and a high allelic frequency. The two
allelic variants CYP2C9*2 and *3 have allelic frequencies of ~13
and 7%, and lower enzyme activity in comparison to the wild-type
(CYP2C9*1) (23). In the present
study, the allelic variant CYP2C9*2 was not detected and is
uncommon in the Han population. CYP2C9*3 (rs1057910) is located at
amino acid position 359 in exon 7, and is defined by an A-to-C
nucleotide substitution (1075 A>C), resulting in a
non-synonymous substitution amino acid change from leucine to
isoleucine. The allelic frequency of CYP2C9*3 in this study was
7.8%, and is similar with previous studies, who showed 4.5% allelic
frequency of CYP2C9*3 in the Chinese population (24). The present study also identified that
subjects with the CYP2C9*1/*3 genotype had a lower mean warfarin
daily dose requirement compared with subjects with wild-type
CYP2C9*1/*1. This suggests that in CYP2C9*1/*3 genotype subjects,
the enzyme activity was reduced and metabolism and clearance of
warfarin was delayed, thus enhancing their sensitivity to warfarin
and decreasing the warfarin daily dose requirement.
VKORC1 is responsible for the transformation of
epoxide (vitamin K-2,3-epoxide) to its reduced form (vitamin K
hydroquinone), this process is required for the γ-carboxylation of
the vitamin K-dependent coagulation factors (factors II, VII, IX
and X). Warfarin inhibits the above process catalyzed by the VKORC1
enzyme complex, thus preventing the regeneration of the reduced
form of vitamin K (25). Mutations in
VKORC1 can lead to warfarin resistance (26,27).
rs9923231 (−1639 G>A) is the most common SNP of VKORC1 gene. In
the Caucasian population, GG is the highest genotype and AA is the
lowest (3). However, in the Chinese
population, AA is the highest genotype and its frequency is >83%
(24,28). The present study confirmed this finding
and detected 115 subjects with the AA genotype in 122 cases.
Furthermore, subjects with the AG+GG genotype of VKORC1 had a
higher mean warfarin daily dose requirement than subjects with
wild-type AA genotype. This indicates that the G allele produces
VKORC1 with a lower activity and results in warfarin resistance and
a higher warfarin daily dose requirement. The present results are
in accordance with another study showing that subjects with the GG
genotype showed a higher warfarin dose compared to the subjects
with the AG or AA genotype (29).
The present study showed that the warfarin
maintenance dose can be best estimated by variables, such as age,
height, and CYP2C9 and VKORC1 genotypes. A dosing algorithm was
developed and its efficacy was further validated in an unrelated
cohort of AF patients undergoing warfarin therapy. A prospective
clinical study was performed to assess the dosing algorithm in the
initiation stage of warfarin therapy in AF patients. During the
50-day follow-up, more patients received the warfarin maintenance
dose in the experimental group (86.7%) compared to the control
group (63.3%). Among all the patients acquiring the warfarin
maintenance dose, Pearsons correlation analysis showed that the
predicted dose was prominently correlated with the actual dose,
making effective prediction of the actual warfarin maintenance dose
by the warfarin-dosing algorithm. The predicted warfarin
maintenance dose, which is based on a combination of genetic
factors and non-genetic factors of AF patients, could effectively
reduce the time elapse from initiation until stable warfarin
maintenance dose. Due to the limited cases and observation time,
our warfarin-dosing algorithm requires further investigation and
clinical application. With more warfarin-related genes identified
and confirmed, and more non-genetic factors revealed, the warfarin
maintenance dose predicted from the algorithm would be more
accurate, thereby improving the efficacy and safety of warfarin
therapy.
In conclusion, a new warfarin-dosing algorithm was
developed based on age, height, and CYP2C9 and VKORC1 genotypes,
and it can shorten the time elapse from initiation until warfarin
maintenance dose in AF patients. This warfarin-dosing algorithm can
promote patients acquiring warfarin maintenance dose during the
follow-up, and shorten the time elapse from initiation until
warfarin maintenance dose. Further study is required to expand the
sample size and assess the clinical application in AF patients
undergoing warfarin therapy.
Acknowledgements
The present study was funded by the Pudong Health
and Family Planning Commission (grant no. PW2013A-24) and the
Talents Training Program of Shanghai Seventh People's Hospital
(grant no. XX2013-001)
References
|
1
|
Ansell J, Hirsh J, Poller L, Bussey H,
Jacobson A and Hylek E: The pharmacology and management of the
vitamin K antagonists: The Seventh ACCP Conference on
Antithrombotic and Thrombolytic Therapy. Chest. 126(Suppl 3):
204S–233S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsh J, Guyatt G, Albers GW and
Schunemann HJ: Proceedings of the Seventh ACCP Conference on
Antithrombotic and Thrombolytic Therapy: Evidence-based guidelines.
Chest. 126(Suppl 3): 172S–696S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lam MP and Cheung BM: The pharmacogenetics
of the response to warfarin in Chinese. Br J Clin Pharmacol.
73:340–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hawes EM and Viera AJ: Anticoagulation:
Monitoring of patients receiving anticoagulation. FP Essent.
422:24–30. 2014.PubMed/NCBI
|
|
5
|
No authors listed: Effect of long-term
oral anticoagulant treatment on mortality and cardiovascular
morbidity after myocardial infarction. Anticoagulants in the
Secondary Prevention of Events in Coronary Thrombosis (ASPECT)
Research Group. Lancet. 343:499–503. 1994.PubMed/NCBI
|
|
6
|
Cannegieter SC, Rosendaal FR, Wintzen AR,
van der Meer FJ, Vandenbroucke JP and Briët E: Optimal oral
anticoagulant therapy in patients with mechanical heart valves. N
Engl J Med. 333:11–17. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fihn SD, McDonell M, Martin D, Henikoff J,
Vermes D, Kent D and White RH: Warfarin Optimized Outpatient
Follow-up Study Group: Risk factors for complications of chronic
anticoagulation. A multicenter study. Ann Intern Med. 118:511–520.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
No authors listed: Adjusted-dose warfarin
versus low-intensity, fixed-dose warfarin plus aspirin for
high-risk patients with atrial fibrillation: Stroke prevention in
atrial fibrillation III randomised clinical trial. Lancet.
348:633–638. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirsh J, Dalen J, Anderson DR, Poller L,
Bussey H, Ansell J and Deykin D: Oral anticoagulants: Mechanism of
action, clinical effectiveness, and optimal therapeutic range.
Chest. 119(Suppl 1): 8S–21S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi H and Echizen H:
Pharmacogenetics of warfarin elimination and its clinical
implications. Clin Pharmacokinet. 40:587–603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Approves Updated Warfarin FDA (Coumadin)
Prescribing information: New genetic information may help providers
improve initial dosing estimates of the anticoagulant for
individual patients. http://www.fda.gov/bbs/topics/NEWS/2007/NEW01684.htmlAccessed.
February 19–2009
|
|
12
|
Mazzaccara C, Conti V, Liguori R, Simeon
V, Toriello M, Severini A, Perricone C, Meccariello A, Meccariello
P, Vitale DF, et al: Warfarin anticoagulant therapy: A Southern
Italy pharmacogenetics-based dosing model. PLoS One. 8:e715052013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bodin L, Verstuyft C, Tregouet DA, Robert
A, Dubert L, Funck-Brentano C, Jaillon P, Beaune P, Laurent-Puig P,
Becquemont L, et al: Cytochrome P450 2C9 (CYP2C9) and vitamin K
epoxide reductase (VKORC1) genotypes as determinants of
acenocoumarol sensitivity. Blood. 106:135–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vecsler M, Loebstein R, Almog S, Kurnik D,
Goldman B, Halkin H and Gak E: Combined genetic profiles of
components and regulators of the vitamin K-dependent
gamma-carboxylation system affect individual sensitivity to
warfarin. Thromb Haemost. 95:205–211. 2006.PubMed/NCBI
|
|
15
|
Aithal GP, Day CP, Kesteven PJL and Daly
AK: Association of polymorphisms in the cytochrome P450 CYP2C9 with
warfarin dose requirement and risk of bleeding complications.
Lancet. 353:717–719. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ekladious SM, Issac MS, El-Atty Sharaf SA
and Abou-Youssef HS: Validation of a proposed warfarin dosing
algorithm based on the genetic make-up of Egyptian patients. Mol
Diagn Ther. 17:381–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Liu R, Luo ZY, Yan H, Huang WH, Yin
JY, Mao XY, Chen XP, Liu ZQ, Zhou HH, et al: Comparison of the
predictive abilities of pharmacogenetics-based warfarin dosing
algorithms using seven mathematical models in Chinese patients.
Pharmacogenomics. 16:583–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scibona P, Redal MA, Garfi LG, Arbelbide
J, Argibay PF and Belloso WH: Prevalence of CYP2C9 and VKORC1
alleles in the Argentine population and implications for
prescribing dosages of anticoagulants. Genet Mol Res. 11:70–76.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voora D, Eby C, Linder MW, Milligan PE,
Bukaveckas BL, McLeod HL, Maloney W, Clohisy J, Burnett RS, Grosso
L, et al: Prospective dosing of warfarin based on cytochrome P-450
2C9 genotype. Thromb Haemost. 93:700–705. 2005.PubMed/NCBI
|
|
20
|
Senoo K, Lane D and Lip GY: Stroke and
bleeding risk in atrial fibrillation. Korean Circ J. 44:281–290.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lip GY and Lane DA: Stroke prevention in
atrial fibrillation: A systematic review. JAMA. 313:1950–1962.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou SF, Zhou ZW and Huang M:
Polymorphisms of human cytochrome P450 2C9 and the functional
relevance. Toxicology. 278:165–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vogl S, Lutz RW, Schönfelder G and Lutz
WK: CYP2C9 genotype vs. metabolic phenotype for individual drug
dosing - a correlation analysis using flurbiprofen as probe drug.
PLoS One. 10:e01204032015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Zou Y, Wang X, Huang X, Sun Y, Wang
Y, Dong L and Jiang H: Warfarin dosage response related
pharmacogenetics in Chinese population. PLoS One. 10:e01164632015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oldenburg J, Watzka M, Rost S and Müller
CR: VKORC1: Molecular target of coumarins. J Thromb Haemost.
5(Suppl 1): 1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rost S, Fregin A, Ivaskevicius V,
Conzelmann E, Hörtnagel K, Pelz HJ, Lappegard K, Seifried E,
Scharrer I, Tuddenham EG, et al: Mutations in VKORC1 cause warfarin
resistance and multiple coagulation factor deficiency type 2.
Nature. 427:537–541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harrington DJ, Gorska R, Wheeler R,
Davidson S, Murden S, Morse C, Shearer MJ and Mumford AD:
Pharmacodynamic resistance to warfarin is associated with
nucleotide substitutions in VKORC1. J Thromb Haemost. 6:1663–1670.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Ge W, Yu F and Zhu H: Impact of
VKORC1 gene polymorphism on interindividual and interethnic
warfarin dosage requirement-a systematic review and meta analysis.
Thromb Res. 125:e159–e166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sconce EA, Khan TI, Wynne HA, Avery P,
Monkhouse L, King BP, Wood P, Kesteven P, Daly AK and Kamali F: The
impact of CYP2C9 and VKORC1 genetic polymorphism and patient
characteristics upon warfarin dose requirements: Proposal for a new
dosing regimen. Blood. 106:2329–2333. 2005. View Article : Google Scholar : PubMed/NCBI
|