Introduction
A previous study has shown that the disequilibrium
of the microenvironment in melanocytes (MCs) and the regression of
cell adhesion and migration, which are caused by psychological,
nerve and immunity factors, are the main pathogeneses of vitiligo
(1). In the initial effective stage of
vitiligo treatment, the pigmentation occurs from the hickie
follicular orifice, and extends to be flakiness. There are
amelanotic melanocytes (AMMCs) in the hair follicle outer root
sheath, which are the most important MC reservoir for skin lesion
repigmentation (2). During the
repigmentation course, MCs experience the stage of activation,
migration, adhesion and proliferation (3). The dynamic equilibrium of aggregation and
disaggregation of actin cytoskeleton is the important regulating
factor for cell adhesion and migration.
Fructus Ligustri Lucidi (FLL), as a member of
Chinese herbs, has an important role in the treatment of disease.
The active ingredients of FLL include oleanolic acid, butyl
alcohol, tyrosol, quercetin, palm acid, stearic acid,
polysaccharide, oleic acid, flax and linum (4). Quercetin and oleanolic acid are two most
important monomers of FLL extract. Oleanolic acid has the
anti-inflammatory, antimutagenic, antioxidant, anti-virus and liver
protection activity (5,6). Quercetin has the function of
antioxidation, anti-inflammation, lowering blood pressure,
anti-platelet aggregation, antitumor, anti-atherosclerosis and
regulating immunity (7–9). Previous studies have reported that the
FLL extract and its monomers oleanolic acid and quercetin can
improve the tyrosinase activity of MCs, and stimulate the
melanogenesis (10–14). However, their effects on adhesion,
migration of MCs and the intracellular actin are seldom reported.
In the present study, the effects of the FLL extract and its
monomers oleanolic acid and quercetin on the adhesion and migration
of human epidermal MCs and intracellular actin were investigated,
and the associated mechanism was discussed. The aim was to provide
a basis for further application of FLL to treatment of
vitiligo.
Materials and methods
Materials
The FLL extract was prepared by ethanol extraction
from FLL (China's National Institute for the Control of
Pharmaceutical and Biological Products, Beijing, China). FLL was
soaked in 10 ml of 90% ethanol (Sigma-Aldrich, St. Louis, MO, USA)
for 1 week at room temperature. Following filtration, the
extraction solution was obtained. The ethanol was removed by
concentration, and the cream mixture was obtained. The 200 mg/ml
ethanol solution of the FLL extract was prepared. Using fresh M254
medium (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou,
China), the concentration of the FLL extract was adjusted to 0.6,
0.3, 0.15, 0.075 and 0.0375 mg/ml, respectively. Quercetin and
oleanolic acid were provided by China's National Institute for the
Control of Pharmaceutical and Biological Products. The
concentration of quercetin was adjusted to 80, 40, 20, 10 and 5 µM,
respectively, and the concentration of oleanolic acid was adjusted
to 24, 12, 6, 3 and 1.5 µM, respectively.
Culture and identification of MCs
The circumcision specimens were obtained from
10–25-year-old normal adolescent males who underwent circumcision
in the Department of Urology (The Fourth Affiliated Hospital of
Jinan University, Guangzhou Red Cross Hospital, Guangzhou,
Guangdong, China). The patients had no history of pigmentation
disorder such as vitiligo. The study was approved by the ethics
committee of the Fourth Affiliated Hospital of Jinan University.
Written informed consent was obtained from the patients. The
circumcision specimens were soaked in 70% alcohol for 5–10 min,
followed by rinsing with phosphate-buffered saline (PBS;
Sigma-Aldrich) 6–12 times. The specimens were transferred to the
sterile petri dish containing a small amount of PBS. The dermis and
subcutaneous fatty tissue were removed. The remaining specimens
were separated into square pieces (2×2 mm), and tiled in the
sterile petri dish. 0.25% Dispase II (Fuzhou Maixin Biotechnology
Development Co., Ltd.) was added for digestion at 4°C for 16–24 h,
followed by incubation at 37°C for 1 h. The epidermis was separated
and was washed with PBS 3–5 times. Following digestion for 5 min,
M254 complete medium containing 10% fetal calf serum (Fuzhou Maixin
Biotechnology Development Co., Ltd.) was added to terminate
digestion. The MCs were pipetted from the epidermis, and the cell
suspension was obtained, followed by filtering with a 200-mesh
sieve (Fuzhou Maixin Biotechnology Development Co., Ltd.).
Following centrifugation at 225 × g for 3 min, cell counting was
performed. After 24 h of culture using M254 culture medium (Fuzhou
Maixin Biotechnology Development Co., Ltd.), the first culture
medium was changed, followed by a change of culture medium every 3
days. After primary culture for 8–15 days, the MCs were 80%
confluent. The cells were inoculated to the new sterile flasks for
continued culture. The 3–5 generation of MCs were used for further
experiments.
MCs were identified by the L-DOPA staining and
HMB-45 immunochemical staining method. For the former method, the
cell climbing slice was prepared and was fixed with 4%
paraformaldehyde (Sigma-Aldrich) for 25 min. The slice was
incubated in L-DOPA staining solution (Shanghai Sangon Biological
Engineering Co., Ltd., Shanghai, China) for 4 h, followed by
washing with water for 5 min. For the latter method, the cell
climbing slice was fixed with 4% paraformaldehyde for 25 min,
followed by washing with PBS for 5 min. The mouse anti-human HMB-45
monoclonal antibody (cat no. SG7870; used 1:50; Shanghai Sangon
Biological Engineering Co., Ltd.) was used as the primary antibody,
and the anti-mouse IgG-HRP (cat no. SC2023; used 1:500; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used as the secondary
antibody. The diaminobenzidine coloration, counterstain,
dehydration, transparency and mounting were performed according to
the manufacturer's protocol. PBS replacing the primary antibody was
used as the negative control.
Determination of cell proliferation
activity with the XTT assay
MCs were inoculated in 96-well plates
(5×104 cell/ml), 100 µl for each well, followed by
culture for 24 h. The complete culture medium containing different
concentrations of the drugs was added. The culture medium without
the drug was used as the control. After culture for 24, 48 and 72
h, the cell morphology was observed under a DVM6 optical microscope
(Leica Science Lab, Berlin, Germany). XTT/PMS solution (50 µl;
Sigma-Aldrich) was added to the well, followed by incubation at
37°C for 4 h. The absorbance value was determined at 490 nm. The
concentration without any cytotoxicity was selected for the cell
adhesion and migration determination assays.
Determination of cell adhesion
MCs were cultured with complete culture medium
containing different concentrations of the drugs for 3 days. M254
medium (50 µl) containing 20 µg/ml fibronectin (Shanghai Sangon
Biological Engineering Co., Ltd.) was added to each well, followed
by incubation at 37°C for 24 h. Following washing with PBS, the
cells were inoculated in 96-well plate coated with fibronectin
(5×105 cell/ml), followed by incubation at 37°C for 4 h.
MCs without drug treatment were used as the control. The cells
without adhesion were rinsed with PBS, and subsequently 50 µl
XTT/PMS solution was added to the well, followed by incubation at
37°C for 4 h. The absorbance value (A) was measured at 490 nm. The
cell adhesion rate was expressed as (A490treatment
group/A490control group) × 100.
Determination of cell migration
The Transwell microporous membrane was coated with
fibronectin (20 µg/ml), followed by drying in the super-clean bench
overnight and washing with PBS. The MCs [100 µl of 2×105
cell/ml, diluted with M254 medium containing 0.1% bovine serum
albumin (BSA)] were added to the upper chamber and 500 µl complete
medium containing different drugs was added to the lower chamber.
The complete medium without drugs was used as the control. After
culture for 24 h, the chamber was removed and the cells in the
upper chamber surface were grazed by a swab. The cells in the lower
chamber were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30
min. The membrane was cut using an operating knife blade, followed
by hematoxylin and eosin staining for 10 min. Three visual fields
were randomly selected under a microscope, and the MCs migrating to
the lower membrane were counted.
Observation of structure and
distribution of actin in MCs
MCs were cultured with complete culture medium
containing drugs for 3 days. MCs without drug treatment were used
as the control. The density of the cells was adjusted to
2×104 cell/ml. The cell climbing slices were prepared,
followed by fixation with paraformaldehyde. Following rupture of
the membrane at room temperature using 0.1% Triton X-100/PBS
(Fuzhou Maixin Biotechnology Development Co., Ltd.), the cells were
blocked using PBS containing 1% BSA for 30 min. Fluorescein
isothiocyanate-phalloidin (5 µg/ml; Fuzhou Maixin Biotechnology
Development Co., Ltd.) was added, followed by staining at room
temperature for 30 min. Following fluorescence mounting, the slices
were observed with an FV1200 confocal laser scanning microscopy
(Olympus Corp., Tokyo, Japan) at 488 nm. Three visual fields were
randomly selected for semiquantitative analysis fluorescence
intensity. The mean optical density (MOD) was expressed as total
cell integral optical density/total cell area.
Statistical analysis
All the statistical analyses were carried out using
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data are
presented as mean ± standard deviation. Comparisons between two
groups were performed using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Culture and identification of normal
MCs
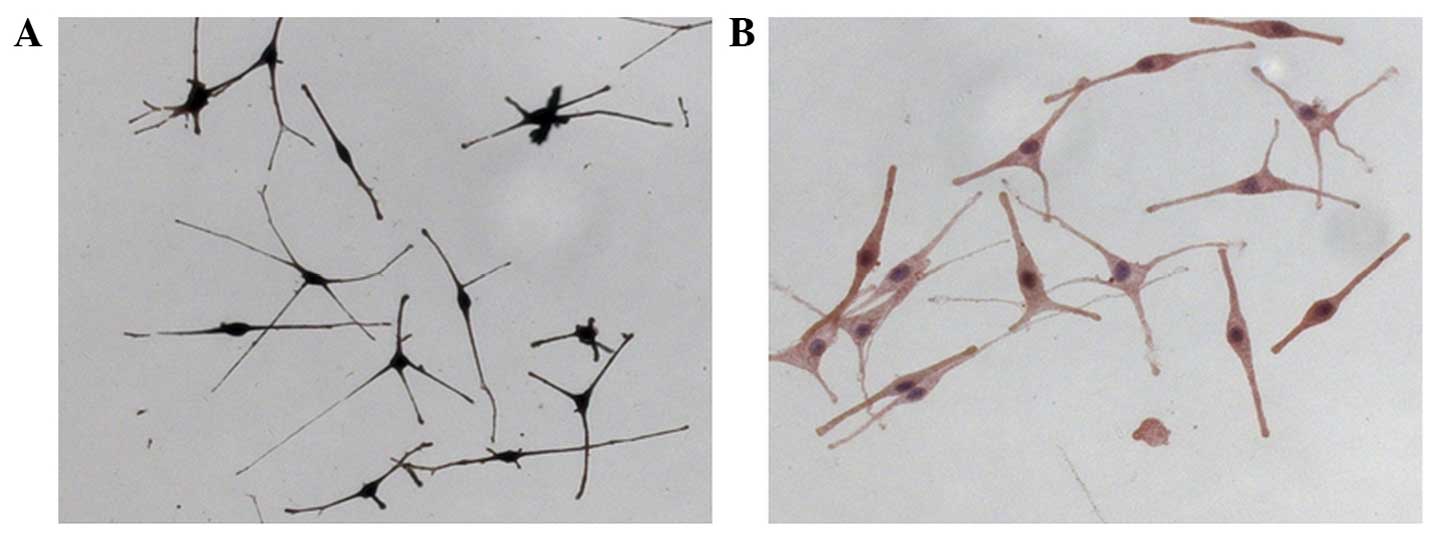
Following culture, the adherent MCs presented
slender dendrites, with fusiform shape or polygon shape. The
proportion of caryon to endochylema was large. There was strong
halation around the cell body caused by refraction, and the cells
were reticulated. Following L-DOPA staining, the endochylema and
dendrite presented brown or black (positive staining) (Fig. 1A). The HMB-45 immunocytochemical stain
was brown, which indicated that the mature MCs had the function of
synthesizing melanin (Fig. 1B).
Cytotoxicity
The result of the XTT assay showed that 0.0375–0.3
mg/ml of the FLL extract, 5–40 µM quercetin and 1.5–12 µM oleanolic
acid had no evident cytotoxicity to MCs. The 0.15 mg/ml FLL
extract, 40 µM quercetin and 12 µM oleanolic acid did not evidently
promote cell proliferation in 24 h (P>0.05), and these
concentrations were selected for the cell migration experiment.
Effects of the FLL extract, quercetin
and oleanolic acid on the adhesion of MCs
Compared with the control group, 0.0375–0.3 mg/ml of
the FLL extract significantly improved the adhesion rate of MCs
(P<0.05) in a dose-dependent manner. When the concentration
researched 0.6 mg/ml, the adhesion rate descended, which may be
caused by cytotoxicity due to a high drug concentration. Quercetin
at 40 µM also significantly improved the adhesion rate compared
with the control group (P<0.05). However, no significance
difference was identified between each oleanolic acid group and
control group (P>0.05) (Table
I).
 | Table I.Effect of the FLL extract, quercetin
and oleanolic acid on the adhesion rate of melanocytes. |
Table I.
Effect of the FLL extract, quercetin
and oleanolic acid on the adhesion rate of melanocytes.
| Group | Concentration | Adhesion rate, % |
|---|
| Control | 0 | 100.00 |
| FLL extract,
mg/ml | 0.0375 |
119.60±11.70a |
|
| 0.0750 |
130.80±14.79a |
|
| 0.1500 |
142.70±14.23b |
|
| 0.3000 |
153.43±19.48b |
|
| 0.6000 |
125.63±15.56a |
| Quercetin, µM | 5 | 88.93±9.93 |
|
| 10 | 98.33±17.40 |
|
| 20 | 110.70±23.29 |
|
| 40 |
133.97±13.02a |
| Oleanolic acid,
µM | 1.5 | 94.37±11.58 |
|
| 3 | 108.59±14.12 |
|
| 6 | 98.30±10.91 |
|
| 12 | 114.93±22.11 |
Effects of the FLL extract, quercetin
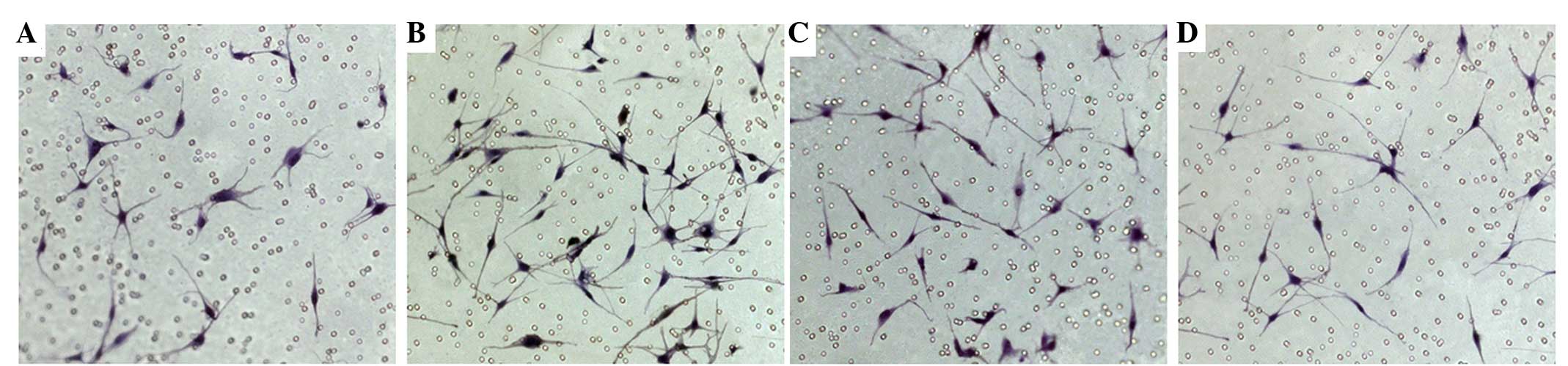
and oleanolic acid on the migration ability of MCs
The number of MCs permeating the microporous
membrane in the 0.15 mg/ml FLL extract and 12 µM oleanolic acid
groups were 43.7 and 30.3, respectively, which were significantly
increased compared with the control group (P<0.01). The number
of cells permeating the microporous membrane in the 40 µM quercetin
group was 23.3, and no significant difference was identified when
compared with the control group (P>0.05) (Fig. 2).
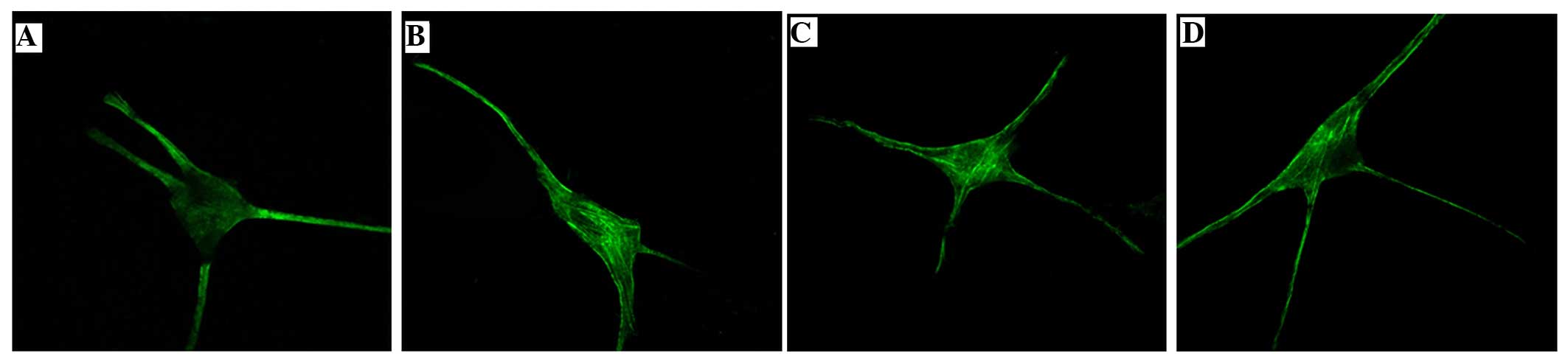
Effects of the FLL extract, quercetin
and oleanolic acid on the structure and distribution of actin in
MCs
In the control group, the fluorescence density was
low and was uniformly and sparsely distributed. This indicated that
the intracellular actin was less, and the stress fiber structure
was not clear. The fluorescence in the 0.15 mg/ml FLL extract, 12
µM oleanolic acid and 40 µM quercetin groups was mainly distributed
around the inner side of the cell membrane and caryon, and numerous
bunched stress fibers could be observed. This indicated that there
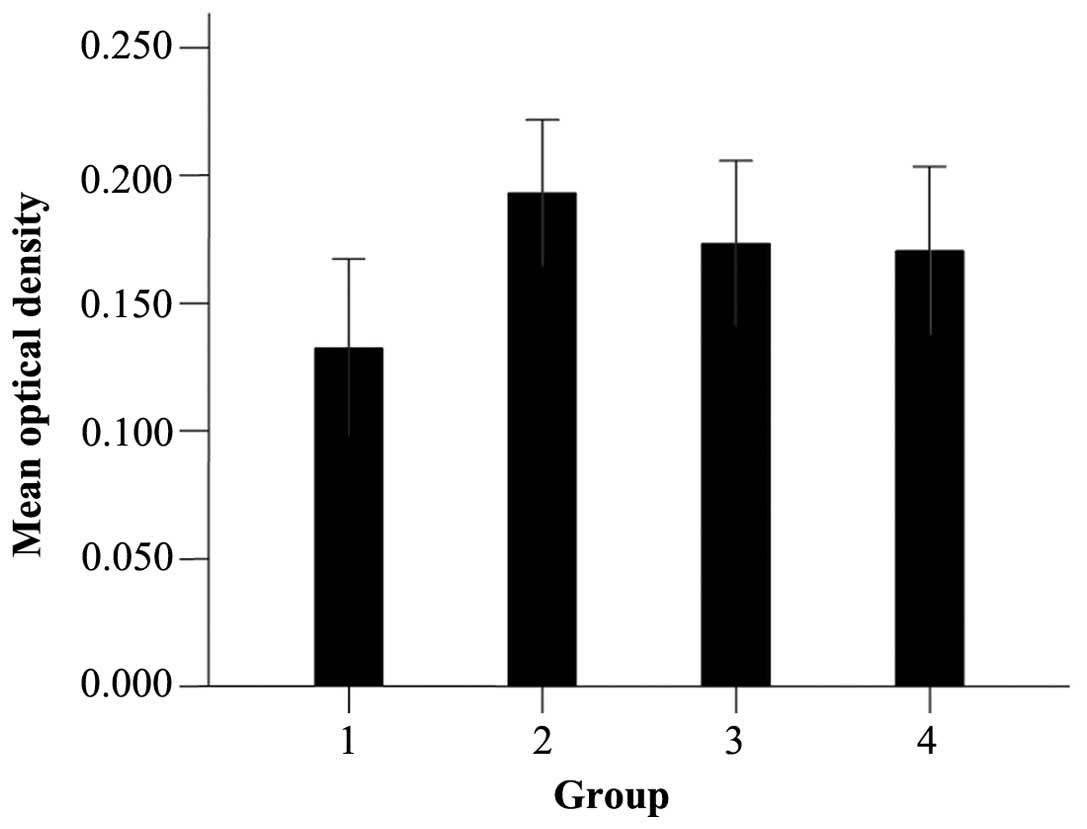
was an aggregation of filamentous fibrous actin (Fig. 3). The MOD of actin expression in the
0.15 mg/ml FLL extract, 12 µM oleanolic acid and 40 µM quercetin
groups was higher compared to the control group (P<0.05)
(Fig. 4).
Discussion
The present study investigated the effects of the
FLL extract and its monomers oleanolic acid and quercetin on the
adhesion and migration of MCs and the structure and distribution of
intracellular actin. The results show that the FLL extract has a
significant stimulatory effect on the adhesion and migration of
MCs. Quercetin can promote the adhesion of MCs and oleanolic acid
can induce the migration of MCs. Liu et al (10) reported that the FLL extract can improve
the tyrosinase activity of MCs. The study by Chang et al
(11) shows that the FLL extract can
significantly promote the proliferation of B10BR MCs, which
indicates that the treatment of vitiligo using the FLL extract may
be associated with its promotion of keratinocyte secreting
cytokine, and a change of the epidermic microenvironment. Zhang
et al (12) identified that
oleanolic acid can increase the expression levels of MC tyrosinase
and tyrosinase-related protein 1 (TRP-1) mRNA, and improve the
activity of tyrosinase and melanin synthesis. In addition,
oleanolic acid can promote the proliferation of MCs. Takeyama et
al (13) studied the influence of
quercetin on melanin formation in MCs and reported that quercetin
can induce the increase of tyrosinase activity of epidermic cells
and increase the melanin content. In addition, the ultrastructure
study shows that following quercetin treatment, more mature
melanosomes assemble at the stratum basale MC with extended cell
dendrites. The study by Nagata et al (14) observed that quercetin can improve the
tyrosinase activity of in vitro-cultured melanoma cells and
normal MCs, increase the synthesis of melanin and decrease the
level of other melanin formation inhibitors. In addition, the
activation of tyrosinase by quercetin can be blocked by
actinomycin-D and cycloheximide, which indicates that quercetin can
regulate the transcription and translation level for melanin
formation. Nylander et al (15)
reported that tyrosinase and TRP-1 can be activated by P53 albumen
associated with ultraviolet ray. Quercetin can activate P53
albumen, so it is presumed that upregulation of melanin synthesis
by quercetin is due to its promotion of P53 albumen activation
(16). The aforementioned studies have
provided evidence for further application of the FLL extract,
oleanolic acid and quercetin to the clinical treatment of
vitiligo.
During the repigmentation in vitiligo treatment, the
pigment island appears firstly on the follicular orifice of
leukoplakia, and subsequently extends to be flakiness. The pigment
is the darkest on the follicular orifice, and is the lightest on
the recovering zone. A previous study has reported that there are
HMB-45, TYR, TRP1 and TRP2-negative AMMCs in the hair follicle
outer root sheath (17). In this
position AMMCs are not damaged, and are activated by the
specificity factors. They move to the epidermis and extend outward
from the follicular orifice, for pigment regeneration. AMMCs are
the most important reservoir in the course of pigment regaining
(2). Another study has shown that the
activation and adhesion of AMMCs in the hair follicle outer root
sheath and their migration to the lesion position is key for
regaining pigment (18).
Cell adhesion and migration is an initiative course
of consuming energy, which requires cytoskeleton participation. The
main components of the cytoskeleton are the microtubule,
microfilament and intermediate filament. The microfilament can form
a stress fiber. The fibrous actin is formed by polymerization of
the monomer globular actin. Actin is the most important structural
component of lamellar pseudopodium of motor cells. The review by
Miao (19) reported that following
treatment with cytochalasin D, a drug which can specifically
inhibit the polymerization of actin cytoskeleton, the oriented
movement of cells is inhibited. The movement recovered subsequent
to releasing the drug indicating that the dynamic change of the
actin cytoskeleton is extremely important to cell migration. Chen
et al (20) identified that the
change of cell adhesion is also closely connected with the change
of actin. Therefore, certain investigators believe that the
aggregation and disaggregation of intracellular actin can promote
the migration of MCs (21).
The results of the present study show that when
compared with the control group, 0.0375–0.3 mg/ml of the FLL
extract can significantly improve the adhesion rate of MCs in a
dose-dependent manner. In addition, 0.15 mg/ml of the FLL extract
can significantly promote the migration of MCs. Oleanolic acid has
no clear effect on the adhesion of MCs, and only 12 µM oleanolic
acid promoted the migration of MCs. Compared with the control
group, 40 µM quercetin significantly improved the cell adhesion;
however, its affect on the migration of MCs was not clear. In
addition, the actin in the MCs treated with 0.15 mg/ml of the FLL
extract, 12 µM oleanolic acid and 40 µM quercetin exhibited an
aggregation change at different levels. The stress fiber fasciculus
is mainly distributed inside the cell membrane and around the
nucleus. The MOD in these 3 groups was significantly higher
compared to the control group. It is speculated that the FLL
extract and its monomers oleanolic acid and quercetin can activate
the precursor MCs in hair follicle reservoir, activate actin and
induce the aggregation of cellular actin cytoskeleton. The monomers
promote the cell adhesion and migration to the lesion position for
the production of melanin and formation of pigment island
surrounding the hair follicle. Oleanolic acid may be the active
ingredient in the FLL extract, which promotes the MC migration, and
quercetin may be the active ingredient in FLL, which enhances the
adhesion of MCs. There may be another action mechanism of the FLL
extract on MCs, which should be explored in future studies. In
addition, in the present study the effects of oleanolic acid on the
adhesion of MCs and the effects of quercetin on cell migration are
not clear, which should be further investigated.
Acknowledgements
The present study was supported by a grant from
Natural Science Foundation of Guangdong Province (no.
9151051501000006).
References
|
1
|
Moretti S, Spallanzani A, Amato L,
Hautmann G, Gallerani I, Fabiani M and Fabbri P: New insights into
the pathogenesis of vitiligo: Imbalance of epidermal cytokines at
sites of lesions. Pigment Cell Res. 15:87–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshida H, Kunisada T, Grimm T, Nishimura
EK, Nishioka E and Nishikawa SI: Review: Melanocyte migration and
survival controlled by SCF/c-kit expression. J Investig Dermatol
Symp Proc. 6:1–5. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vickaryous MK and Hall BK: Human cell type
diversity, evolution, development, and classification with special
reference to cells derived from the neural crest. Biol Rev Camb
Philos Soc. 81:425–455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng M and Hu ZH: The Ligustrum
biological and chemical composition of progress. Chin Tradit Herbal
Drugs. 41:1219–1221. 2010.(In Chinese).
|
|
5
|
Tian LT, Ma L and Du NS: Survey of
pharmacology of oleanolic acid. Zhongguo Zhong Yao Za Zhi.
27:884–886, 901. 2002.(In Chinese). PubMed/NCBI
|
|
6
|
Chouaïb K, Hichri F, Nguir A, Daami-Remadi
M, Elie N, Touboul D, Ben Jannet H and Hamza MA: Semi-synthesis of
new antimicrobial esters from the natural oleanolic and maslinic
acids. Food Chem. 183:8–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoek-van den Hil EF, van Schothorst EM,
van der Stelt I, Hollman PC, Keijer J and Rietjens IM: Quercetin
tests negative for genotoxicity in transcriptome analyses of liver
and small intestine of mice. Food Chem Toxicol. 81:34–39. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wiczkowski W, Szawara-Nowak D, Topolska J,
et al: Metabolites of dietary quercetin: Profile, isolation,
identification, and antioxidant capacity. J Funct Foods.
11:121–129. 2014. View Article : Google Scholar
|
|
9
|
Cai LV and Zhang J: Pharmacological
effects of the quercetin. World Phytomedicines. 20:108–112.
2005.(In Chinese).
|
|
10
|
Liu ZL, Tu CX, Ren F and Lin XR: Research
the role of the 56 herbs alcohol extract to tyrosinase activity and
animals pigments. Chin J Dermatol. 34:284–285. 2001.(In
Chinese).
|
|
11
|
Chang SB, Xu AE, Li YW, Zhang DM and Wei
XD: Effects of extracts from seven kinds of traditional Chinese
medicine on the proliferation and melanogenesis of a melanocyte
cell line B10BR by interaction with HaCaT cells. Chin J Dermatol.
40:409–411. 2007.(In Chinese).
|
|
12
|
Zhang DM, Li YW, Wei XD and Xu AE: Effects
of Fructus Ligustri Lucidi on the tyrosinase activity and
melanogenesis of cultured human melanocytes. Chin J Dermatol.
39:197–199. 2006.(In Chinese).
|
|
13
|
Takeyama R, Takekoshi S, Nagata H, Osamura
RY and Kawana S: Quercetin-induced melanogenesis in a reconstituted
three-dimensional human epidermal model. J Mol Histol. 35:157–165.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagata H, Takekoshi S, Takeyama R, Homma T
and Yoshiyuki Osamura R: Quercetin enhances melanogenesis by
increasing the activity and synthesis of tyrosinase in human
melanoma cells and in normal human melanocytes. Pigment Cell Res.
17:66–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nylander K, Bourdon JC, Bray SE, Gibbs NK,
Kay R, Hart I and Hall PA: Transcriptional activation of tyrosinase
and TRP-1 by p53 links UV irradiation to the protective tanning
response. J Pathol. 190:39–46. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plaumann B, Fritsche M, Rimpler H,
Brandner G and Hess RD: Flavonoids activate wild-type p53.
Oncogene. 13:1605–1614. 1996.PubMed/NCBI
|
|
17
|
Ma HJ, Yue XZ, Wang DG, Li CR and Zhu WY:
A modified method for purifying amelanotic melanocytes from human
hair follicles. J Dermatol. 33:239–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui J, Shen LY and Wang GC: Role of hair
follicles in the repigmentation of vitiligo. J Invest Dermatol.
97:410–416. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao L: Review: Cell movement, cell
migration, and cytoskeletal research progress. Acta Biophys Sin.
23:281–288. 2007.(In Chinese).
|
|
20
|
Chen HQ, Tian W, Chen YS, Li L, Raum J and
Sung KL: Effect of steady and oscillatory shear stress on F-actin
content and distribution in neutrophils. Biorheology. 41:655–664.
2004.PubMed/NCBI
|
|
21
|
Wehrle-Haller B: The role of Kit-ligand in
melanocyte development and epidermal homeostasis. Pigment Cell Res.
16:287–296. 2003. View Article : Google Scholar : PubMed/NCBI
|