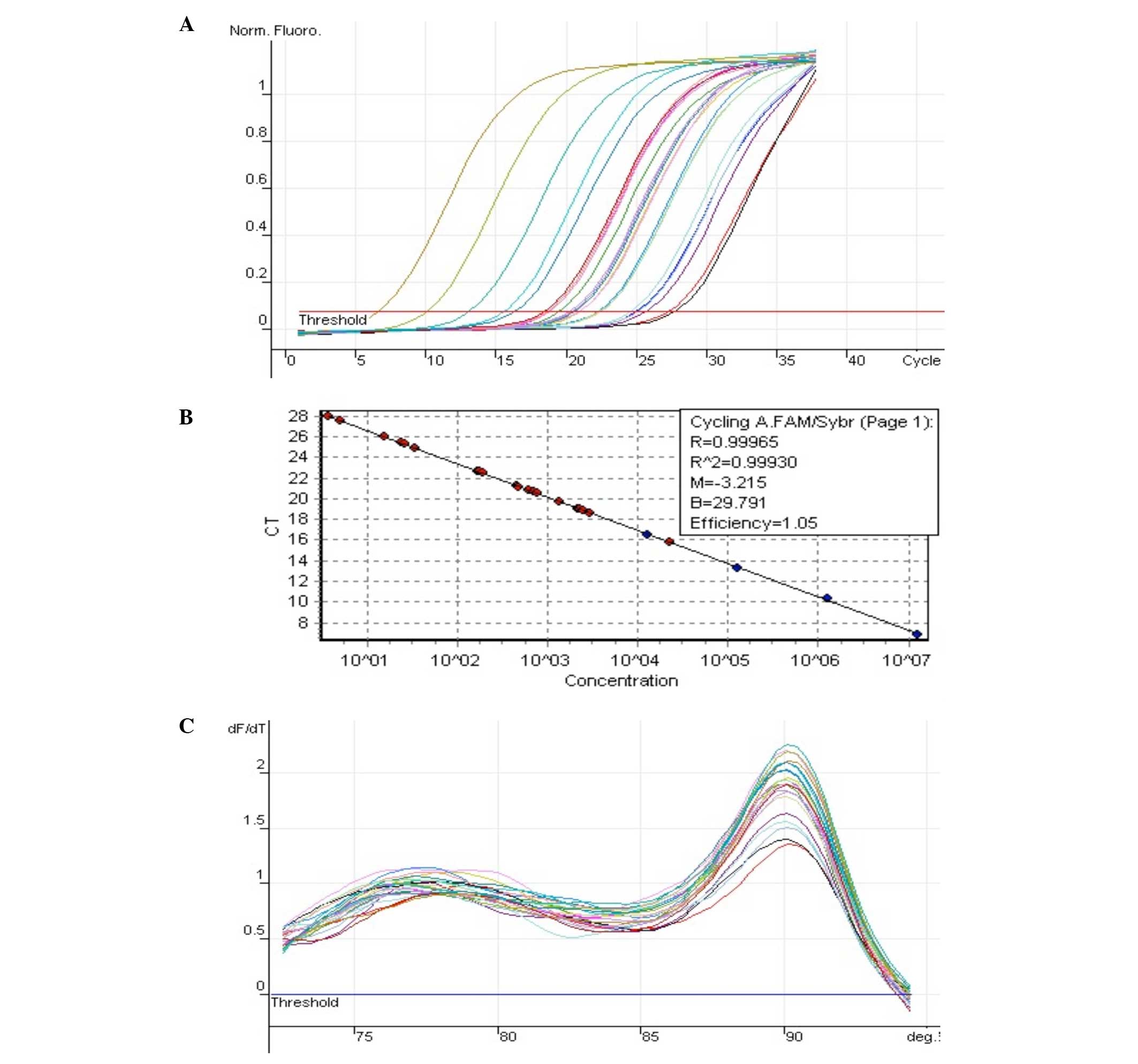
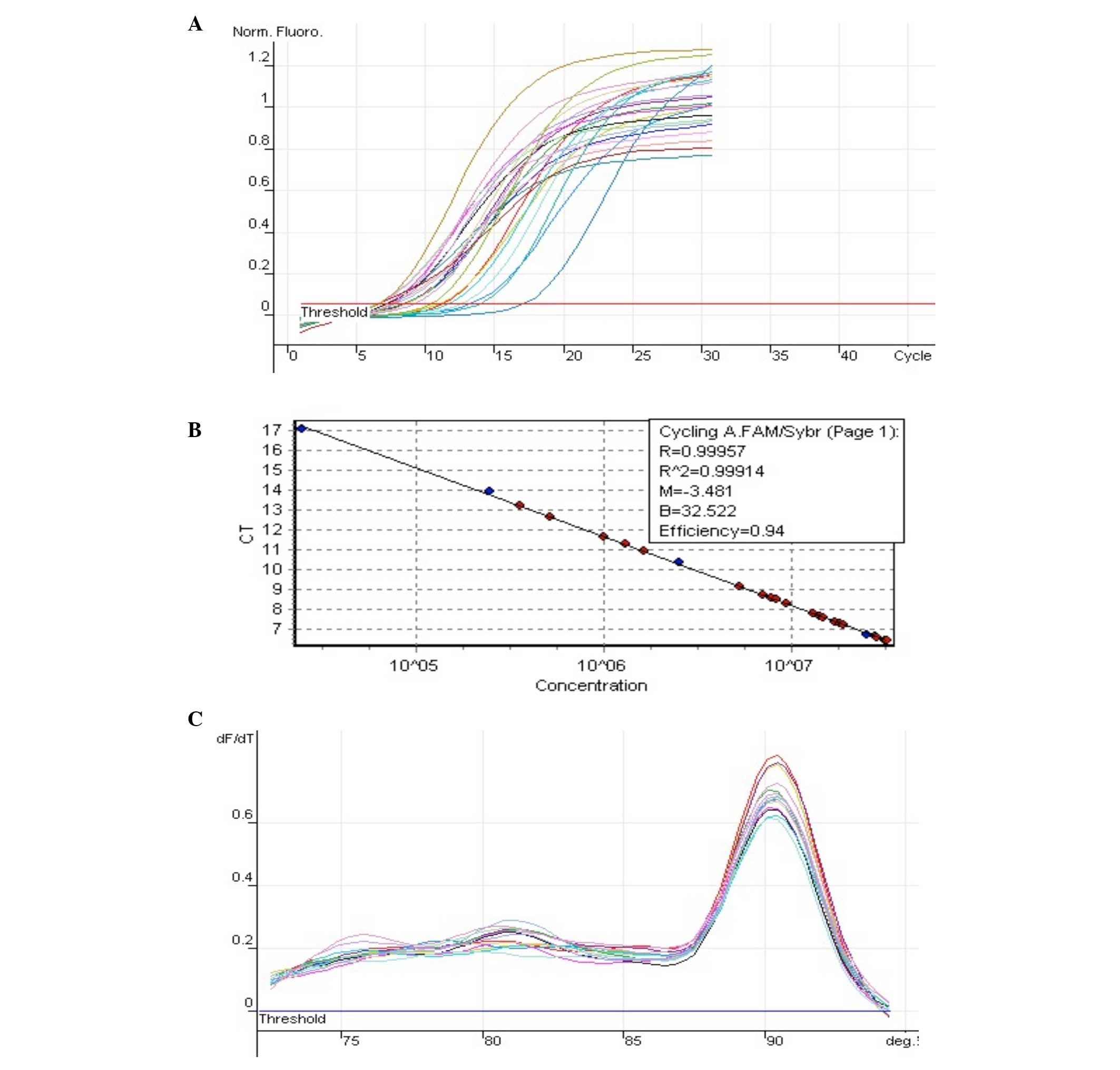
Introduction
Hepatic portal occlusion (HPO) is one of the
commonly used methods to control intraoperative bleeding; however,
serious ischemia reperfusion injury (IRI) often occurs when the
flow regains. Present study on brain, heart, kidney, retina and
other tissues shows that a large number of free radicals are
produced during IRI when endogenous radical scavengers are not in
abundance, causing cell damage. Therefore, increasing attention in
liver surgery has focused on identifying effective drugs to
alleviate liver function damage in IRI and improve the
postoperative survival rate.
Melatonin (MT) is a type of neural endocrine hormone
secreted by the pineal gland, with antioxidant, anti-toxic,
anti-stress and anti-inflammatory effects (1,2). Studies
found that MT has a protective role in IRI of brain, heart, kidney
and retina; however, its protective role in hepatic IRI and its
mechanism remain to be elucidated. The present study aimed to
investigate whether MT can effectively reduce the HPO and the
subsequent hepatic IRI and its action mechanism.
Materials and methods
Experimental animals and grouping
A total of 66 healthy male Sprague-Dawley rats were
purchased from the Experimental Animal Department, Shanghai
Jiaotong University (Shanghai Second Medical University, Shanghai,
China). The rats (3–6 months; body weight, 220±30 g) were divided
into 3 groups: i) The normal control group (group N), ii) the
ischemia reperfusion group (IR group) and iii) melatonin treatment
group (MT group). Groups IR and MT were subdivided into 5 groups
(n=6/each group): 35 min of ischemia, and 2, 4, 8 and 24 h
reperfusion.
Main instruments and reagents
Main instruments
The main instruments used included animal surgical
equipments, electronic balance, centrifuge (3K15; Sigma-Aldrich,
St. Louis, MO, USA), RM2165 paraffin slicing machine, microscope
(IX71; Leica Microsystems, Ltd., Milton Keynes, UK), polymerase
chain reaction (PCR) and reverse transcription-quantitative PCR
(RT-qPCR; ABI 7500 RealTime system; Applied Biosystems Life
Technologies, Waltham, MA, USA).
Main reagents
MT (Sigma-Aldrich), the hematoxylin and eosin
(H&E) staining kit (Shanghai Beyotime Biological Technology
Co., Ltd., Shanghai, China), interleukin-1β (IL-1β) double antibody
sandwich ABC-propidium iodide enzyme-linked immunosorbent assay
(ELISA) (Shanghai Usen Biological Technology Co., Ltd., Shanghai,
China) and RT reaction kit (Fermentas Co.; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) were used in the present
study.
Experimental method
Modeling and processing
Pringle's method was used to totally block the first
porta hepatis with a non-image small artery clip, thus causing
complete occlusion of the portal vein, hepatic artery and common
bile duct. The clip was unblocked 35 min after occlusion and the
hepatic blood flow regained. Rats of the MT group were administered
an intraperitoneal injection of MT (10 mg/kg, 1 ml) at 70 and 35
min before ischemia, early reperfusion, and 1 and 2 h after
reperfusion, respectively (3,4).
For each animal group, 1 ml of blood was taken from
the portal vein of the rat at the normal time-point (prior to any
processes), 35 min before ischemia, 2, 4, 8 and 24 h after
reperfusion (6 rats at each time-point). Subsequently, the blood
was set in sterile pyrogen-free EP tubes for 20 min and centrifuged
at 4,000 × g for 10 min. Serum was separated and stored at −30°C
until required for analysis. In total, 1 g of liver tissue was
washed by physiological saline (0°C) and stored in a liquid
nitrogen tank for measurement. A total of 1 g of the liver tissue
was fixed with 10% formaldehyde solution for analysis.
Pathology inspection of the liver tissue
Liver tissue was fixed with 10% formaldehyde.
Following conventional paraffin section and H&E staining, the
pathological changes of the liver were observed under
microscopy.
Serum IL-1β expression by ELISA
The anti-mouse IL-1β single antibody (cat. no.
ab2105; Abcam, Cambridge, UK) was used to cover the ELISA plate.
IL-1β of the standard and sample was combined with the single
antibody. The biotinylated anti-mouse IL-1β antibody was added to
form an immune complex, which was connected to the plate.
Horseradish peroxidase-labeled streptavidin was combined with
biotin. The enzyme substrate OPD was added and a yellow color was
produced. The reaction was terminated when liquid sulfuric acid was
added and the color became dark. The optical density (OD) value was
measured at 492 nm and as the IL-1β concentration was positively
correlated with the OD value, the IL-1β concentration in the
specimen could be obtained by a standard curve.
Interleukin-1 receptor antagonist (IL-1Ra) gene
expression by RT-qPCR
Total RNA was extracted and RNA was reverse
transcribed into cDNA according to the manufacturer's protocol of
the reverse transcription kit (Applied Biosystems Life
Technologies). Real-time PCR primers were designed and synthesized
by Takara Co. (Otsu, Japan) according to IL-1Ra (NM_022194) and rat
18S gene sequences. Amplification was carried out in accordance
with the manufacturer's protocol. The sequences were as follows:
Rat-18S-s, 5-CGGCTACCACATCCAAGGAA-3; rat-18S-a,
5-GCTGGAATTACCGCGGCT-3. The amplification conditions were: 95°C for
2 min, 95°C for 15 sec, 60°C for 20 sec, 72°C for 20 sec, for a
total of 37 cycles. The LightCycler (Roche Diagnostics Co.,
Indianapolis, IN, USA) was used for the amplification reaction and
Cq value. The formula ΔCq=Cq
(IL-1Ra)-Cq (18S) was used and relative expression of
the IL-1Ra mRNA was 2−ΔΔCt.
Statistical analysis
SPSS 10.0 (SPSS, Inc., Chicago, IL, USA) was used
for the statistical analysis. Quantitative data are presented as
mean ± standard deviation. Analysis of variance was used to compare
between groups. The Student-Newman-Keuls test was used for pairwise
comparison between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pathological changes of liver
tissue
Pathological sections of liver tissue specimens from
every time-point after reperfusion were observed for morphology
(Fig. 1). The results showed similar
pathological changes between the IR group, 2 h IR group and MT
group. Evident piecemeal necrosis and serious fusion necrosis were
observed 4 h after reperfusion in the specimens of the IR group,
while only piecemeal necrosis was observed in the MT group. Only
piecemeal necrosis was observed in the MT group 8 h after
reperfusion. No fusion necrosis was observed in each specimen. With
the extension of reperfusion time, degeneration and necrosis degree
of liver cells in the IR group and damage scope of hepatic cords
were gradually expanded. A peak was reached at 24 h after
reperfusion. Piecemeal necrosis and fusion necrosis were observed
in each sample of the IR group; however, this change was not
evident in the MT group. Piecemeal necrosis was observed in each
sample of the MT group; however, fusion necrosis was not
observed.
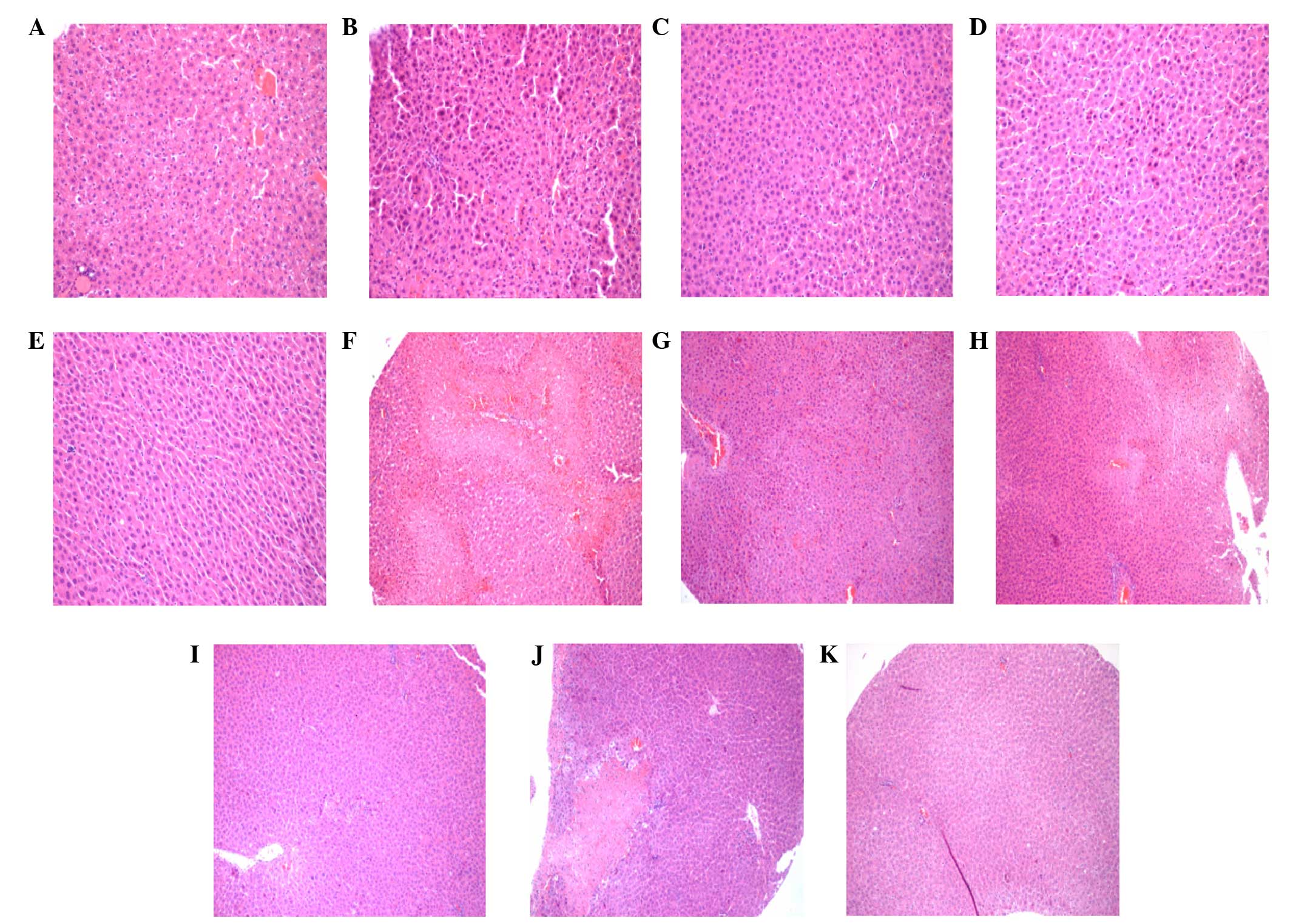 | Figure 1.Hematoxylin and eosin (H&E)
staining of the different rat groups. H&E staining results of
the (A) normal rats, (B) 0 IR group, (C) 0 MT group, (D) 2 h IR
group, (E) 2 h MT group, (F) 4 h IR group, (G) 4 h MT group, (H) 8
h IR group, (I) 8 h MT group, (J) 24 h IR group and (K) 24 h MT
group. [(A-E) magnification, ×200; (F-K) magnification, ×100]. MT,
melatonin treatment; IR, ischemia reperfusion. |
Examination results of IL-1β
IL-1β values at each ischemic time-point of the IR
and MT groups were higher than that of group N (P<0.05). IL-1β
values at 35 min of hepatic ischemia, 2, 4, 8 and 24 h reperfusion
of the MT group were significantly lower than that of the IR group
(P<0.05). The results are shown in Table I.
 | Table I.Changes of interleukin-1β activity in
ischemia reperfusion injury rats. |
Table I.
Changes of interleukin-1β activity in
ischemia reperfusion injury rats.
|
|
| Reperfusion time,
h |
|---|
|
|
|
|
|---|
| Groups | Normal time-point,
h | 0 | 2 | 4 | 8 | 24 |
|---|
| N | 17.68±4.78 | – | – | – | – | – |
| IR | – |
45.76±3.39a |
50.22±7.81a |
61.40±10.76a |
62.77±15.05a |
57.45±16.98a |
| MT | – |
28.38±4.76a,b |
30.47±3.56a,b |
38.14±6.92a,b |
37.03±6.59a,b |
40.60±11.24a |
Expression results of the IL-1Ra
gene
The expression of NM_022194 (IL-1Ra) is shown in
Fig. 2 and 18S is shown in Fig. 3.
Relative expression of IL-1Ra
Relative quantitative results showed that IL-1Ra
mRNA expression in the 2 h MT group was higher compared to the 2 h
IR group by 4.85-fold and IL-1Ra mRNA expression in the 4 h MT
group was higher compared to the 4 h IR group by 9.34-fold.
Differences between the two groups at other time-points were
<2-fold. The IL-1Ra gene expression level was similar between
the MT and IR groups at 24 h after reperfusion (Table II).
 | Table II.Relative expression of IL-1Ra. |
Table II.
Relative expression of IL-1Ra.
| Groups | IL-1Ra |
|---|
| MT0/IR0 | 0.456871016 |
| MT2/IR2 | 4.858945705 |
| MT4/IR4 | 9.342555162 |
| MT8/IR8 | 0.620242510 |
| MT24/IR24 | 0.992904616 |
Discussion
Liver resection is a main treatment for a variety of
liver diseases. The liver has a rich blood supply, so HPO is often
adopted during surgery to reduce wound bleeding. However this
method reduces the intraoperative bleeding while causing hepatic
IRI.
During IR, hepatic neutrophil infiltration, release
of inflammatory mediators and oxygen free radical formation are the
important factors leading to liver damage. Among them, IL-1 is an
important initiator for inflammation, which has an important role
in IRI of multiple systems (5–7). Therefore, with IL-1 as an important entry
point, the present study examined IL-1 expression prior and
subsequent to reperfusion and MT intervention. The results showed
that IL-1β, the main active forms of IL-1, was evidently increased
in IR rats. IL-1β was clearly higher at 35 min of ischemia and 2,
4, 8 and 24 h after reperfusion compared to IL-1β prior to HPO, and
the most significant increase of IL-1β was at 8 h after
reperfusion. Possible mechanisms causing the increase of IL-1β in
liver IR included: Neutrophils, mononuclear macrophages,
lymphocytes and endothelial cells, which secrete more IL-1
(8); liver cells can also express and
synthesize IL-1 under ischemia and hypoxia stimulation (9,10); other
inflammatory factors, such as IL-6 and intercellular adhesion
molecule-1, can also stimulate mononuclear macrophages and
neutrophils to produce IL-1; and IL-1 itself can stimulate the
synthesis and release of IL-1 for feedback (11,12).
In the present study, it was found that IL-1β
following IR was significantly higher compared to normal rats, as
IL-1 is one of the initiators of inflammation. Blocking IL-1 can
reduce the liver IRI (13,14). Previous experiments on heart and
kidneys have confirmed that IL-1 could be reduced and an
inflammatory response could be initiated by directly administrating
IL-1 receptor blockers or increasing the IL-1 receptor inhibition
gene via gene transfection (15,16). In
addition, it can reduce IL-1β-induced apoptosis (17). Therefore, if the excessive release of
IL-1 can be inhibited following hepatic IR, IRI could be reduced.
In the present study, rats treated by MT had significantly lower
IL-1β at each time-point of reperfusion 35 min after HPO compared
to the rats not treated by MT (P<0.05). MT can block a series of
IL-1β-mediated inflammatory reactions by reducing IL-1β in rat
hepatic reperfusion, so as to reduce liver structure and function
injury due to IR. Previous studies have shown that the nitric oxide
(NO) gene was closely associated with the production of IL-1 in
liver IR (18,19), and MT was confirmed to effectively
downregulate nuclear factor-κB expression and inhibit the activity
of inducible NO synthase in rat liver IR (20,21), so as
to moderately adjust NO generation. The latter was closely
associated with the release of IL-1β (22). Therefore, MT may reduce the release of
IL-1β by adjusting NO.
This is the most ideal method to reduce IRI by
endogenous protective reaction to enhance the tolerance of the
liver itself. IL-1 is one of the important regulatory factors for
acute inflammation (23). IL-1Ra can
inhibit the roles of IL-1α and IL-1β through competitive
combination with type I and II IL-1 receptors, thus reducing
inflammation (24). Recombinant human
(Rh) IL-1Ra was the first identified natural cytokine antagonists.
It has a certain degree of homology with IL-1α and IL-1β and it can
competitively combine with type I and type II IL-1 receptors
without producing IL-1-like effects. Animal studies showed that
RhIL-1Ra can effectively block the effect of IL-1, treat certain
inflammatory diseases that were associated with the disorder of
cytokines, and return the excessive IL-6 and tumor necrosis
factor-α level to normal without interfering with the internal
environment balance (25). RhIL-1Ra
has been clinically trialed on rheumatoid arthritis and autoimmune
disease, and has achieved good curative effects. No adverse effects
have been identified in long-term application (26). Previous cardiac and renal experiments
have confirmed that IL-1Ra expression, which is increased by the
direct administration of IL-1 receptor blockers or gene
transfection, can reduce the inflammatory response (15,16). In
addition, IL-1Ra can reduce IL-1β-induced apoptosis (17). Therefore, if overexpression of IL-1Ra
can be induced following liver IR, it would help to reduce IRI.
Therefore, the present study detected two groups of
rat liver specimens, which were administered reperfusion 35 min
after HPO and found that IL-1Ra genes, which were almost not
detected in normal rats, were significantly increased. IL-1Ra
expression in the 2 h MT group was higher than that of the IR group
by 4-fold, and IL-1Ra expression in the 4 h MT group was higher
than that of the IR group by 9-fold. Expression differences between
the two groups at other time-points were all within 2-fold. Barrier
et al (27) reported the
upregulation of IL-1Ra in liver pretreated by ischemia with
gene-chip technology. They believed that protective mechanisms of
ischemic preconditioning on liver IRI were associated with
overexpression of IL-1Ra. However, the mechanism of increased
IL-1Ra following IR remains to be elucidated. In the present study,
IL-1Ra in the group pre-treated by MT was 9-fold higher than that
of IR following IR, and further experiments are required to prove
whether MT can induce upregulation of IL-1Ra, similar to ischemic
preconditioning, thus producing protective effects.
In conclusion, the present study suggested that MT
can protect the liver and reduce IRI not only by reducing the
production of IL-1, but also by blocking IL-1 receptors, namely
raising IL-1Ra gene expression. As the serious side effects of MT
have not been reported yet, this may be a new way for protecting
IRI in clinical studies.
References
|
1
|
Li Y, Yang Y, Feng Y, Yan J, Fan C, Jiang
S and Qu Y: A review of melatonin in hepatic ischemia/reperfusion
injury and clinical liver disease. Ann Med. 46:503–511. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu LF, Qin Q, Qian ZH, Shi M, Deng QC,
Zhu WP, Zhang H, Tao XM and Liu Y: Protective effects of melatonin
on ischemia-reperfusion induced myocardial damage and hemodynamic
recovery in rats. Eur Rev Med Pharmacol Sci. 18:3681–3686.
2014.PubMed/NCBI
|
|
3
|
Rodríguez-Reynoso S, Leal C, Portilla E,
Olivares N and Muñiz J: Effect of exogenous melatonin on hepatic
energetic status during ischemia/reperfusion: Possible role of
tumor necrosis factor-α and nitric oxide. J Surg Res. 100:141–149.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deprés-Brummer P, Metzger G, Morin D,
Urien S, Touitou Y, Tillement JP, Claustrat B and Lévi F:
Pharmacokinetically guided melatonin scheduling in rats with
circadian system suppression. Eur J Pharmacol. 312:171–178. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simeoni E, Dudler J, Fleury S, Li J,
Pagnotta M, Pascual M, von Segesser LK and Vassalli G: Gene
transfer of a soluble IL-1 type 2 receptor-Ig fusion protein
improves cardiac allograft survival in rats. Eur J Cardiothorac
Surg. 31:222–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sener G, Sehirli O, Velioğlu-Oğünç A,
Cetinel S, Gedik N, Caner M, Sakarcan A and Yeğen BC: Montelukast
protects against renal ischemia/reperfusion injury in rats.
Pharmacol Res. 54:65–71. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Menger MD, Richter S, Yamauchi J and
Vollmar B: Role of microcirculation in hepatic ischemia/reperfusion
injury. Hepatogastroenterology. 46(Suppl 2): 1452–1457.
1999.PubMed/NCBI
|
|
8
|
Galea J, Armstrong J, Gadsdon P, Holden H,
Francis SE and Holt CM: Interleukin-1 beta in coronary arteries of
patients with ischemic heart disease. Arterioscler Thromb Vasc
Biol. 16:1000–1006. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deten A, Volz HC, Briest W and Zimmer HG:
Cardiac cytokine expression is upregulated in the acute phase after
myocardial infarction. Experimental studies in rats. Cardiovasc
Res. 55:329–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herskowitz A, Choi S, Ansari AA and
Wesselingh S: Cytokine mRNA expression in postischemic/reperfused
myocardium. Am J Pathol. 146:419–428. 1995.PubMed/NCBI
|
|
11
|
Neumann FJ, Marx N, Gawaz M, Brand K, Ott
I, Rokitta C, Sticherling C, Meinl C, May A and Schömig A:
Induction of cytokine expression in leukocytes by binding of
thrombin-stimulated platelets. Circulation. 95:2387–2394. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prabhu SD, Chandrasekar B, Murray DR and
Freeman GL: beta-adrenergic blockade in developing heart failure:
Effects on myocardial inflammatory cytokines, nitric oxide, and
remodeling. Circulation. 101:2103–2109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashimoto K, Nishizaki T, Yoshizumi T,
Uchiyama H, Okano S, Ikegami T, Yanaga K and Sugimachi K:
Beneficial effect of FR167653 on cold ischemia/reperfusion injury
in rat liver transplantation. Transplantation. 70:1318–1322. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi J, Takeyoshi I, Ohwada S,
Iwanami K, Matsumoto K, Muramoto M and Morishita Y: The effects of
FR167653 in extended liver resection with ischemia in dogs.
Hepatology. 28:459–465. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harada H, Wakabayashi G, Takayanagi A,
Shimazu M, Matsumoto K, Obara H, Shimizu N and Kitajima M: Transfer
of the interleukin-1 receptor antagonist gene into rat liver
abrogates hepatic ischemia-reperfusion injury. Transplantation.
74:1434–1441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki K, Murtuza B, Smolenski R, Sammut
IA, Suzuki N, Kaneda Y and Yacoub MH: Overexpression of
interleukin-1 receptor antagonist provides cardio protection
against ischemia-reperfusion injury associated with reduction in
apoptosis. Circulation. 104(Suppl 1): 308–313. 2001.
|
|
17
|
Maedler K, Sergeev P, Ehses JA, Mathe Z,
Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA and Donath MY:
Leptin modulates beta cell expression of IL-1 receptor antagonist
and release of IL-1beta in human islets. Proc Natl Acad Sci USA.
101:8138–8143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kohli V, Gao W, Camargo CA Jr and Clavien
PA: Calpain is a mediator of preservation-reperfusion injury in rat
liver transplantation. Proc Natl Acad Sci USA. 94:9354–9359. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li SQ, Liang LJ, Huang JF and Li Z:
Ischemic preconditioning protects liver from hepatectomy under
hepatic inflow occlusion for hepatocellular carcinoma patients with
cirrhosis. World J Gastroenterol. 10:2580–2584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM,
Seok JH and Lee JH: Hepatic ischemia/reperfusion in rats induces
iNOS gene transcription by activation of NF-kappaB. Biochem Biophys
Res Commun. 261:917–922. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong S, She H, Takeuchi H, Han B,
Engelhardt JF, Barton CH, Zandi E, Giulivi C and Tsukamoto H:
Signaling role of intracellular iron in NF-kappaB activation. J
Biol Chem. 278:17646–17654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koeppel TA, Thies JC, Schemmer P, Trauner
M, Gebhard MM, Otto G and Post S: Inhibition of nitric oxide
synthesis in ischemia/reperfusion of the rat liver is followed by
impairment of hepatic microvascular blood flow. J Hepatol.
27:163–169. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raz R, Durbin JE and Levy DE: Acute phase
response factor and additional members of the interferon-stimulated
gene factor 3 family integrate diverse signals from cytokines,
interferons, and growth factors. J Biol Chem. 269:24391–24395.
1994.PubMed/NCBI
|
|
24
|
Gabay C, Smith MF, Eidlen D and Arend WP:
Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase
protein. J Clin Invest. 99:2930–2940. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pang SF, Dubocovich ML and Brown GM:
Melatonin receptors in peripheral tissues: A new area of melatonin
research. Biol Signals. 2:177–180. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding WH, Wu FX and Li DY: The change of
plasma interleukin-6 level and cardiac protective effect of
monoclonal antibody to IL-6 during myocardial infarction. Zhonghua
Xin Xue Guan Bing Za Zhi. 27:29–32. 1999.(In Chinese).
|
|
27
|
Barrier A, Olaya N, Chiappini F, Roser F,
Scatton O, Artus C, Franc B, Dudoit S, Flahault A, Debuire B, et
al: Ischemic preconditioning modulates the expression of several
genes, leading to the overproduction of IL-1Ra, iNOS, and Bcl-2 in
a human model of liver ischemia-reperfusion. FASEB J. 19:1617–1626.
2005. View Article : Google Scholar : PubMed/NCBI
|