Introduction
Fatigue is a term used for physical exhaustion,
which is highly prevalent within the stressed modern human
societies. Fatigue is a symptom of the body not being able to
sustain a given level of activity. Fatigue has a negative effect on
various aspects of human life including physical strength, work
performance, domesticity and social life (1,2).
Increasing evidence has proved that fatigue is
closely associated with oxidative stress (3,4). In 1982,
the study by Davies et al (5)
confirmed that radicals increase significantly following exhaustive
exercise, which supports the theory that exercise may induce an
increased production of radicals. An increased generation of
radicals during exercise and decreased exercise capacity are most
closely associated with the performance of radicals and the lipid
peroxidation in the biofilm of various organs. Oxidative stress can
be attributed to exercise whereas the reactive oxygen species (ROS)
are the foremost cause of exercise-induced disturbances. By
contrast, beneficial effects of antioxidants occur during
anti-fatigue (6,7). As a result, a number of studies
investigating functional foods focus on ROS generation and their
performance on antioxidant activity and to demonstrate the role of
fatigue resistance.
Deep sea water (DSW) is extracted from >200
meters below the sea level. DSW has received increasing attention
for various beneficial characteristics, such as low temperature,
nutrition, sterility, stability and safety (8–10). It is
known that high atmospheric pressure contributes to small and
stable water molecules, ionization state and easy absorption of
DSW.
More than 90 types of mineral, such as magnesium
(Mg), calcium, sodium and potassium, as well as microelements, such
as cobalt, selenium, chromium and vanadium, have been found in DSW
(11). Numerous studies have confirmed
that DSW has pharmacological effects, including protecting the
cardiovascular system (12,13), improving metabolic processes (14,15),
anti-osteoporosis properties (16),
treating atopic dermatitis skin lesions (17), accelerating recovery from physical
fatigue (18), providing intestinal
protection against duodenal ulcers (9)
and inhibiting the metastatic potential of human breast cancer cell
lines (19).
Although DSW has already been studied for its
alleviating fatigue effect in Wistar rats, the beneficial effects
isolated in that study were only attributed to the minerals of DSW
(20). In addition, the mechanism
behind the beneficial effect has not yet been fully clarified. As
aforementioned, fatigue is associated with oxidative stress. The
present study aimed to determine whether DSW achieved this effect
by antioxidant properties. To test this hypothesis, the antioxidant
and antifatigue effects of DSW in imprinting control region (ICR)
mice were assessed.
Materials and methods
Materials
DSW was provided by Pacific Deep Ocean Biotech Co.,
Ltd. (Hualian, Taiwan), which was collected from the West Pacific
Ocean (662 meters in depth). Microbes and macromolecules were
filtered out through reverse osmosis. Table I shows the composition of DSW
subsequent to being concentrated, and the content was assessed by
SGS Institut Fresenius GmbH (Dresden, Germany). Glycogen, blood
lactate (BLA), superoxide dismutase (SOD), glutathione peroxidase
(GSH-Px) and malondialdehyde (MDA) kits were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Blood urea
nitrogen (BUN), lactate dehydrogenase (LDH) and creatine kinase
(CK) levels were measured by the Clinical Laboratory of Traditional
Chinese Medicine Integrated Hospital of Southern Medical University
(Guangzhou, China).
 | Table I.Mineral content of deep sea water used
in the study. |
Table I.
Mineral content of deep sea water used
in the study.
| Parameter | Content, mg/l |
|---|
| Magnesium | 45,300 |
| Calcium | 51.5 |
| Sodium | 11.6 |
| Kalium | 14,800 |
| Ammonium | 13,400 |
| Chloride | 13,200 |
| Bromide | 2,400 |
| Iodine | 4.4 |
| Nitrite | 0.38 |
| Nitrate | 100 |
| Sulfate | 45,700 |
| Manganese | <0.05 |
| Iron | 0.05 |
| Chromium | 0.07 |
| Cobalt | 0.007 |
| Copper | 0.044 |
| Nickel | 0.16 |
| Selenium | 0.002 |
| Rubidium | 4.7 |
| Zinc | <0.05 |
| Chromium | 0.07 |
| Boron | 135 |
Animals
The male ICR mice, each weighing 32±2 g (specific
pathogen-free), were purchased from Hunan SJA Laboratory Animal
Co., Ltd. (Hunan, China). The mice were housed within a temperature
of 22–26°C, humidity of 40–60%, and a light/dark cycle of 12 h,
respectively. All the procedures involving laboratory animal use
were performed in accordance with the guidelines of the Institute
of Animal Care and Use Committee of Southern Medical
University.
Methods
Prior to conducting the experiment, mice were
allowed to adapt to the new environment for 1 week. A total of 192
mice were divided into the following 4 groups: i) Control; ii)
DSW-high dose; iii) DSW-middle dose; and iv) DSW-low dose groups.
Mice in group I were forced to participate in a weight-loaded
swimming test (n=48). BLA was measured in the mice in group II
(n=48). BUN, LDH and CK levels were measured in the mice in group
III (n=48). Hepatic glycogen and muscle glycogen levels were
measured in the mice in group IV (n=48). In each group, mice were
further evenly subdivided into 4 subgroups (n=12): i) Control
group, distilled water; ii) DSW-high dose group, 2.3 mg/ml Mg2+;
iii) DSW-middle dose group, 0.92 mg/ml Mg2+; and iv) DSW-low dose
group, 0.46 mg/ml Mg2+.
Every morning, the mice received a 0.8-ml dose of
distilled water or DSW by intragastric administration. After a
period of 30 min following the intragastric administration, all the
mice were trained to swim for 15 min once and three times a week.
This experiment lasted for 4 weeks. The night before forced
swimming or blood collection, all the mice were fasted overnight.
All experiments were conducted according to the National Institutes
of Health Guide for Care and Use of Laboratory Animals.
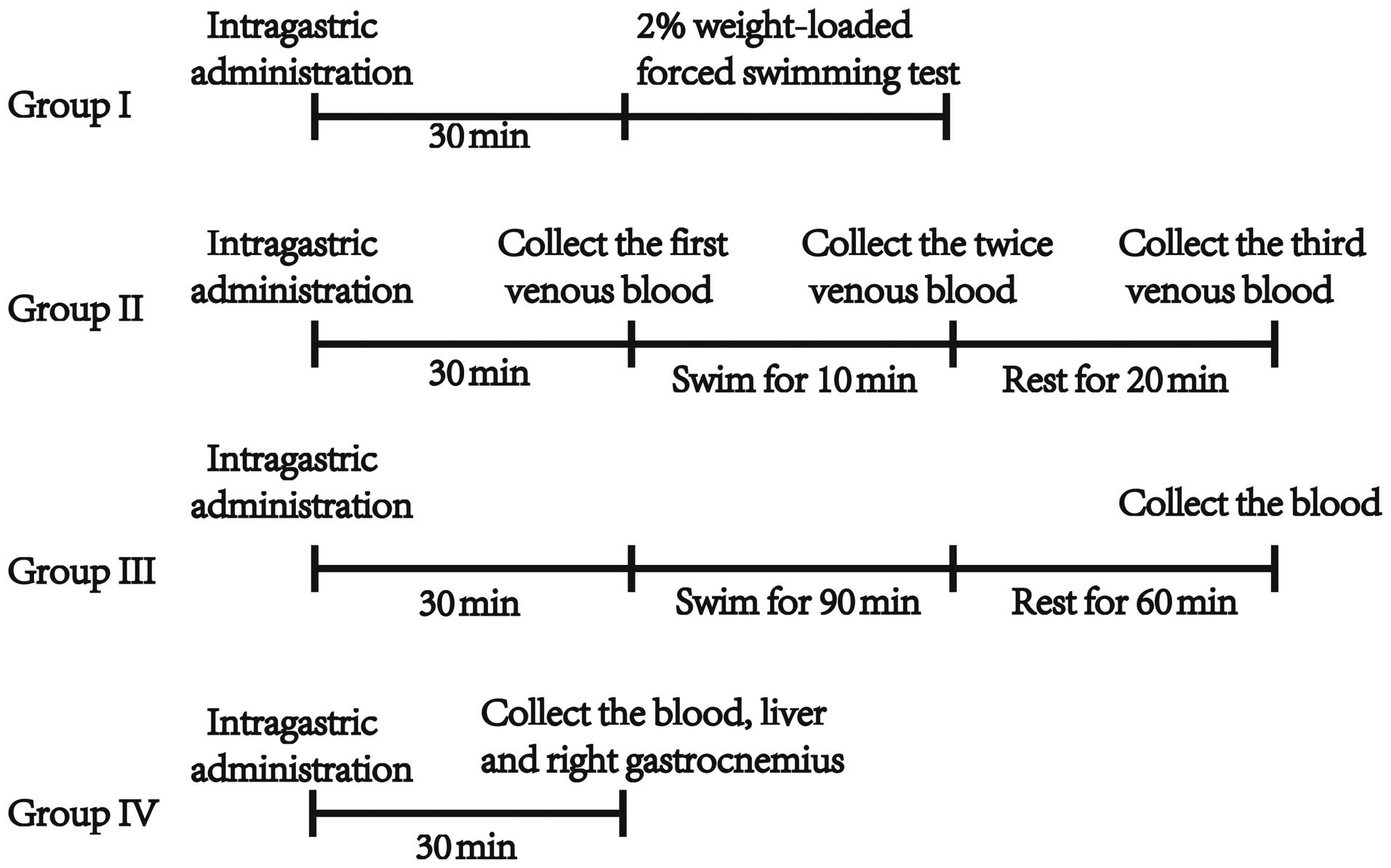
The experimental plan is exhibited as a flow diagram
in Fig. 1.
Weight-loaded forced swimming test
(group I)
After a period of 30 min following the last
intragastric administration, the mice were individually placed in a
swimming pool (45×45×55 cm) that was 40 cm in depth, and had a
temperature of 25±1°C. A tin wire (2% of the body weight) was
attached to the tail root of the mouse. The pool was too deep for
the mice to make contact with the bottom of the pool. The time from
the mice being placed into the pool to when they failed to rise to
the surface of the water for 10 sec was recorded.
Determination of BLA (group II)
After a period of 30 min following the last
intragastric administration, 20 µl of venous blood was collected
from the posterior orbit of each mouse. The mice were subsequently
placed into the pool (at 25±1°C) to swim for 10 min in the case of
non-weighted-loading. Following swimming, two samples of venous
blood were immediately collected from the posterior orbit. A third
sample of blood was collected after a period of rest for 20 min.
The area under the BLA curve was calculated as 5× (BLA content
prior to swimming + 3× following swimming + 2× resting for 20 min).
The formula was as described previously, in the Chinese Technical
Standards for Testing and Assessment of Health Food (2003)
(21).
Determination of BUN, LDH and CK
(group III)
After a period of 30 min following the last
intragastric administration, mice were individually forced to swim
for 90 min with non-weight-loading. The mice subsequently rested
for 60 min. Following rest, whole blood samples were collected by
extirpating the left eyeball in tubes without anticoagulant and the
mice were sacrificed. The blood sample was placed at 4°C for 3 h,
and centrifuged at 1,800 × g for 15 min. BUN, LDH and CK levels of
each serum sample were tested by the Roche Cobas Integra 6000
modular biological immune analyzer (Roche Diagnostics, Basel,
Switzerland) in the Clinical Laboratory of Traditional Chinese
Medicine Integrated Hospital of Southern Medical University.
Determination of glycogen, SOD, GSH-PX
and MDA (group IV)
After a period of 30 min following the last
intragastric administration, the whole blood samples were collected
by extirpating the left eyeball in tubes without anticoagulant. The
liver and right gastrocnemius muscle were collected immediately,
and dried with absorbent paper following washing with physiological
saline. The samples were maintained at −80°C until analysis of
glycogen content. Simultaneously, the blood sample was placed at
4°C for 3 h, and centrifuged at 1,800 × g for 15 min. In addition,
the plasma SOD and GSH-Px activities and MDA levels was measured in
the serum. All these methods followed the manufacturer's protocols
in the commercially available kits from Nanjing Jiancheng
Bioengineering Institute.
Statistical analysis
The results are shown as mean ± standard deviation
values. SPSS 21.0 statistical software (IBM, Corp., Armonk, NY,
USA) was used for statistical analysis of data. The difference
between the control group and each DSW-treated group was accessed
using one-way analysis of variance, followed by post hoc Dunnett's
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of DSW on body weight
No significant difference in the initial or final
body weight was identified between the control and DSW-treated
groups (Table II).
 | Table II.Effects of DSW on the body weight of
imprinting control region mice. |
Table II.
Effects of DSW on the body weight of
imprinting control region mice.
|
| Body weight, g |
|---|
|
|
|
|---|
|
| Group I | Group II | Group III | Group IV |
|---|
|
|
|
|
|
|
|---|
| Group | Initial | Final | Initial | Final | Initial | Final | Initial | Final |
|---|
| Control | 32.1±0.8 | 35.0±1.5 | 31.6±0.9 | 37.2±2.0 | 31.3±1.1 | 36.1±1.0 | 31.9±0.8 | 36.5±1.6 |
| DSW-H | 32.3±0.7 | 36.7±2.4 | 31.1±1.1 | 36.5±1.2 | 31.0±1.5 | 35.1±2.2 | 32.0±0.8 | 37.0±2.4 |
| DSW-M | 32.1±1.1 | 35.5±1.9 | 31.9±1.2 | 36.5±1.6 | 31.4±0.8 | 34.8±0.8 | 31.9±1.2 | 37.2±1.5 |
| DSW-L | 31.1±0.9 | 35.2±1.8 | 31.7±1.1 | 36.1±1.7 | 31.1±1.1 | 35.3±1.8 | 31.9±1.0 | 35.2±1.6 |
Effect of DSW on weight-loaded forced
swimming test (group I)
The DSW-high dose group mice were able to swim for
longer compared to the control group (P<0.05) (Table III). The mean duration of the
swimming time in the control group was 2,517.8 sec, while the time
in the DSW-H group was 3,240.3 sec. However, no significant
difference was identified between the control group and the DSW-M
or DSW-L groups.
 | Table III.Effects of DSW on the weight-loaded
forced swimming test of imprinting control region mice. |
Table III.
Effects of DSW on the weight-loaded
forced swimming test of imprinting control region mice.
| Group | Swimming time,
sec |
|---|
| Control | 2,517.8±574 |
| DSW-H |
3,240.3±875a |
| DSW-M | 2,503.7±612 |
| DSW-L | 2,523.0±513 |
Effect of DSW on BLA (group II)
Swimming increased BLA and a period of rest reversed
this exercise-induced increase (Table
IV). The accumulation levels of BLA (including pre-swimming,
post-swimming and post-rest) were significantly decreased in the
DSW-treated groups compared to those in the control groups
(Table IV).
 | Table IV.Effects of DSW on BLA in imprinting
control region mice. |
Table IV.
Effects of DSW on BLA in imprinting
control region mice.
|
| BLA, mmol/l |
|---|
|
|
|
|---|
| Group | Pre-exercise | Post-exercise | Post-rest | Area under BLA
curve |
|---|
| Control | 6.6±1.3 | 11.4±1.6 | 9.5±1.0 | 298.6±34.0 |
| DSW-H |
5.5±1.3a |
8.9±1.1b |
6.4±1.2b |
228.2±23.2b |
| DSW-M |
5.5±1.2a |
8.7±1.6b |
6.9±1.2b |
227.2±37.0b |
| DSW-L |
4.4±1.1b |
9.7±1.5b |
6.8±1.5b |
247.5±43.4b |
Effect of DSW on BUN, LDH, CK (group
III)
In general, endurance exercise increases BUN, LDH
and CK levels. Table V demonstrates
the effect of DSW on BUN, LDH and CK levels in ICR mice after
consumption with different concentrations of DSW for 4 weeks. The
BUN levels (9.5±1.2 mmol/l) of the DSW-H group were significantly
different to the control group. Furthermore, the LDH and CK levels
of the DSW-treated groups were significantly lower compared to the
control group.
 | Table V.Effects of DSW on BUN, LDH and CK
levels in imprinting control region mice. |
Table V.
Effects of DSW on BUN, LDH and CK
levels in imprinting control region mice.
| Group | BUN, mmol/l | LDH, U/l | CK, mmol/l |
|---|
| Control | 11.4±1.7 | 1,611.8±46.3 | 4,068.5±11.9 |
| DSW-H |
9.5±1.2a |
1,374.9±67.4b |
1,667.6±92.6b |
| DSW-M | 10.9±1.2 |
1,210.2±52.8b |
2,991.9±75.8b |
| DSW-L | 10.8±0.8 |
1,280.7±39.9b |
2,854.0±94.7b |
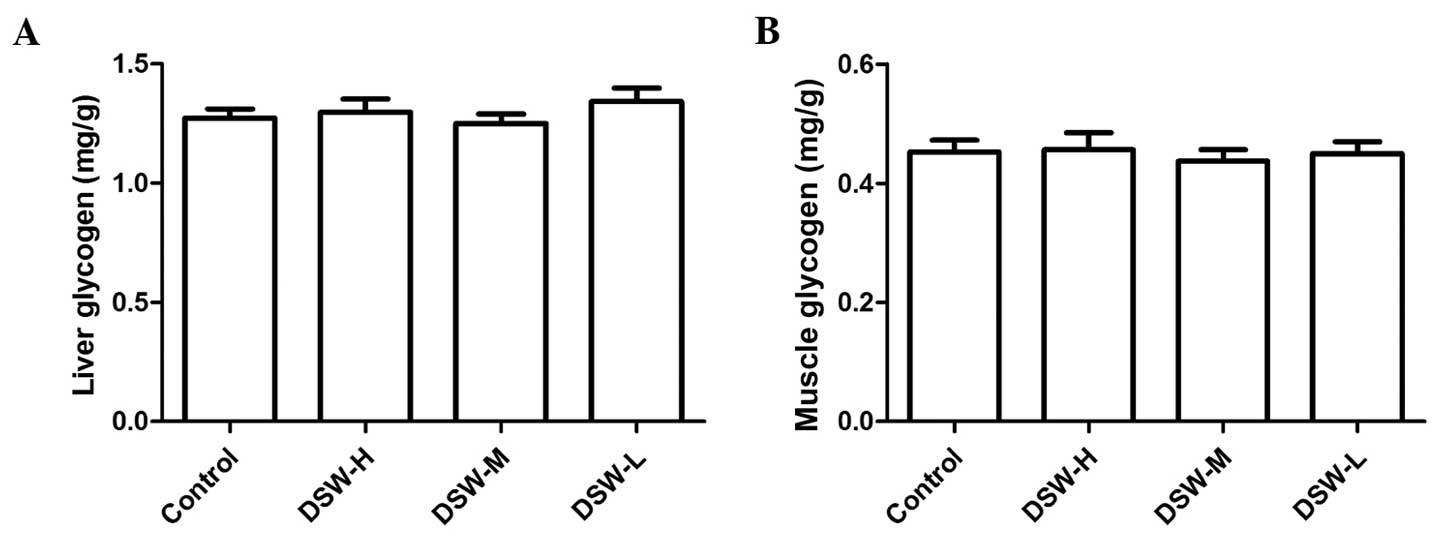
Effect of DSW on glycogen (group
IV)
No significant difference was observed for the
storage content of glycogen in the liver and right gastrocnemius
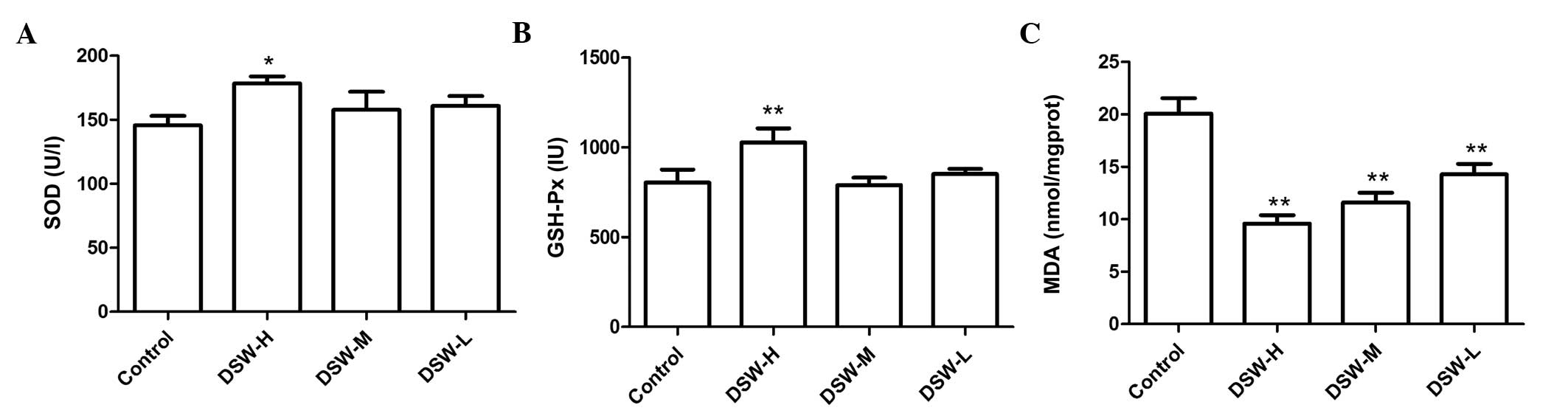
between each group (Fig. 2). MDA, as
one of the thiobarbituric acid-reactive substances, is an indicator
of lipid peroxidation. As the result, the DSW-treated groups
exhibited significantly lower plasma MDA concentrations compared to
that in the control group. By contrast, the DSW-H group showed
significantly higher SOD and GSH-Px activities compared to the
control group (Fig. 3).
Discussion
The results showed that DSW improved exercise
capacity and accelerated the recovery from physical fatigue, which
was associated with a decreased BLA curve, MDA levels,
post-swimming concentrations of BUN, LDH and CK levels, and an
increase of antioxidant enzyme activity such as SOD and GSH-Px.
Physical exhaustion is a special type of fatigue.
The mice swam until the muscles could not sustain movement, which
was associated with their physical condition. Physical condition
was therefore one of the evaluation indexes of delaying fatigue
during swimming. The present study clearly showed that DSW at a
high concentration extended the exhaustive swimming time in the
weight-loaded swimming test, which proved that this concentration
of DSW could enhance exercise capacity.
The accumulation of BLA in muscle and blood is one
of the dominant causes of physical fatigue (22). As the product of glycolysis under
anaerobic conditions, the increase in BLA would reduce the internal
pH, which may cause an aching muscle pain. Boron, a trace element
in DSW, has been found to reduce an exercise-induced rise in plasma
lactate (23). Certain changes in
enzyme activity, such as LDH and CK, can be used as an indication
of damage of the membrane permeability in skeletal muscle and the
recovery of sensitive biochemical indicators (24). It is known that the higher the BUN
level increases following exercise, the worse the capability to
adapt to exercise-loading is. As the product of energy metabolism,
BUN is an important indicator of fatigue status, which reflects the
ability to adapt to exercise (25). It
has been proved that rats subjected to a rubidium-free diet showed
higher BUN levels compared to the rats fed with rubidium (26). Rubidium is also an important element of
DSW. Glycogen is one of the storage forms of energy in the living
body. During strenuous exercise, the capability of glycogen storage
prior to exercise can directly affect the ability to exercise
(27). However, in the current study,
no significant difference was observed between the control and
DSW-treated groups.
Investigations of the connection between ROS and
diseases have recently attracted increasing attention. ROS can be
controlled by numerous types of antioxidant enzymes, such as SOD
and GSH-Px (28). Alternatively, MDA
as a cause of lipid peroxidation may lead to membranous dysfunction
of the cells and mitochondria (29).
According to Harman's theory of radicals, strenuous exercise can
disrupt the balance of the oxidative and antioxidant system,
resulting in the accumulation of reactive radicals, which limits
the continuation of exercise (30).
The increased level of metabolism, energy consumption, oxygen
consumption and body oxygen demand exceeds the actual oxygen uptake
during exercising. Simultaneously, the body is in a relatively
hypoxic state, leading to an increase in radicals. The accumulation
of oxidative metabolite, along with the relative decline of
antioxidant enzyme activity may cause lipid peroxidation, leading
to oxidative damage.
Previous studies have identified the anti-fatigue
effect of DSW in rats and humans (18,20). In the
present study, ICR mice were used as an alternative model to
determine the anti-fatigue effect of DSW and its mechanism. As
reported in certain previous studies, the increased generation of
radicals during exercise and the decreased exercise capacity are
most closely associated with the performance of radicals and lipid
peroxidation in the biofilm of various organs. GSH, SOD and
catalase (CAT) levels of the mice fed with antioxidants, such as
carvedilol and melatonin, were increased while the lipid
peroxidation was attenuated (31).
From the aforementioned results, DSW demonstrated
the properties of fatigue resistance and oxidizability. In the view
of the association between fatigue and oxidative damage, we
speculate that DSW may perform the anti-fatigue effect by
increasing the antioxidant activity, possibly as follows. Mg, which
participates in energy metabolism, catalyzing or activating >300
types of enzymes, is a necessary cofactor of a series of adenosine
triphosphate (ATP) enzymes, and has an extremely important role in
the promotion of protein synthesis. In addition, Mg is involved in
signal transduction, affecting parathyroid secretion. The study by
Rock et al (32) study
mentioned that free radical-mediated injury could contribute to
skeletal muscle lesions resulting from Mg deficiency. Mg deficiency
has been closely associated with production of ROS, cytokines and
eicosanoids, along with vascular compromise in vivo
(33). The activity center of GSH-Px
is selenocysteine, and selenium is a necessary part of GSH-Px
(34). There is a direct association
between inoxidizability and trace elements. Determination of GSH-Px
can be used as a measure of the vitality of the selenium levels of
a biochemical indicator. Coenzyme Q has an anti-fatigue effect in
mice (35). The compound of coenzym Q
requires selenium, and its enzyme activity is associated with the
content of selenium. Coenzyme Q is the important electronic
delivery vector of mitochondrial respiratory chain redox and
contributes to mitochondrial ATP synthesis, as well as
non-enzymatic antioxidants (35).
Lithium can increase the capability for scavenging free radical in
animals, and increases the resilience of a cell against destructive
free radical attack (18,36). In addition, trace elements such as
copper and zinc are highly correlated to antioxidants (37). CuZn-SOD is an SOD that can scavenge
superoxide anion radicals and protect cells from damage. Copper and
zinc constitute the prosthetic group of CuZn-SOD. Zinc is
associated with the stability of the enzyme whereas copper is
associated with the activity of the enzyme. The long issimus muscle
area and percentage of muscling were increased in pigs fed by
chromium picolinate (38), which
indicates that chromium may strengthen muscles.
By contrast, the main ingredients of DSW, taken from
the west rim of the Pacific Ocean in the present study, were Mg,
calcium, potassium, cobalt, selenium, zinc, boron, chromium and
vanadium. Therefore, DSW can supply a variety of minerals and trace
elements required for the antioxidant system, which serve to
improve the activity of antioxidant enzymes to reduce lipid
peroxidation level, resist exercise-induced oxidative stress and
ultimately cause an anti-fatigue effect to promote physical
recovery within sports.
The objective of the present study was to determine
the anti-fatigue and antioxidant effect of DSW. According to the
results of the present study, DSW may perform the anti-fatigue
effect by increasing antioxidant activity. However, even though DSW
is rich in a wide variety of trace elements, the type and content
of elements are different in different ocean areas and at different
depths of the same ocean. In addition, as DSW is a natural compound
that contains numerous types of minerals and trace element, it is a
challenge to determine the effect of every aspect in each element.
Despite the results suggesting the association between fatigue
resistance and inoxidizability, further study to demonstrate the
antioxidant effect of DSW is required. It has been reported that
antioxidants could improve the capability of exercise (7). In addition, the present results showed
the inoxidizability of DSW in the pre-exercise stage, so therefore
the inoxidizability contributed to fatigue resistance. Future
research should investigate the inoxidizability from further
aspects, such as lipid hydroperoxide and CAT. Furthermore, a
comparison of the inoxidizability of pre-exercise and post-exercise
is necessary.
In conclusion, DSW has the ability to alleviate
physical fatigue of ICR mice by decreasing BLA, BUN, LDH, CK and
MDA, and enhancing antioxidant capacity such as SOD and GSH-Px.
Although the present paper provides certain inoxidizability
evidence of DSW to explain the anti-fatigue mechanisms, it requires
more advanced research to identify the further mechanisms.
Acknowledgements
The authors would like to thank Pacific Deep Ocean
Biotech Co., Ltd., for providing DSW. The present study was
supported by the National Natural Science Foundation of China
(grant nos. 81302892, 81373520 and 81400231), Guangdong Natural
Science Foundation (grant nos. S2013040016226 and S2013010014777)
and the Science and Technology Planning Project of Guangdong
Province (grant no. 2014A020221013).
References
|
1
|
Mehta RK and Agnew MJ: Influence of mental
workload on muscle endurance, fatigue, and recovery during
intermittent static work. Eur J Appl Physiol. 112:2891–2902. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JC, Kao JY, Kuo DH, Liao CF, Huang CH,
Fan LL and Way TD: Antifatigue and antioxidant activity of
alcoholic extract from Saussurea involucrata. J Tradit
Complement Med. 1:64–68. 2011.PubMed/NCBI
|
|
3
|
Kennedy G, Spence VA, McLaren M, Hill A,
Underwood C and Belch JJ: Oxidative stress levels are raised in
chronic fatigue syndrome and are associated with clinical symptoms.
Free Radic Biol Med. 39:584–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coombes JS, Rowell B, Dodd SL, Demirel HA,
Naito H, Shanely RA and Powers SK: Effects of vitamin E deficiency
on fatigue and muscle contractile properties. Eur J Appl Physiol.
87:272–277. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davies KJ, Quintanilha AT, Brooks GA and
Packer L: Free radicals and tissue damage produced by exercise.
Biochem Biophys Res Commun. 107:1198–1205. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powers SK, DeRuisseau KC, Quindry J and
Hamilton KL: Dietary antioxidants and exercise. J Sports Sci.
22:81–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bak JP, Kim YM, Son J, Kim CJ and Kim EH:
Application of concentrated deep sea water inhibits the development
of atopic dermatitis-like skin lesions in NC/Nga mice. BMC
Complement Altern Med. 12:1082012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang CC, Yao CA, Lin YR, Yang JC and Chien
CT: Deep-sea water containing selenium provides intestinal
protection against duodenal ulcers through the upregulation of
Bcl-2 and thioredoxin reductase 1. PLoS One. 9:e960062014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuchiya Y, Watanabe A, Fujisawa N, Kaneko
T, Ishizu T, Fujimoto T, Nakamura K and Yamamoto M: Effects of
desalted deep seawater on hematologic and blood chemical values in
mice. Tohoku J Exp Med. 203:175–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheu MJ, Chou PY, Lin WH, Pan CH, Chien
YC, Chung YL, Liu FC and Wu CH: Deep sea water modulates blood
pressure and exhibits hypolipidemic effects via the AMPK-ACC
pathway: An in vivo study. Mar Drugs. 11:2183–2202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katsuda S, Yasukawa T, Nakagawa K, Miyake
M, Yamasaki M, Katahira K, Mohri M, Shimizu T and Hazama A:
Deep-sea water improves cardiovascular hemodynamics in Kurosawa and
Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull.
31:38–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li PC, Pan CH, Sheu MJ, Wu CC, Ma WF and
Wu CH: Deep sea water prevents balloon angioplasty-induced
hyperplasia through MMP-2: An in vitro and in vivo study. PLoS One.
9:e969272014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He S, Hao J, Peng W, Qiu P, Li C and Guan
H: Modulation of lipid metabolism by deep-sea water in cultured
human liver (HepG2) cells. Mar Biotechnol (NY). 16:219–229. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ha BG, Park JE, Shin EJ and Shon YH:
Effects of balanced deep-sea water on adipocyte hypertrophy and
liver steatosis in high-fat, diet-induced obese mice. Obesity
(Silver Spring). 22:1669–1678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu HY, Liu MC, Wang MF, Chen WH, Tsai CY,
Wu KH, Lin CT, Shieh YH, Zeng R and Deng WP: Potential osteoporosis
recovery by deep sea water through bone regeneration in SAMP8 mice.
Evid Based Complement Alternat Med. 2013:1619762013.PubMed/NCBI
|
|
17
|
Hataguchi Y, Tai H, Nakajima H and Kimata
H: Drinking deep-sea water restores mineral imbalance in atopic
eczema/dermatitis syndrome. Eur J Clin Nutr. 59:1093–1096. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou CW, Tsai YS, Jean WH, Chen CY, Ivy JL,
Huang CY and Kuo CH: Deep ocean mineral water accelerates recovery
from physical fatigue. J Int Soc Sports Nutr. 10:72013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim S, Chun SY, Lee DH, Lee KS and Nam KS:
Mineral-enriched deep-sea water inhibits the metastatic potential
of human breast cancer cell lines. Int J Oncol. 43:1691–1700.
2013.PubMed/NCBI
|
|
20
|
Wang ST, Hwang DF, Chen RH, Chen YC, Liang
CW, Lin CS and Tsai ML: Effect of deep sea water on the
exercise-induced fatigue of rats. J Food Drug Anal. 17:133–141.
2009.
|
|
21
|
Wang J, Huang HH, Cheng YF and Yang GM:
Structure analysis and laxative effects of oligosaccharides
isolated from bananas. J Med Food. 15:930–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White GE and Wells GD: The effect of
on-hill active recovery performed between runs on blood lactate
concentration and fatigue in alpine ski racers. J Strength Cond
Res. 29:800–806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yazici Z, Kaya Y, Baltaci AK, Mogulkoc R
and Oztekin E: The effects of boron administration on plasma leptin
and lactate levels in ovariectomized rats which had acute swimming
exercise. Neuro Endocrinol Lett. 29:173–177. 2008.PubMed/NCBI
|
|
24
|
Louthrenoo W, Weerayutwattana N,
Lertprasertsuke N and Sukitawut W: Serum muscle enzymes, muscle
pathology and clinical muscle weakness: Correlation in Thai
patients with polymyositis/dermatomyositis. J Med Assoc Thai.
85:26–32. 2002.PubMed/NCBI
|
|
25
|
Wu C, Chen R, Wang XS, Shen B, Yue W and
Wu Q: Antioxidant and anti-fatigue activities of phenolic extract
from the seed coat of Euryale ferox Salisb. and
identification of three phenolic compounds by LC-ESI-MS/MS.
Molecules. 18:11003–11021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yokoi K, Kimura M and Itokawa Y: Effect of
low dietary rubidium on plasma biochemical parameters and mineral
levels in rats. Biol Trace Elem Res. 51:199–208. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin Y, Liu HL, Fang J, Yu CH, Xiong YK and
Yuan K: Anti-fatigue and vasoprotective effects of
quercetin-3-O-gentiobiose on oxidative stress and vascular
endothelial dysfunction induced by endurance swimming in rats. Food
Chem Toxicol. 68:290–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yagmurca M, Erdogan H, Iraz M, Songur A,
Ucar M and Fadillioglu E: Caffeic acid phenethyl ester as a
protective agent against doxorubicin nephrotoxicity in rats. Clin
Chim Acta. 348:27–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu F, Lu S, Yu F, Feng S, McGuire PM, Li R
and Wang R: Protective effects of polysaccharide from Euphorbia
kansui (Euphorbiaceae) on the swimming exercise-induced
oxidative stress in mice. Can J Physiol Pharmacol. 84:1071–1079.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Atamaniuk J, Vidotto C, Tschan H, Bachl N,
Stuhlmeier KM and Müller MM: Increased concentrations of cell-free
plasma DNA after exhaustive exercise. Clin Chem. 50:1668–1670.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh A, Garg V, Gupta S and Kulkarni SK:
Role of antioxidants in chronic fatigue syndrome in mice. Indian J
Exp Biol. 40:1240–1244. 2002.PubMed/NCBI
|
|
32
|
Rock E, Astier C, Lab C, Vignon X, Gueux
E, Motta C and Rayssiguier Y: Dietary magnesium deficiency in rats
enhances free radical production in skeletal muscle. J Nutr.
125:1205–1210. 1995.PubMed/NCBI
|
|
33
|
Wiles ME, Wagner TL and Weglicki WB:
Effect of acute magnesium deficiency (MgD) on aortic endothelial
cell (EC) oxidant production. Life Sci. 60:221–236. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rahmanto AS and Davies MJ:
Selenium-containing amino acids as direct and indirect
antioxidants. IUBMB Life. 64:863–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu X, Ji R and Dam J: Antifatigue effect
of coenzyme Q10 in mice. J Med Food. 13:211–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adipudi V and Reddy VK: Effect of chronic
lithium chloride on membrane adenosine triphosphatases in certain
postural muscles of rats. Eur J Pharmacol. 259:7–13. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nazıroğlu M and Yürekli VA: Effects of
antiepileptic drugs on antioxidant and oxidant molecular pathways:
Focus on trace elements. Cell Mol Neurobiol. 33:589–599. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Page TG, Southern LL, Ward TL and Thompson
DL Jr: Effect of chromium picolinate on growth and serum and
carcass traits of growing-finishing pigs. J Anim Sci. 71:656–662.
1993.PubMed/NCBI
|