Introduction
In recent years, it has become increasingly common
for cancer chemotherapy to be administered even to patients with
underlying diseases. However, there is limited clinical data for
drug-drug interactions between anticancer agents and other disease
therapeutics.
Paclitaxel has been widely used as a key drug for a
number of cancers including ovarian cancer and lung cancer. The
major metabolic pathway of paclitaxel is via cytochrome P5402C8
(CYP2C8), which deactivates it to 6α-hydroxide (1). Clopidogrel is a common antiplatelet agent
used to inhibit blood clots. Clopidogrel is metabolized in the
liver to acyl-β-D-glucuronide, which has been recently shown to
inhibit CYP2C8 (2). Therefore, the
potential exists for paclitaxel and clopidogrel to cause drug
interactions, but the combination has rarely been examined
clinically (3).
In the present study, patients who had been
administered the combination of paclitaxel and clopidogrel were
investigated. To the best of our knowledge, this is the first
retrospective study investigating the potential interaction between
these two drugs.
Materials and methods
Background
Patients who were administered a combination of
paclitaxel and clopidogrel (75 mg/day) at Ogaki Municipal Hospital
(Ogaki, Gifu, Japan) between January 2009 and October 2015 were
identified. The gender, age, cancer type, regimen, paclitaxel dose,
kidney function, liver function and any adverse events were
extracted from the electronic medical record. The severity of
adverse events was evaluated using the Common Terminology Criteria
for Adverse Events (version 4.0; http://evs.nci.nih.gov/ftp1/CTCAE/About.html). The
present study was conducted with the approval of the Ogaki
Municipal Hospital ethics committee.
Adverse events and discontinuation
rate
The incidence of adverse events was analyzed,
including the discontinuation rate due to adverse events. These
rates were compared with typical clinical trials (4,5).
Comparison of average neutrophil count
prior and subsequent to administration of clopidogrel and
paclitaxel
Neutrophil counts and neutrophil reduction rate were
recorded and compared in patients prior and subsequent to the
combined administration of clopidogrel and paclitaxel. Neutrophil
reduction rate was calculated by this formula: [1 - (day 8
neutrophil counts/day 1 neutrophil counts)] × 100 (%).
Statistical analysis
Average neutrophil counts prior and subsequent to
co-administration of clopidogrel and paclitaxel were compared using
an unpaired t-test, and the neutrophil reduction rate was compared
using Mann-Whitney U-test with P<0.05 considered to indicate a
statistically significant difference. The statistical analysis
software used was EZR version 1.26 (6).
Results
Background
Patient characteristics and background are shown in
Table I. A total of 5 patients
received paclitaxel and clopidogrel concomitantly. The therapeutic
regimen for the patients included was carboplatin (nedaplatin) +
paclitaxel (4 cases), paclitaxel alone (1 case), carboplatin +
paclitaxel + radiation therapy (2 cases), or carboplatin +
paclitaxel (1 case). A total of 8 cases were analyzed. The only
drug used that influences CYP2C8 was clopidogrel. None of the 8
cases had any notable problems regarding blood cell counts prior to
chemotherapy.
 | Table I.Background information for the
patients administered a combination of paclitaxel and
clopidogrel. |
Table I.
Background information for the
patients administered a combination of paclitaxel and
clopidogrel.
| Patient | Case no. | Gender | Age, years | Reason for
clopidogrel use | PS | Liver
functiona | Creatinin clearance,
ml/minb | Cancer | Regimen | Paclitaxel, dose
mg/week | Total times of
paclitaxel administration |
|---|
| A | 1 | Female | 62 | Myocardial
infarction | 0 | Normal | 44 | Ovarian cancer
(following surgery) |
Carboplatin+paclitaxel every (AUC=5,
175mg/m2, 3 weeks, 3 courses) | 86.3 | 3 |
| B | 2 | Female | 77 | Cerebral
infarction | 1 | Normal | 68 | Ovarian cancer
(following surgery) |
Carboplatin+paclitaxel every (AUC=5,
175mg/m2, 3 weeks, 3 courses) | 70.0 | 1 |
| C | 3 | Female | 78 | Myocardial
infarction | 0 | Normal | 45 | Lung squamous cell
carcinoma (stage IIa) |
Carboplatin+paclitaxel+radiation therapy
(AUC=2, 40 mg/m2, weekly, 6 courses) | 60.0 | 3 |
| D | 4 | Male | 74 | Myocardial
infarction | 1 | Normal | 97 | Lung adenocarcinoma
(stage IIIa) |
Carboplatin+paclitaxel+radiation therapy
(AUC=2, 40 mg/m2, weekly, 6 courses) | 70.0 | 4 |
|
| 5 |
| 74 |
| 1 | Normal | 95 | Recurrent lung
adenocarcinoma |
Carboplatin+paclitaxel (AUC=6, 200
mg/m2, 21 daily) | 94.9 | 1 |
| E | 6 | Female | 62 | Angina pectoris
(following PCI4) | 0 | Normal | 62 | Second recurrent
ovarian cancer | Nedaplatin+paclitaxel
(75 and 180 mg/m2, respectively, every 3 weeks) | 96.0 | 6 |
|
| 7 |
| 65 |
| 0 | Normal | 69 | Third recurrent
ovarian cancer | Paclitaxel (80
mg/m2, weekly) | 132.8 | 15 |
|
| 8 |
| 65 |
| 0 | Normal | 69 | Third recurrent
ovarian cancer | Nedaplatin+paclitaxel
(75 and 180 mg/m2, respectively, every 3 weeks) | 96.0 | 5 |
Adverse events and discontinuation
rate
A summary of the adverse events for each case is
shown in Table II. Grade 3 (<1,000
to 500 counts/mm3) or higher neutropenia presented in
all cases, occurring from the first course of treatment in 5 cases.
Grade 4 neutropenia (<500 counts/mm3) was observed in
5 cases (88%) and 4 cases showed febrile neutropenia (50%).
Treatment was discontinued due to adverse events in 4 cases (50%).
Reduction of paclitaxel dose or skipping a course of treatment was
required in 6 cases (75%).
 | Table II.Summary of the adverse events
(AE). |
Table II.
Summary of the adverse events
(AE).
|
|
|
| Average cell count
after 8 days of paclitaxel, administration
counts/mm3 | Minimum cell count in
all courses, counts/mm3 |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Case no. | Received courses,
n | Dose reduction or
skip | Leucocyte | Neutrophil | Leucocyte | Neutrophil | Febrile
neutropenia | Administration end
reason |
|---|
| 1 | 3 | Yes | 1,243 | 140 | 960 | 100 | Yes | Protocol
finished |
| 2 | 1 | No | – | – | 860 | 340 | Yes | Discontinued for
AE |
| 3 | 1 | Yes | 2,097 | 1,047 | 1,350 | 710 | No | Discontinued for
AE |
| 4 | 1 | Yes | 1,655 | 1,040 | 620 | 300 | Yes | Protocol
finished |
| 5 | 1 | Yes | – | – | 640 | 190 | Yes | Discontinued for
AE |
| 6 | 6 | No | 1,148 | 370 | 840 | 230 | No | Completely
response |
| 7 | 7 | Yes | 1,781 | 875 | 850 | 250 | No | Progressive
disease |
| 8 | 9 | Yes | 818 | 200 | 410 | 50 | No | Discontinued for
AE |
Comparison of average neutrophil
counts prior and subsequent to combination of clopidogrel and
paclitaxel
The data include the findings for 1 patient who
underwent 6 courses of paclitaxel + nedaplatin therapy for a second
recurrent ovarian cancer and 3 years later had 6 courses of
paclitaxel + nedaplatin therapy for a third recurrence. Data were
not available on potential bone marrow suppressive therapy between
courses of chemotherapy.
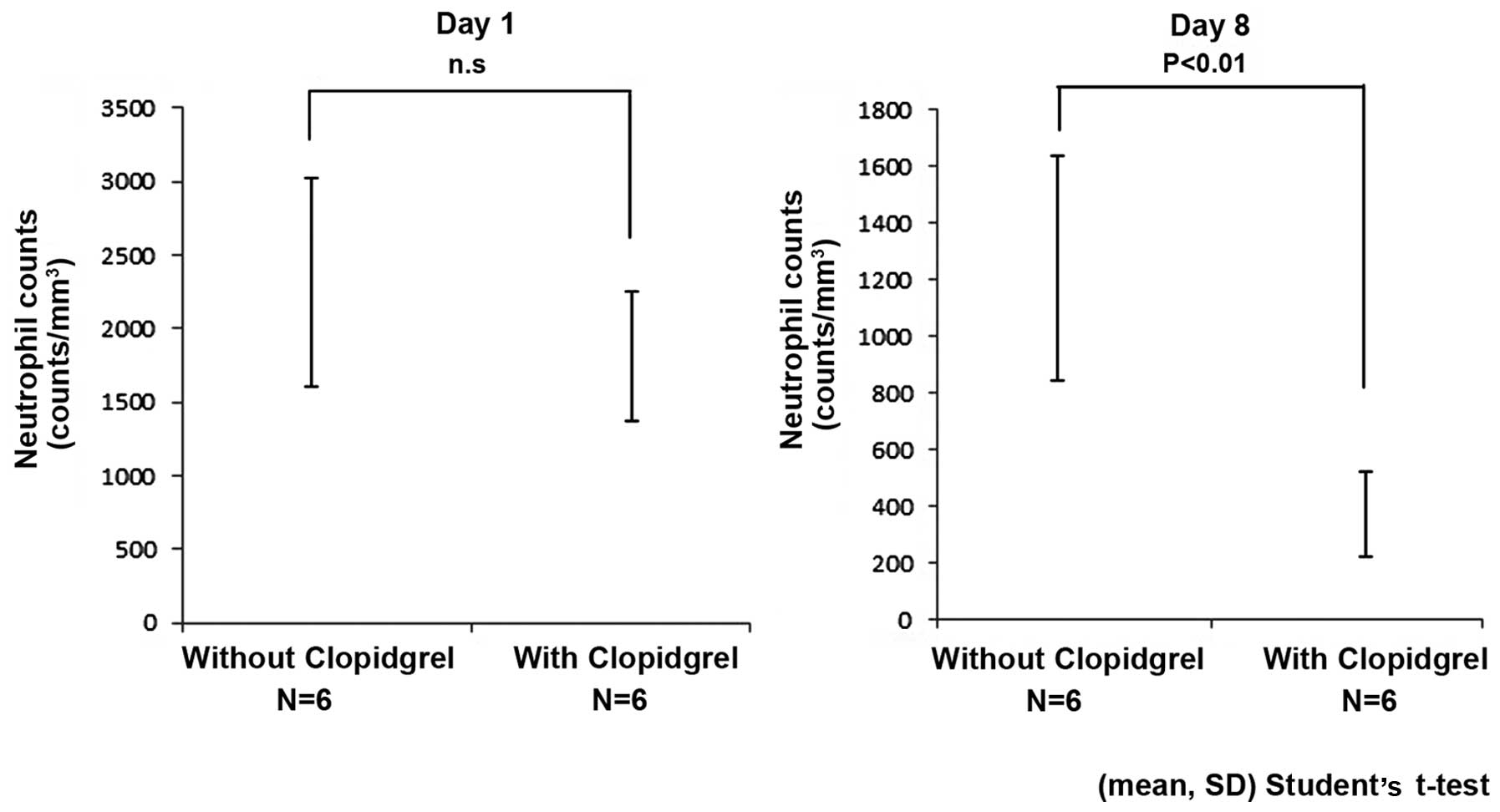
On the initial day of paclitaxel administration (day
1), average neutrophil counts showed no significant difference with
or without clopidogrel administration (without clopidogrel,
2,312±712 counts/mm3; with clopidogrel, 1,815±438
counts/mm3, mean ± standard deviation; P=0.18). At day 8
of paclitaxel administration, the average neutrophil counts with
clopidogrel co-administration were significantly reduced (without
clopidogrel, 1,240±395 counts/mm3; with clopidogrel,
370±148 counts/mm3, mean ± standard deviation;
P<0.01) (Fig. 1).
The neutrophil reduction rate was significantly
higher in combination with clopidogrel compared to without
clopidogrel [without clopidogrel: 54.1% (39.5–59.9%); with
clopidogrel: 82.6% (60.5–89.4%), median (range); P<0.01].
Discussion
Paclitaxel is believed to be mainly inactivated to
6α-hydroxide by CYP2C8 (1). Poor
metabolism by CYP2C8 reduces the clearance of paclitaxel by 11%. An
increased neuropathy risk by 2–3 times has been reported (7–9). In
addition, this has been shown to increase the risk of leucopenia
(10,11). Therefore, drug interaction with CYP2C8
inhibitors is assumed to increase the incidence and severity of
adverse events of paclitaxel.
Clopidogrel is metabolized in the liver and the
active metabolite has been shown to inhibit CYP2C8 (2). It has been reported that the activity of
CYP2C8 is inhibited 60–85% by continuous administration of 75 mg
clopidogrel/day; thus, its inhibitory effect is shown to be strong.
When clopidogrel is used in combination with a drug metabolized by
CYP2C8, it may decrease the drug clearance. The interaction of
paclitaxel and clopidogrel was previously reported by Bergmann
et al (3) in a case study; the
clearance of paclitaxel was reduced 38% by the co-administration of
clopidogrel. Despite this warning of the potential drug
interactions of the two agents, follow-up clinical studies are
lacking.
The variation in the background of the cases in this
study makes a simple comparison difficult. Paclitaxel + carboplatin
therapy (TC therapy) was used as the standard regimen for treatment
of ovarian cancer and lung cancer. TC therapy is associated with a
relatively high rate of neutropenia compared to other paclitaxel
regimens (grade 3 or 4 leukopenia: 59%, grade 3 or 4 neutropenia:
89–92%, febrile neutropenia: 9%) (4,5). However, TC
therapy administration for >6 courses has reported a rate of 87%
neutropenia and is well tolerated (4).
Although comparisons between different regimens are difficult, in
previous studies, patients received more carboplatin (AUC 6 and
7.5) compared to the patients in the present study. The paclitaxel
doses were similar to previous studies (175 and 180
mg/m2) and the majority of patients in the present
study. Therefore, the neutropenia risk is considered lower compared
to these studies. However, in the present study, neutropenia of
grade 3 or higher presented in all cases, and 50% discontinued
treatment with severe adverse events such as febrile neutropenia.
This suggests that the adverse events are amplified by the drug
interactions of paclitaxel and clopidogrel. A larger study that can
control for patient background is required in order to further
quantify this drug interaction.
For the 1 case involving paclitaxel + nedaplatin
therapy, it was possible to compare the average neutrophil counts
prior and subsequent to clopidogrel administration. The case also
used aspirin, atorvastatin and lansoplazole, following percutaneous
coronary intervention. Except for clopidogrel, these drugs cannot
be considered to influence drug interaction with paclitaxel, and
bone marrow suppression. The neutrophil reduction rate was
significantly higher following the combination treatment of
clopidogrel and paclitaxel compared to prior to clopidogrel
administration. Infection did not occur in this case, but the
average number of neutrophils at day 8 was <500
counts/mm3 with clopidogrel. In general, infection rates
increase when neutrophil counts fall <500 counts/mm3,
and the frequency and severity of infections are inversely
proportional to the number of neutrophils (12). Thus, when neutropenia is severe due to
the administration of clopidogrel, it is likely that the risk of
infection is also greatly increased.
The present study has certain limitations. One of
them is the small sample size (8 cases). Patient backgrounds were
not matched in each case, due to the different regimens.
Additionally, only 1 patient could be evaluated who received
paclitaxel with and without clopidogrel. Therefore, the impact of
aging is evident in prior and subsequent comparison of a single
case. Furthermore, the study was not a pharmacogenetic and
pharmacokinetic study. Therefore, more studies are required.
The drug interaction of paclitaxel and clopidogrel
cannot be clinically negligible, as the data suggest that there is
an increased risk of severe adverse events. Therefore, therapeutic
strategies should be considered to avoid the combination of these
two agents where possible. When a combination is required, it is
necessary to monitor for adverse events carefully.
References
|
1
|
Rahman A, Korzekwa KR, Grogan J, Gonzalez
FJ and Harris JW: Selective biotransformation of taxol to 6
alpha-hydroxytaxol by human cytochrome P450 2C8. Cancer Res.
54:5543–5546. 1994.PubMed/NCBI
|
|
2
|
Tornio A, Filppula AM, Kailari O, Neuvonen
M, Nyrönen TH, Tapaninen T, Neuvonen PJ, Niemi M and Backman JT:
Glucuronidation converts clopidogrel to a strong time-dependent
inhibitor of CYP2C8: A phase II metabolite as a perpetrator of
drug-drug interactions. Clin Pharmacol Ther. 96:498–507. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergmann TK, Filppula AM, Launiainen T,
Nielsen F, Backman J and Brosen K: Neurotoxicity and low paclitaxel
clearance associated with concomitant clopidogrel therapy in a 60
year old Caucasian woman with ovarian carcinoma. Br J Clin
Pharmacol. Oct 7–2015.(Epub ahead of print).
|
|
4
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katsumata N, Yasuda M, Takahashi F,
Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura
E, et al: Japanese Gynecologic Oncology Group: Dose-dense
paclitaxel once a week in combination with carboplatin every 3
weeks for advanced ovarian cancer: A phase 3, open-label,
randomised controlled trial. Lancet. 374:1331–1338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergmann TK, Brasch-Andersen C, Gréen H,
Mirza M, Pedersen RS, Nielsen F, Skougaard K, Wihl J, Keldsen N,
Damkier P, et al: Impact of CYP2C8*3 on paclitaxel clearance: A
population pharmacokinetic and pharmacogenomic study in 93 patients
with ovarian cancer. Pharmacogenomics J. 11:113–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hertz DL, Roy S, Motsinger-Reif AA,
Drobish A, Clark LS, McLeod HL, Carey LA and Dees EC: CYP2C8*3
increases risk of neuropathy in breast cancer patients treated with
paclitaxel. Ann Oncol. 24:1472–1478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hertz DL, Motsinger-Reif AA, Drobish A,
Winham SJ, McLeod HL, Carey LA and Dees EC: CYP2C8*3 predicts
benefit/risk profile in breast cancer patients receiving
neoadjuvant paclitaxel. Breast Cancer Res Treat. 134:401–410. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huizing MT, Vermorken JB, Rosing H, ten
Bokkel Huinink WW, Mandjes I, Pinedo HM and Beijnen JH:
Pharmacokinetics of paclitaxel and three major metabolites in
patients with advanced breast carcinoma refractory to anthracycline
therapy treated with a 3-hour paclitaxel infusion: A European
Cancer Centre (ECC) trial. Ann Oncol. 6:699–704. 1995.PubMed/NCBI
|
|
11
|
Nakajima M, Fujiki Y, Kyo S, Kanaya T,
Nakamura M, Maida Y, Tanaka M, Inoue M and Yokoi T:
Pharmacokinetics of paclitaxel in ovarian cancer patients and
genetic polymorphisms of CYP2C8, CYP3A4, and MDR1. J Clin
Pharmacol. 45:674–682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Comprehensive Cancer Network:
Prevention and treatment of cancer-related infections. Version
1.2013. http://oralcancerfoundation.org/treatment/pdf/infections.pdfAccessed.
February 05–2013
|