Introduction
Telomeres are the specialized nucleoprotein
structures found at the ends of the linear chromosomes of
eukaryotic cells. They are comprised of tandem arrays of short DNA
sequence repeats. Human telomeres are composed of several kilobases
of 5′-TTAGGG-3′ sequences on what is referred to as the G-rich
strand. The majority of the telomere repeats are found as duplexes,
but the 3′ end of the G-rich strand extends as a single strand.
Telomeres do not encode proteins. Instead, their repeat sequences
partly serve as a ‘buffer’ that can be lost when the 5′ terminal
RNA primers cannot be replaced following DNA replication [reviewed
in (1)]. The telomere length is
normally maintained in embryonic cells and stem cells by the
activity of telomerase. However, telomeres are shortened with each
successive round of DNA replication in cells that no longer express
telomerase, such as terminally differentiated skin fibroblasts.
Generally, once telomeres reach a critically short length, cells
become senescent (2).
Shelterin is a six-member protein complex that
associates with telomeric DNA in mammals [reviewed in (3)]. In cells with telomerase activity,
shelterin has a role in maintaining telomere length. This complex
is generally required to protect the ends of the chromosomes from
proteolytic degradation and prevent them from being recognized as
double-stranded DNA breaks. Without the protection of shelterin,
DNA repair mechanisms would be triggered, fusing the chromosomes
end-to-end resulting in genomic instability (3).
The six subunits of shelterin are telomeric repeat
factor 1 (TRF1), TRF2, TERF1-interacting nuclear factor 2 (TIN2),
protection of telomeres protein 1 (POT1), tripeptidyl peptidase 1
(TPP1) and repressor/activator protein 1 (RAP1). Among the
subunits, TRF1 and TRF2 bind to the duplex region of the repeat
array (4,5). Although structurally similar (6), TRF1 and TRF2 have extremely different
roles at the telomeres and bind to distinct sets of partner
proteins [reviewed in (7)]. TRF1
negatively regulates telomere length (8) and facilitates telomere DNA replication
(9), while TRF2 is essential for
telomere capping (3) and protecting
the telomeres from DNA damage repair mechanisms (10). TRF1 and TRF2 interact with TIN2. TIN2
stabilizes the TRF1-TRF2 interaction onto telomeric DNA (6). TRF2 also binds to RAP1, but TRF1 does not
(11).
The POT1 and TPP1 subunits of the complex are
interacting partners (12,13), and the two proteins are represented in
equal amounts in cells (14). The POT1
protein is found at the 3′ end of the G-rich strand, bound to the
single-stranded TTAGGG repeats (15).
TPP1 is required for the stability of POT1 (16). The TPP1-POT1 complex prevents a DNA
damage repair response at the telomeric overhang site (17,18). TPP1 is
also involved in connecting POT1 to TIN2 (13,19,20). As TIN2 also binds to TRF1 and TRF2, it
serves to connect all of the DNA binding activities of shelterin
(21–23).
Of the six shelterin subunits, the highly conserved
RAP1 protein is the only subunit that is not essential in mice
(24,25). As part of shelterin, RAP1 contributes
to the maintenance of genome stability by protecting telomeric DNA
ends from non-homologous end joining (26,27) and from
homologous recombination that can alter telomere length (24). RAP1 is recruited to telomeric repeats
by TRF2, and in mouse cells, RAP1 levels are dependent on TRF2
(10). In humans, RAP1 is more
abundant than TRF2, and knocking down TRF2 resulted in a reduction
in RAP1 (14). By contrast,
immunodepletion of RAP1 resulted in a loss of TRF2 from cell
extracts (26,27).
RAP1 has non-telomeric activities as well. In the
nucleus, mouse RAP1 associates with non-telomeric chromatin, and a
knockout leads to an alteration in the expression of a set of
genes, about a third of which contained TTAGGG sequences in their
promoter regions (25). In human
cells, RAP1 binds to selective telomeric sequences located at
interstitial sites and regulate gene transcription (28). In addition to its nuclear functions,
RAP1 has been found in the cytoplasm of human cells. RAP1 is
required for inhibitor of nuclear factor (NF)-κB kinase (IKK)
phosphorylation of the p65 subunit of NF-κB, making p65 competent
for transcriptional activation (29).
The telomere is a dynamic structure, and the
interaction between its proteins and DNA is key to the integrity of
the genome. Progressive shortening of telomeres that occurs in
normal, somatic cells suggests that telomere stability is disrupted
over time since there should be fewer available binding sites for
shelterin to bind. A previous study assessed whether there was a
correlation between various lengths of telomere sequences and the
levels of the shelterin components (14). However, the study was performed using
immortalized cells with varying length telomeres that maintained
those lengths generation after generation. A quantitative analysis
of shelterin components in cells that undergo normal telomere
attrition would be informative in understanding the natural aging
process. In the present study, the progression of normal, somatic
cells was followed through their natural life span using neonatal
human dermal fibroblasts.
Telomere lengths and cellular phenotypes of young
cells (low population doubling) and old cells (high population
doubling) were observed and the levels of shelterin components in
the two populations were compared. In addition, the expression
levels of RAP1 were evaluated in the nucleus and the cytoplasm of
cells that were naturally or prematurely aged by hydrogen peroxide
(H2O2)-induced oxidative stress or via serum
deprivation.
Materials and methods
Cell lines
Human dermal fibroblasts derived from neonatal
foreskin (HDFn) and growth medium were obtained from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). HDFn cells were
grown at 37°C with 5% CO2 in medium 106 supplemented
with low serum growth supplement. Cells were passaged according to
the manufacturer's protocol. The population doubling levels (PDLs)
of cells were calculated as follows: PDL = 3.32 ×
(logXharvest - logXseeding) + starting PDL,
where Xharvest is the number of cells at the time of
harvest and Xseeding is the number of cells seeded
(30). The U251 glioblastoma cell line
was kindly provided by Dr Prakash Chinnaiyan from the H. Lee
Moffitt Cancer Center and Research Institute (Tampa, FL, USA). U251
cells were propagated in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) in 5% CO2
environment at 37°C. At each passage, the cells were grown to
80–100% confluence and were subsequently split at a 1:5 ratio.
Telomere length analysis
To isolate genomic DNA, HDFn cells were grown in
100-mm plates. When the cells were ~95% confluent, the genomic DNA
from a desired PDL was isolated using the DNeasy® Blood
and Tissue kit (Qiagen, Valencia, CA, USA). The lengths of
telomeres were analyzed using the TeloTAGGG Telomere Length assay
from Roche Life Sciences US (Indianapolis, IN, USA) according to
the manufacturer's protocol. Briefly, equal amounts (1.5 µg) of
genomic DNA from young and old cells were digested with RsaI
and HinfI, which digest non-telomeric sequences but leave
telomere intact. The generated DNA fragments were resolved on a
0.8% agarose gel for 4 h at 80 V. Transfer of the DNA fragments
onto a nylon membrane by capillary action occurred for ~24 h. DNA
was cross-linked with UV to the nylon membrane using a Stratalinker
(Stratagene, Santa Clara, CA, USA) according to the manufacturer's
protocol. Both prehybridization and hybridization were performed at
42°C overnight with gentle agitation. Detection of the signal was
performed using autoradiography. Calculation of the mean telomere
lengths was determined using Telometric (31).
Cell extract preparation and
fractionation
For whole cell extracts, HDFn cells were grown in
100-mm dishes to ~95% confluence. At the desired population
doubling, cells were washed with cold 1X phosphate-buffered saline
(PBS) and lysed in radioimmunoprecipitation assay buffer [150 mM
NaCl, 1.0% NP-40, 0.5% deoxycholate, 0.1% SDS and 50 mM Tris-HCl
(pH 8.0)] containing protease inhibitors (cOmplete EDTA-free
Protease Inhibitor Cocktail Tablet; Sigma-Aldrich, St. Louis, MO,
USA) for 2 min. The cells were scraped from the surface of the
flasks, transferred to microcentrifuge tubes and centrifuged at
20,000 × g for 5 min. The soluble fractions were transferred to new
tubes. For cellular fractionation, the cells were washed with cold
1X PBS and fractionated as previously described (32). Protein concentrations of all samples
were determined using the Pierce® BCA Protein assay kit
(Thermo Fisher Scientific, Inc., Chicago, IL, USA).
Induction of oxidative stress
Oxidative damage was introduced to HDFn cells by
treatment with H2O2 as follows. The cells
were grown in 100-mm dishes to ~95% confluence to a PDL of 9.
Short-term oxidative stress was induced by incubating cells in
medium containing a final concentration of 20 µM
H2O2. The cells were exposed to the
H2O2 for 2 h at 37°C. Cells without
H2O2 treatment were run in parallel as a
control. After the incubation, treated and untreated cells were
washed with 1X PBS and fresh growth medium was added. Four days
post-treatment, images of the cells were captured, and the were
subsequently fractionated as previously described (32).
U251 cells were stressed using
H2O2 or serum deprivation. The cells were
grown in 100-mm dishes to ~80% confluence in DMEM/10% FBS.
Short-term oxidative stress was induced by adding
H2O2 at a final concentration of 1 mM for 3 h
at 37°C. For serum-starvation, cells were grown in DMEM/0.5% FBS at
37°C overnight. Cells without H2O2 treatment,
grown in DMEM/10% FBS were run in parallel as a control. Following
the incubation, cells were washed with 1X PBS and harvested, lysed
and fractionated as previously described (32).
Immunoblotting
The samples were resolved on 10% SDS-polyacrylamide
gels and were transferred onto nitrocellulose membranes. Blocking
and incubation with antibodies was performed in 5% non-fat dry
milk/10 mM Tris (pH 7.5), 150 mM NaCl and 0.05% Tween-20 (TBST).
Membranes were washed with TBST. The primary antibodies used in the
study were obtained from the following vendors: mouse monoclonal
anti-human TRF2 (cat. no. 05-521) from Calbiochem (Billerica, MA,
USA) used at 1:1,000 dilution; rabbit polyclonal anti-human RAP1
(cat. no. A300-306A) from Bethyl Laboratories (Montgomery, TX, USA)
used at 1:2,000 dilution; rabbit polyclonal anti-human TIN2 (cat.
no. ab82998) from Abcam Inc. (Cambridge, MA, USA) used at 1:1,000
dilution; rabbit polyclonal anti-human POT1 (cat. no. NB500-176)
from Novus Biologicals (Littleton, CO, USA) used at 1:1,000
dilution; and goat polyclonal anti-human actin (cat. no. sc-1616)
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) used at
1:2,000 dilution. Secondary antibodies conjugated to horseradish
peroxidase were used in conjunction with the SuperSignal West Pico
chemiluminescent kit (Thermo Fisher Scientific, Inc.) for detection
of the proteins in Figs. 1B, 2 and 3. For
Fig. 1A, protein detection was
performed using the Odyssey (LI-COR Biosciences, Lincoln, NE, USA)
with the secondary antibodies IRDye 800CW (cat. no. 925-32211) and
IRDye 680RD (cat. no. 925-68070) used at 1:50,000 dilutions. The
intensities of the bands were determined either densitometrically
using ImageJ (33) or using the
Odyssey CLx imager quantitation program.
Results
HDFn cells at increased PDLs exhibit
hallmarks of aging
The present study aimed to determine the fate of the
shelterin subunits in normal, differentiated human cells with a
finite number of divisions as they age using HDFn cells. The cells
grew for specific numbers of PDL and young cells of only a few PDL
were compared with old cells isolated following numerous doublings.
Before analyzing the shelterin complex, cell aging was confirmed by
examining the cellular morphologies and telomere lengths of young
and old cells. HDFn cells isolated at 3 or 6 PDL were considered to
be young, and cells at PDL28, PDL32 and PDL38 were considered old.
These PDLs were chosen to represent old cells to avoid using
senescent cells that have telomeres that have become critically
short and are no longer dynamically changing. At PDL6, the cells
appeared long and slender with spindle-like features characteristic
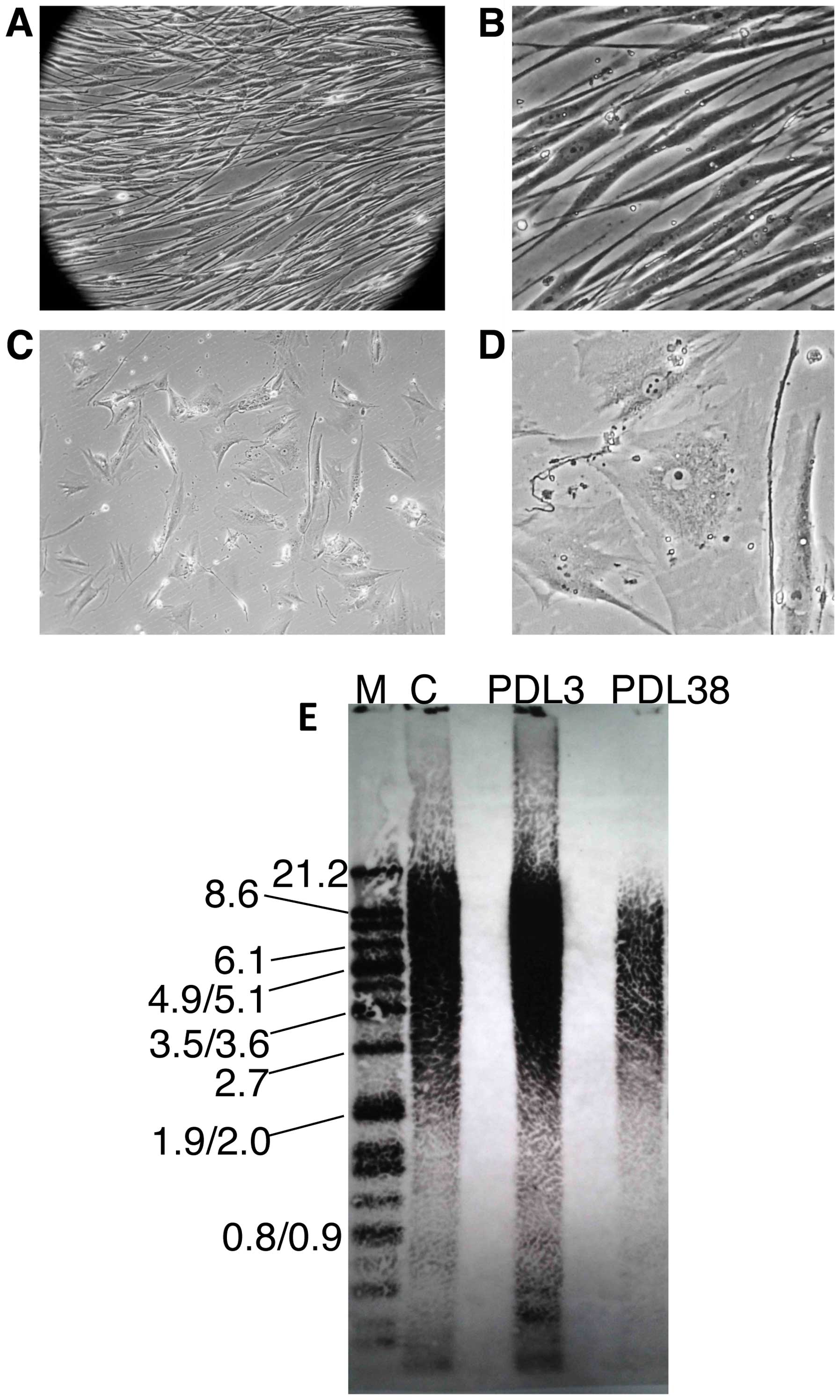
of young fibroblasts (Fig. 4A and B).
By contrast, cells at PDL38 were irregularly shaped, more flattened
with the presence of large vacuoles and had an increased cytoplasm
volume (Fig. 4C and D). As HDFn cells
are terminally differentiated, their telomeres are expected to
shorten with each round of DNA replication. The telomere lengths
were measured in young (PDL3) and old (PDL38) HDFn cells. As
expected, the telomere lengths were heterogeneous (Fig. 4E). The mean terminal restriction
fragment length for the HDFn cells at PDL3 was ~10.5 kilobase pair
(kbp) while it was ~5.1 kbp for the HDFn cells at PDL38. In
addition to the decreased length of the telomeres, the telomeric
signal of the DNA isolated from the old cells was significantly
reduced compared to the younger cells, indicating less telomeric
DNA in the older population.
Abundance of shelterin components as
telomeres shorten in aging cell populations
The levels of various shelterin subunits in young
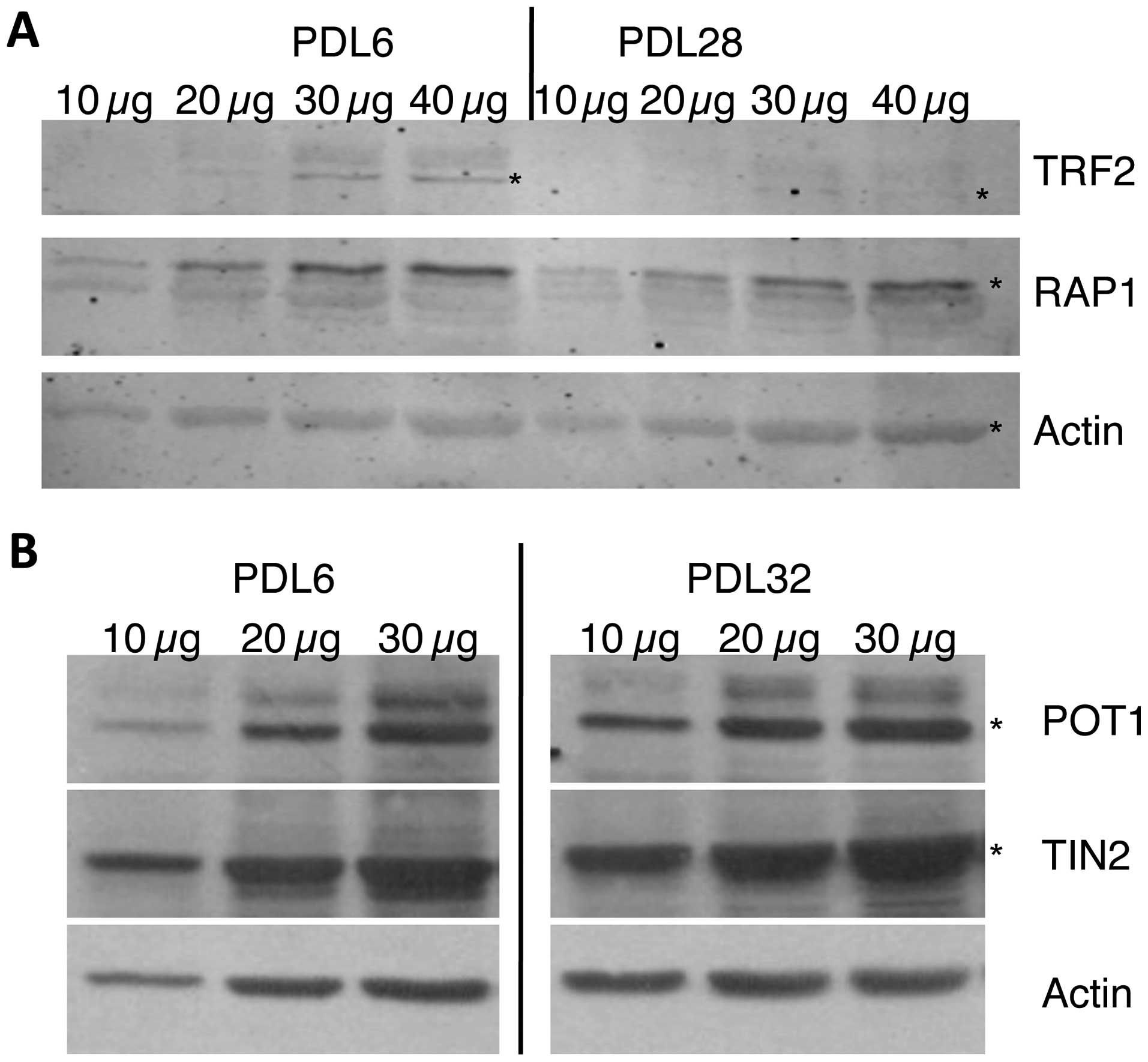
and old HDFn cells were measured. When comparing TRF2 levels of
older (PDL28) and younger (PDL6) cells, the amounts of TRF2 were
diminished in older cells to approximately one-third of those
observed in the younger cells (Fig.
1A). However, when RAP1, which requires TRF2 to direct it to
the telomeres, was measured from the same samples, the level in the
older cells was 75% that of the younger cells.
The POT1 subunit binds to the single-stranded
repeats at the end of the G-rich strand. The TIN2 protein forms a
bridge between POT1 and the rest of the shelterin complex. Levels
of the single-stranded DNA binding protein POT1 and its interacting
partner TIN2 were compared between young (PDL6) and old (PDL32)
cell populations. The POT1 and TIN2 proteins appeared to be at
approximately the same levels in the young and old cells (Fig. 2B).
Subcellular localization of RAP1 and
TRF2 in young versus old cell populations
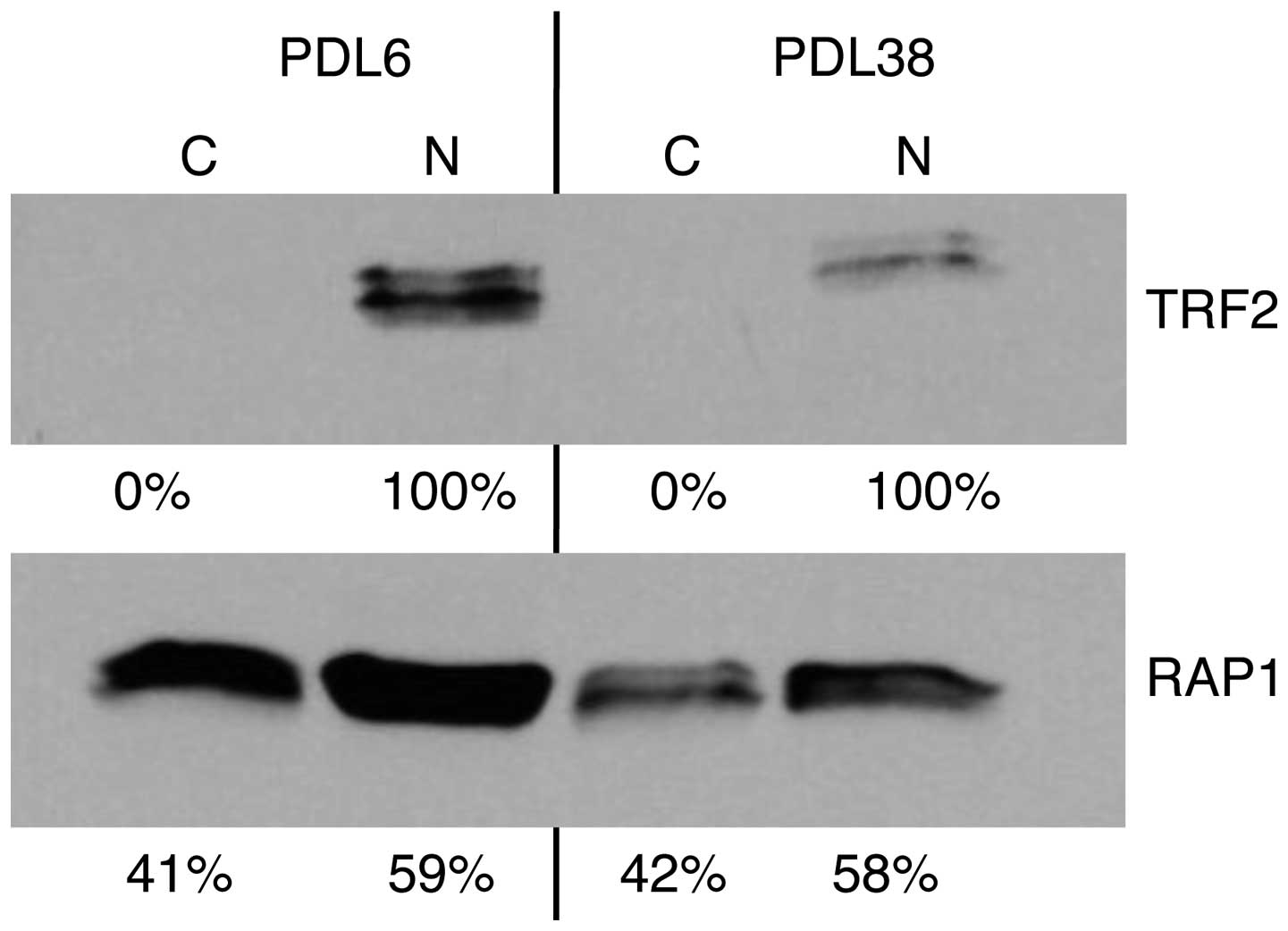
To determine where in the cells the RAP1 and TRF2
proteins were located, nuclear and cytoplasmic fractions of young
(PDL3) and old (PDL38) HDFn cell lysates were analyzed. As
expected, TRF2 was only found in the nucleus (Fig. 2, upper panel). RAP1 was found in the
nuclear and cytoplasmic fractions (Fig.
2, lower panel). When measuring total levels of TRF2 and RAP1
by adding the amounts for each fraction together and subsequently
comparing old and young cells, the TRF2 and RAP1 levels at PDL38
(Fig. 2) had continued to decline as
the cells aged from PDL28 (Fig. 1A).
In the older (PDL38) cells, TRF2 levels were ~26% of those from the
PDL6 population, while RAP1 levels of PDL11 were ~53% of those of
PDL6. Although decreased, the levels of RAP1 continued to remain
higher in the older population. However, the decrease in RAP1
levels was distributed between the nuclear and cytoplasmic
pools.
Effects of oxidative stress on
RAP1
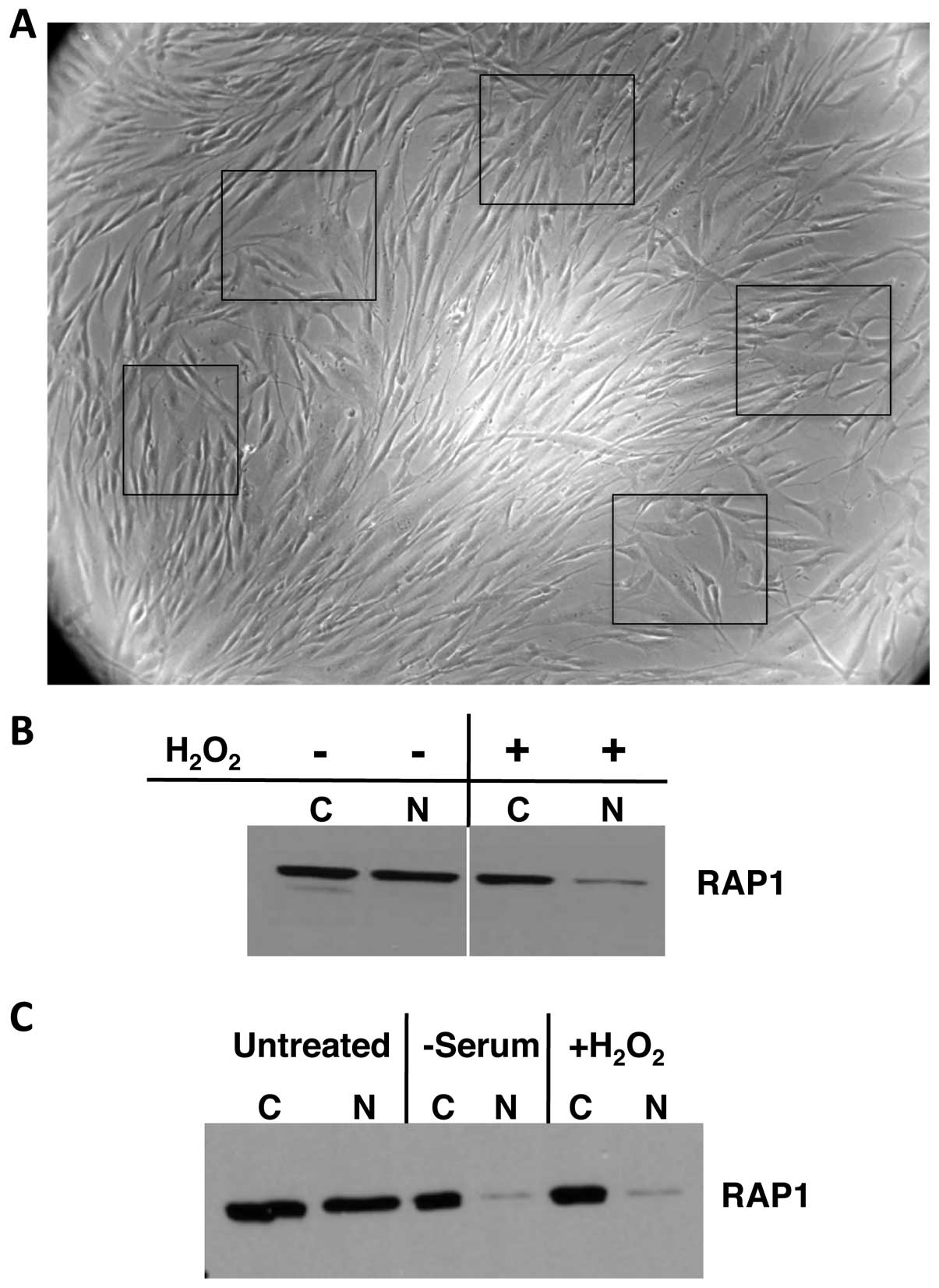
When young HDFn cells were treated with
H2O2 they showed typical aged-cell
morphologies (Fig. 3A compared to
cells at PDL38 in Fig. 4C and D).
To determine the effects of oxidative stress on
RAP1, HDFn cells were treated with H2O2 and
fractionated into cytoplasmic and nuclear portions. In the
untreated cells, RAP1 levels in the cytoplasm and nucleus were
similar, but H2O2 treatment resulted in a
loss of the nuclear RAP1 with no effect on the cytoplasmic pool
(Fig. 3B). To determine whether this
phenomenon was a general feature of all cells or just those without
telomerase activity as in the HDFn cells, U251 glioblastoma cells
were exposed to H2O2. Similar to the results
of the H2O2-treated HDFn cells, the nuclear
RAP1 was severely depleted (Fig. 3C).
Withdrawing serum, which results in oxidative stress, from U251
cells led to RAP1 levels in the cytoplasm and nucleus that were
similar to the levels in cells treated with
H2O2 (Fig.
3C).
Discussion
Telomeres protect the ends of linear chromosomes,
providing genomic stability. The DNA repeats of the telomere serve
as a binding platform for the shelterin complex. As opposed to the
rest of the genome in normal, terminally differentiated cells, the
telomere is a dynamic structure. As cells replicate their DNA for
cell division, the telomere shortens due to an inability to replace
the terminal RNA primers. Thus, the length of the telomere limits
the number of replication cycles in cells without telomerase, the
enzyme that normally extends telomeres in embryonic and stem
cells.
As cells progress through numerous divisions,
telomere shortening is believed to result in a reduced number of
binding sites for the shelterin complex, leading to the expectation
that shelterin subunit levels may decrease concomitantly. A
previous study showed that the levels of the shelterin subunits did
not reflect telomere lengths (14).
However, those experiments were performed using immortalized cell
lines with telomeres that were stable across generations. To the
best of our knowledge, the present study is the first to compare
levels of shelterin components between younger and older
populations in a single-cell lineage of human dermal fibroblasts as
they age.
HDFn with a finite lifespan were grown and isolated
at early passages (young cells) or after numerous PDLs (old cells).
Particular PDLs for the old cells were chosen, such that the cells
exhibited aging cell morphologies and shortened telomeres (Fig. 4) without being completely senescent, in
order to determine the fate of shelterin as the telomere is
changing. As telomere repeats decrease in number over generations,
the single-stranded DNA overhangs at the ends of the telomere
should remain stable. Congruently, the levels of POT1, which bind
to the single-stranded DNA of the telomere, were the same in young
and old cells (Fig. 1B). TPP1 is one
of the binding partners of POT1 (12,13), and
TPP1 and POT1 are found in a 1:1 stoichiometry in shelterin
(14), thus the levels of TPP1 were
not assessed. TIN2 connects POT1/TPP1 to the remainder of shelterin
(21–23). TIN2 levels in the present study also
did not change as cells aged (Fig.
1B).
As the telomere lengths decrease, the levels of TRF2
decreased (Fig. 1A). This result would
support the general hypothesis that the loss of potential binding
sites in the telomere would affect the abundance of telomeric
protein. Technical difficulties complicated the measurement of
TRF1. However, previous study has shown that TRF1 levels are
substoichiometric compared to the levels of TRF2 (14), so we would speculate that the levels of
TRF1 would also decrease at a rate similar to that of TRF2. The
levels of RAP1, the binding partner of TRF2, also decreased in
older cells, but the decrease was less than that of TRF2 (Fig. 1A). These data are in line with RAP1
being involved in one or more non-telomeric functions, possibly DNA
binding for the control for the control of gene expression in the
nucleus (25,28) and as an effector protein for NF-κB
signaling in the cytoplasm (29) as
cells age.
Since RAP1 has been found to play roles in both the
nucleus and cytoplasm, we undertook experiments to determine where
the decreases in RAP1 levels occurred in older cells. As has been
shown previously (29,14), we only detected TRF2 in the nucleus of
cells, but RAP1 was found in both the nuclear and cytoplasmic
fractions (Fig. 2). Interestingly, the
decrease in the levels of RAP1 was distributed between the nucleus
and the cytoplasm with the levels in each compartment decreasing
the same relative amount. The decrease in the amount of RAP1 in the
nucleus most likely reflects the decrease in TRF2. The RAP1
remaining in the nucleus is probably made up of both RAP1 that is
associated with the telomere repeats that remain and the RAP1 bound
at other chromosomal locations. It was surprising that the
cytoplasmic levels of RAP1 also declined in the older cells. Since
RAP1 has been shown to be a positive regulator of NF-κB signaling
(29), some RAP1 may remain in the
cytoplasm to carry out that function. NF-κB normally provides cells
with protection from apoptosis (34 and references therein). Based
on the finding that knocking down RAP1 sensitized breast tumor
cells to apoptosis (29), it is
possible that RAP1 in the cytoplasm may decrease over successive
generations to allow aged cells to become more susceptible to
apoptosis as well. This may provide a mechanism by which cells that
are old and more likely damaged (e.g., through the accumulation of
ROS and DNA damage) will undergo apoptosis rather than become
immortalized. Another possibility is that the levels of RAP1 may
decrease in older cells so that a different effector protein can
regulate NF-κB signaling. Since increased NF-κB activation makes
fibroblasts less susceptible to reprogramming (35), it seems likely that RAP1 may be
exchanged for some other signaling factor. This may allow the cells
to alter their responses to various stimuli between young and old
cells. Hypothetically, decreased RAP1 levels may help provide a
barrier to cellular reprogramming and sensitize cells to apoptosis.
Additional study in this area seems prudent.
One factor known to affect aging is oxidative
stress. As cells replicate, oxidative damage accumulates, and this
can be seen in cellular phenotypes. We treated our young neonatal
human dermal fibroblasts with H2O2 to cause
premature aging. The levels of H2O2 we used
resulted in cellular morphologies reminiscent of old cells
(Fig. 3A), which correlate with
previous studies, though telomeres did not shorten (36). Using these conditions, nuclear RAP1
levels were greatly diminished while the levels in the cytoplasm
remained relatively stable (Fig. 3B).
Similar results were obtained when U251 glioblastoma cells were
treated with H2O2, indicating that the
decrease in RAP1 levels occurs in cells that express telomerase,
maintaining long telomeres, as well as in the HDFn cells that do
not express telomerase. Serum depletion has been shown to increase
the occurrence of ROS in cells. We depleted serum from the growth
medium of U251 cells, the RAP1 levels in the nucleus decreased, but
those in the cytoplasm remained stable, similarly to the
H2O2 treatment (Fig.
3C). Overall, RAP1 levels in the nucleus decreased when the
cells were treated with H2O2 or
serum-depleted medium, suggesting a possible role for RAP1 in the
response to oxidative stress. The maintenance of RAP1 in the
cytoplasm of the stressed, young HDFn cells and the immortalized
glioblastoma cells seems likely due to maintaining NF-κB signaling
to promote cell survival. The conditions used for the
H2O2 treatment of the HDFn cells do not
result in a loss of telomeric repeats (36), and yet there is a loss of nuclear RAP1.
One possible explanation is that the cytoplasmic RAP1 is turned
over rapidly during NF-κB signaling under such extreme conditions,
and the nuclear RAP1 translocates to maintain the cytoplasmic pool
for continued signaling.
Acknowledgements
The present study was supported by Midwestern
University College of Health Sciences and intramural funding from
Midwestern University.
Glossary
Abbreviations
Abbreviations:
|
HDFn
|
human dermal fibroblasts derived from
neonatal foreskin
|
|
PDL
|
population doubling level
|
References
|
1
|
Campisi J: Aging, cellular senescence, and
cancer. Annu Rev Physiol. 75:685–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blackburn EH: Telomere states and cell
fates. Nature. 408:53–56. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Lange T: Shelterin: The protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bianchi A, Smith S, Chong L, Elias P and
de Lange T: TRF1 is a dimer and bends telomeric DNA. EMBO J.
16:1785–1794. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Broccoli D, Smogorzewska A, Chong L and de
Lange T: Human telomeres contain two distinct Myb-related proteins,
TRF1 and TRF2. Nat Genet. 17:231–235. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Court R, Chapman L, Fairall L and Rhodes
D: How the human telomeric proteins TRF1 and TRF2 recognize
telomeric DNA: A view from high-resolution crystal structures. EMBO
Rep. 6:39–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ancelin K, Brunori M, Bauwens S, Koering
CE, Brun C, Ricoul M, Pommier JP, Sabatier L and Gilson E:
Targeting assay to study the cis functions of human telomeric
proteins: Evidence for inhibition of telomerase by TRF1 and for
activation of telomere degradation by TRF2. Mol Cell Biol.
22:3474–3487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sfeir A, Kosiyatrakul ST, Hockemeyer D,
MacRae SL, Karlseder J, Schildkraut CL and de Lange T: Mammalian
telomeres resemble fragile sites and require TRF1 for efficient
replication. Cell. 138:90–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Celli GB and de Lange T: DNA processing is
not required for ATM-mediated telomere damage response after TRF2
deletion. Nat Cell Biol. 7:712–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Oestreich S and de Lange T:
Identification of human Rap1: Implications for telomere evolution.
Cell. 101:471–483. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xin H, Liu D, Wan M, Safari A, Kim H, Sun
W, O'Connor MS and Songyang Z: TPP1 is a homologue of ciliate
TEBP-beta and interacts with POT1 to recruit telomerase. Nature.
445:559–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu D, Safari A, O'Connor MS, Chan DW,
Laegeler A, Qin J and Songyang Z: PTOP interacts with POT1 and
regulates its localization to telomeres. Nat Cell Biol. 6:673–680.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takai KK, Hooper S, Blackwood S, Gandhi R
and de Lange T: In vivo stoichiometry of shelterin components. J
Biol Chem. 285:1457–1467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baumann P and Cech TR: Pot1, the putative
telomere end-binding protein in fission yeast and humans. Science.
292:1171–1175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hockemeyer D, Palm W, Else T, Daniels JP,
Takai KK, Ye JZ, Keegan CE, de Lange T and Hammer GD: Telomere
protection by mammalian Pot1 requires interaction with Tpp1. Nat
Struct Mol Biol. 14:754–761. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Churikov D and Price CM: Pot1 and cell
cycle progression cooperate in telomere length regulation. Nat
Struct Mol Biol. 15:79–84. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo X, Deng Y, Lin Y, Cosme-Blanco W, Chan
S, He H, Yuan G, Brown EJ and Chang S: Dysfunctional telomeres
activate an ATM-ATR-dependent DNA damage response to suppress
tumorigenesis. EMBO J. 26:4709–4719. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Podell ER, Zaug AJ, Yang Y, Baciu
P, Cech TR and Lei M: The POT1-TPP1 telomere complex is a
telomerase processivity factor. Nature. 445:506–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Houghtaling BR, Cuttonaro L, Chang W and
Smith S: A dynamic molecular link between the telomere length
regulator TRF1 and the chromosome end protector TRF2. Curr Biol.
14:1621–1631. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye JZ, Donigian JR, van Overbeek M, Loayza
D, Luo Y, Krutchinsky AN, Chait BT and de Lange T: TIN2 binds TRF1
and TRF2 simultaneously and stabilizes the TRF2 complex on
telomeres. J Biol Chem. 279:47264–47271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SH, Beausejour C, Davalos AR, Kaminker
P, Heo SJ and Campisi J: TIN2 mediates functions of TRF2 at human
telomeres. J Biol Chem. 279:43799–43804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen LY, Liu D and Songyang Z: Telomere
maintenance through spatial control of telomeric proteins. Mol Cell
Biol. 27:5898–5909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sfeir A, Kabir S, van Overbeek M, Celli GB
and de Lange T: Loss of Rap1 induces telomere recombination in the
absence of NHEJ or a DNA damage signal. Science. 327:1657–1661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinez P, Thanasoula M, Carlos AR,
Gómez-López G, Tejera AM, Schoeftner S, Dominguez O, Pisano DG,
Tarsounas M and Blasco MA: Mammalian Rap1 controls telomere
function and gene expression through binding to telomeric and
extratelomeric sites. Nat Cell Biol. 12:768–780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bae NS and Baumann P: A RAP1/TRF2 complex
inhibits nonhomologous end-joining at human telomeric DNA ends. Mol
Cell. 26:323–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarthy J, Bae NS, Scrafford J and Baumann
P: Human RAP1 inhibits non-homologous end joining at telomeres.
EMBO J. 28:3390–3399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang D, Xiong Y, Kim H, He Q, Li Y, Chen R
and Songyang Z: Human telomeric proteins occupy selective
interstitial sites. Cell Res. 21:1013–1027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teo H, Ghosh S, Luesch H, Ghosh A, Wong
ET, Malik N, Orth A, de Jesus P, Perry AS, Oliver JD, et al:
Telomere-independent Rap1 is an IKK adaptor and regulates
NF-kappaB-dependent gene expression. Nat Cell Biol. 12:758–767.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayflick L: Subculturing human diploid
fibroblast cultures. Tissue Culture Methods and Applications. Kruse
PF Jr and Patterson MK: Academic Press. (New York, NY). 220–223.
1973.
|
|
31
|
Grant JD, Broccoli D, Muquit M, Manion FJ,
Tisdall J and Ochs MF: Telometric: A tool providing simplified,
reproducible measurements of telomeric DNA from constant field
agarose gels. Biotechniques. 31:1314–1316, 1318. 2001.PubMed/NCBI
|
|
32
|
Méndez J and Stillman B: Chromatin
association of human origin recognition complex, cdc6, and
minichromosome maintenance proteins during the cell cycle: Assembly
of prereplication complexes in late mitosis. Mol Cell Biol.
20:8602–8612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abramoff MD, Magalhaes PJ and Ram SJ:
Image Processing with ImageJ. Biophoton Int. 11:36–42. 2004.
|
|
34
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soria-Valles C, Osorio FG,
Gutiérrez-Fernández A, De Los Angeles A, Bueno C, Menéndez P,
Martín-Subero JI, Daley GQ, Freije JM and López-Otín C: NF-κB
activation impairs somatic cell reprogramming in ageing. Nat Cell
Biol. 17:1004–1013. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen QM, Prowse KR, Tu VC, Purdom S and
Linskens MH: Uncoupling the senescent phenotype from telomere
shortening in hydrogen peroxide-treated fibroblasts. Exp Cell Res.
265:294–303. 2001. View Article : Google Scholar : PubMed/NCBI
|