Introduction
High aerobic glycolysis is a predominant feature of
cancer cells, which is termed the Warburg effect (1,2). This
bioenergetic and metabolic characteristic permits cancer cells to
survive under adverse tumor conditions (such as hypoxia) and
enables them to proliferate and invade, subsequently leading to
distant metastasis (3). The control
point of glycolysis is glyceraldehyde 3-phosphate dehydrogenase
that requires nicotinamide adenine dinucleotide (NAD+)
in the glycolytic direction. Cancer cells provide the required
NAD+ via lactate dehydrogenase (LDH), which converts
pyruvate to lactate with concomitant regeneration of
NAD+. The gene expression and activity of LDH is
increased in various types of tumor when compared with healthy
tissue samples (4–6). However, there is another approach that
supplies the required NAD+ for glycolysis, which is
cytosolic malate dehydrogenase (MDH) (7,8). MDH, as a
part of the malate-aspartate shuttle, catalyzes the reversible
reaction of oxaloacetate (OAA) to malate in the presence of NADH.
Typically, the enzyme has two distinct forms, mitochondrial and
cytosolic. The cytosolic isoform is involved in the oxidation of
NADH to NAD+, which is then used for continuing the
progression of glycolysis (9).
Environmental parameters, such as pH, oxygen and
nutrient availability, influence the enzyme kinetics via different
approaches (10). The tumor
microenvironment is defined as a heterogeneous milieu in which the
oxygen pressure, pH and nutrient availability are completely
different from healthy tissues (11).
However, the nature and importance of the tumor environment on
enzyme kinetics has been masked in certain enzyme studies, owing to
the use of cell culture conditions in which pH is in the normal
range, without any fluctuation, and where oxygen and nutrients are
constantly accessible (12).
Alteration of the enzyme characteristics in the tumor
microenvironment, which alters the enzyme kinetics, has received
little attention. Given the potential role of MDH to supply
NAD+ and the effect of stressful tumor microenvironment
on enzyme kinetics, the aim of the current study was to compare the
kinetic parameters of MDH between breast cancer tissue samples and
cell lines (MCF-7 and MDA-MB-231) and healthy mammary tissue
samples. Furthermore, the potential role of MDH for sustaining the
glycolysis pathway in breast cancer cells was investigated.
Materials and methods
Clinical sample collection
Ten human breast tumor samples were obtained from
Apadana Hospital (Ahvaz, Iran) during the mastectomy procedure from
February 2012 to September 2013. Healthy tissue samples away from
the tumor were included as controls. Two independent expert
pathologists from the pathology laboratory of Apadana Hospital
performed the pathological tumor tissue examination. Samples were
immediately preserved in liquid nitrogen, transported to the
laboratory and stored at −80°C. The study was approved by the
ethics committee from Jundishapour Medical University of Ahvaz
(Ahvaz, Iran; associated with Apadana Hospital) and conducted
according to the Guide for Human study by the National Academy of
Sciences (National Institutes of Health), and informed consent was
obtained from all patients involved in the study.
Sample preparation
Frozen tumor and healthy tissue samples were
homogenized (1:5; w:v) in ice cold homogenization buffer (20 mM
Tris-HCl, pH 8.0, 10 mM 2-mercaptoethanol, 10% v:v glycerol, 2 mM
EDTA, 2 mM EGTA and 20 mM β-glycerophosphate) and a few crystals of
phenylmethylsulphonyl fluoride were added at the time of
homogenization. Samples were homogenized using a Miccra homogenizer
(MICCRA GmbH, Müllheim, Germany), centrifuged for 10 min at 10,000
× g at 4°C to remove tissue debris. The supernatant was then
centrifuged for 30 min at 25,000 × g at 4°C to obtain high speed
supernatant that contained the cytoplasmic enzymes (13). Finally, the supernatant was decanted
and held on ice until use. Low molecular weight metabolites and
ions were removed from the supernatant by Sigma-Aldrich Sephadex
G-25 columns (1×5 cm; Merck KGaA, Darmstadt, Germany) equilibrated
in the homogenizing buffer. The samples were then pooled and held
at 4°C until use for subsequent enzyme kinetic
characterization.
Cell culture, cell suspension, cell
homogenate and cytosolic fraction preparation
MCF-7 and MDA-MB-231 cells were obtained from the
Pasteur Institute Collection of Cell Cultures (Tehran, Iran). MCF-7
and MDA-MB-231 cells were maintained at 37°C in the presence of 5%
CO2 in RPMI-1640 supplemented with 10% inactivated fetal bovine
serum, 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin. Prior to each experiment, the culture medium was
removed and the plated MCF-7 and MDA-MB-231 cells were washed with
phosphate-buffered saline (PBS) medium containing 138 mM NaCl, 2.7
mM KCl, 8 mM Na2HPO4, 15 mM
KH2PO4, pH 7.4, and collected by
trypsinization into 1 ml PBS medium. Cells (~30×105,
grown to 80% confluence) were suspended in 3 ml cold isolation
buffer (0.32 M sucrose, 1 mM EDTA and 10 mM Tris-HCl, pH 7.5).
Cytosolic fractions were obtained according to a previous study
(14) with certain modifications.
Briefly, cells were homogenized at 4°C with a Miccra homogenizer
and centrifuged at 25,000 × g for 30 min at 4°C to obtain
supernatant, which contained the cytoplasmic enzymes.
Effect of oxamate on LDH and MDH
activity
To evaluate the effect of oxamate (Appliechem GmbH,
Darmstadt, Germany) on cell proliferation and select the
appropriate oxamate concentration, cells were plated into 96-well,
flat-bottomed plates at 2–4×103 cells/100 µl per well.
After the overnight incubation at 37°C, triplicate wells were
treated with varying concentrations of oxamate, ranging from 5 to
80 mM for 3 days. The relative percentage of metabolically active
cells relative to the untreated controls was then determined on the
basis of the mitochondrial conversion of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to
formazine. The quantity of 3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyltetrazolium bromide that is converted to
formazine indicates the number of viable cells. The results were
assessed in a 96-well format plate reader by measuring the
absorbance at a wavelength of 540 nM (A540 nm). The
percentage of metabolically active cells was compared with the
percentage of control cells growing in the absence of oxamate in
the same culture plate. The half maximal inhibitory concentrations
(IC50) were determined by nonlinear regression analysis
using the equation for a sigmoid plot. The MCF-7 and MDA-MB-231
cells were seeded in 25-cm2 flasks in duplicate. MCF-7
cells were treated (based on the IC50 results) with 30
and 60 mM oxamate, and MDA-MB-231 cells were treated with 10 and 20
mM oxamate for 72 h, with untreated cells serving as controls.
Oxamate was administered at a cell confluency of 70% for each cell
line. After 72 h, cells were harvested and processed for cell
lysate preparation according to the previous method for cell
culture preparation. LDH activity was determined by monitoring the
rate of conversion from NADH to NAD+ in the present of
1.5 mM pyruvate and 0.25 mM NADH. MDH activity was measured using
the same method as for LDH, with 1 mM OAA used instead of pyruvate
as a substrate. The results were normalized to the LDH and MDH
activity per protein content. The protein concentrations were
quantified using the Bradford protein assay (15), and experiments were performed in
triplicate.
Enzyme assay and kinetic
parameters
MDH activity was measured in the presence of OAA
with NADH as substrates for the forward reaction and malate with
NAD+ as substrates for the reverse reaction. The lowest
concentration of each substrate, which demonstrated maximum
velocity, constant rate of product formation and linear regressions
of activities for serial dilutions of enzyme, was assigned as the
optimum substrate concentration.
Reactions were initiated by adding 10 µl of crude
enzyme to a 200-µl total reaction volume by using 20 mM Tris-Hcl
buffer (pH 8) in the microplate well. Activity was monitored at 340
nM (A340 nm) for assessing the conversion of NADH to
NAD+ (or vice versa) by using a BioTek PowerWave X2
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) and
Gen5 version 2.0 software (BioTek Instruments, Inc.; kinetic mode,
reading interval=39 sec).
Data were analyzed using microplate analysis and
kinetics programs 3.51 (16,17). Kinetics 3.51 computer program fitted
data through a nonlinear least squares regression for determination
of the Michaelis-Menten constant (Km; the substrate
concentration resulting in half-maximal activity) and maximum
velocity (Vmax) values.
The Km and Vmax were
calculated from the mean of three separate series of
determinations. Total protein content was measured using the
Bradford method and bovine serum albumin (Sigma-Aldrich; Merck
KGaA) as standard. Due to the potential existence of endogenous
NADH to NAD+ interconversion (i.e., NADH oxidation by
complex І activity) in the crude extract, the NADH to
NAD+ interconversion was surveyed in each sample to
eliminate the possible existence of its effect. This
interconversion was determined by adding the NADH (0.5–1 mM) or
NAD+ (3–5 mM) into the samples and monitoring the
absorbance change at 340 nm.
Statistical analysis
Data were expressed as the mean ± SEM, from
independent determinations on separate preparations of enzymes.
Data were analyzed using Student's t-test and P<0.05 was
considered to indicate a statistically significant difference.
Results
Kinetic properties of MDH and LDH
Optimum assay conditions for MDH in the forward
reaction were 1 mM OAA and 0.25 mM NADH in tumor and healthy tissue
samples, and 1.5 mM pyruvate and 0.25 mM for LDH. It should be
noted that no NADH to NAD+ interconversion activity (or
vice versa) was observed in the crude extract samples.
The maximal activity of MDH in tumor samples for
malate formation (6,978±9.1 mU/g) was significantly greater than
the values in the healthy tissue samples (5,651±12.7 mU/g; Table I) (P<0.05). The Km OAA of
MDH were not identified to be significantly different between the
tumor (0.023±0.004 mM) and healthy (0.02±0.006 mM) samples
(P>0.05). The maximal activity of LDH in the tumor samples for
lactate formation (8,476±7.2 mU/g) was significantly higher than
that of the healthy tissue samples (5,330±9.2 mU/g) (P<0.05;
Table I).
 | Table I.Kinetic parameters of malate
dehydrogenase and lactate dehydrogenase from breast tumor and
healthy tissue samples. |
Table I.
Kinetic parameters of malate
dehydrogenase and lactate dehydrogenase from breast tumor and
healthy tissue samples.
| Kinetic
parameter | Tumor | Healthy |
|---|
| Km OAA
(mM) | 0.023±0.004 | 0.02±0.006 |
| Vmax OAA
(mU/g) |
6,978±9.1a | 5,651±12.7 |
| Vmax
pyruvate (mU/g) |
8,476±7.2a | 5,330±9.2 |
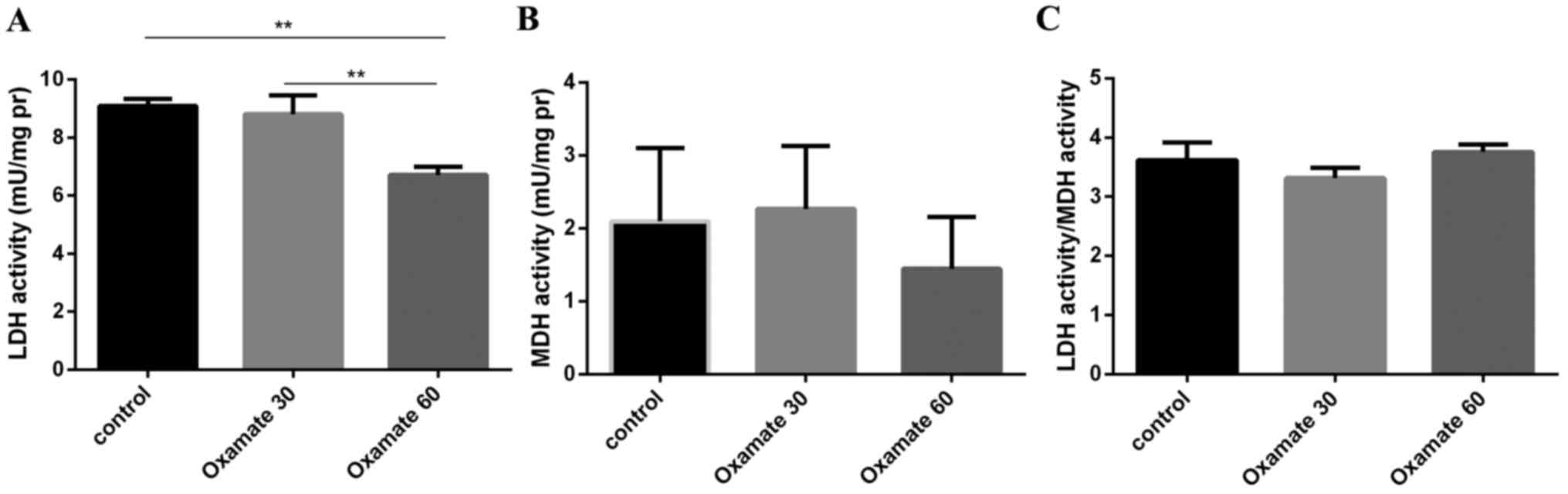
Effect of oxamate on LDH and MDH
activity
The effect of oxamate on LDH and MDH activity in the
MCF-7 cell lines was evaluated at two concentrations, 30 and 60 mM.
Compared with the control (9.087±0.07 mU/mg), LDH activity was not
significantly decreased by 30 mM oxamate after 72 h (8.8±0.20
mU/mg); however, 60 mM oxamate significantly decreased LDH activity
(6.73±0.09 mU/mg). Following treatment with 60 mM oxamate
(P<0.05), MDH activity remained stable and was not significantly
changed in the MCF-7 cell line (Fig.
1; P>0.05).
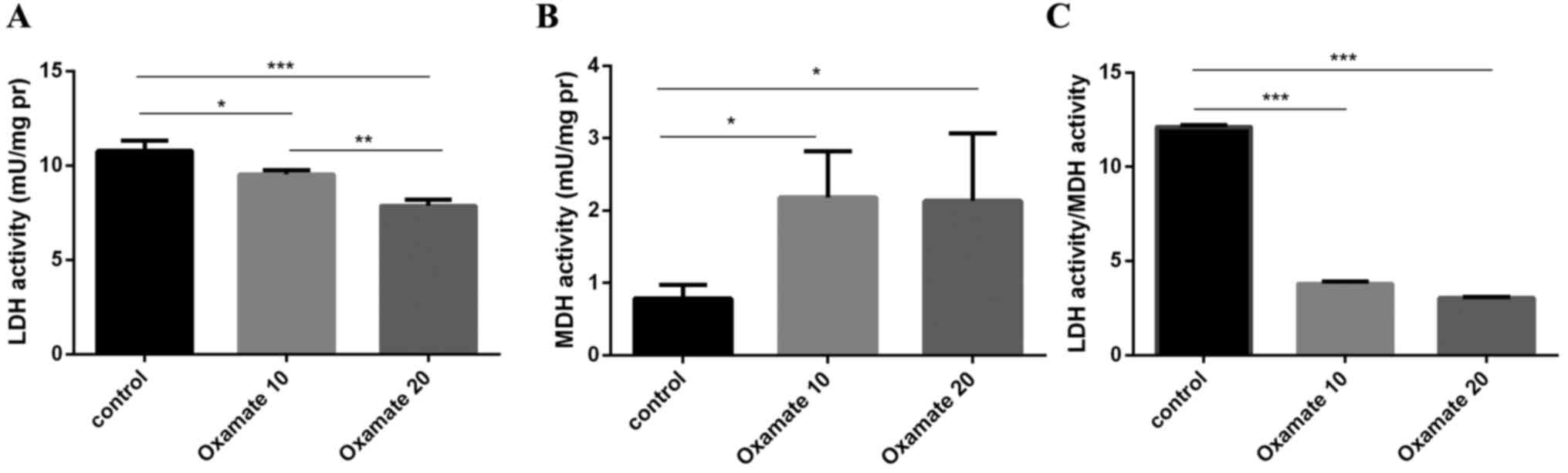
LDH and MDH activity of MDA-MB-231 were measured
following treatment with 10 and 20 mM oxamate for 72 h. Compared
with the control (10.763±0.17 mU/mg), LDH activity of MDA-MB-231
was significantly decreased by treatment with 10 mM (9.53±0.06
mU/mg) and 20 mM (7.87±0.1 mU/mg) oxamate (P<0.05). Notably, MDH
activity was increased by ~3-fold following treatment with 10
(2.183±0.20 mU/mg) and 20 mM (2.153±0.29 mU/mg) oxamate compared
with the control (0.785±0.06 mU/mg; Fig.
2), and no significant difference was identified between the
increase of MDH activity following 10 and 20 mM oxamate treatment
(P>0.05).
Discussion
High aerobic glycolysis is one of the main hallmarks
of cancer cells, which is driven by multiple enzymes. The enzyme
rate is regulated via two different processes as follows: i) Change
in the total quantity of the enzyme present (Vmax); ii)
change in one or more kinetic constants (Km or
Vmax). These processes and the enzyme stability are
strongly influenced by the composition of the intracellular milieu
in which the enzyme operates (10).
Given the different microenvironmental conditions of the tumor
compared with the healthy tissue, the enzyme function may differ
between healthy tissue and tumor samples. The data from the current
study supported the hypothesis and indicated that the kinetics of
MDH, as one of the important enzymes in metabolism, has a
distinctive feature in tumors and may be an alternative approach
for supporting the glycolysis pathway.
The maximum activity measured was assumed to be
primarily due to the cytosolic fraction, as the highest
concentration of OAA used in the assay method (1 mM) was
considerably greater than that (by 0.04 mM), which was known to
inhibit the activity of the mitochondrial fraction (14). Furthermore, to demonstrate that the
samples were free of the mitochondrial enzyme form, the
supernatants, made from specimens, were assessed for NADH oxidase
activity, as the mitochondrial marker and the interrupter of
dehydrogenase. The results clarified that there was no NADH oxidase
activity; therefore, the supernatant was free of mitochondrial
particles.
The present data indicated that the maximum activity
of the cancerous MDH (C-MDH) for producing NAD+ and
malate is higher than the normal MDH (N-MDH). This result is
consistent with the current hypothesis regarding the role of MDH in
supporting NAD+ pool. In addition, a greater
Vmax of MDH causes a higher level of NAD+,
which may be used as a precursor for sustaining a high rate of
aerobic glycolysis in tumors. According to the attained results,
the required NAD+ for the continuous flow of glycolysis
may be supplied by cytoplasmic MDH in addition to eminent LDH in
tumors. In the current study, the LDH kinetics in tumor and healthy
tissue samples were evaluated. The Vmax of C-LDH for the
generation of NAD+ and pyruvate was ~2-fold higher than
N-LDH. This is consistent with previous studies, which demonstrated
markedly higher LDH activity and gene expression in tumors when
compared to the relevant healthy samples (5,18,19). In the current study, while LDH and MDH
had the same maximum activity in the healthy samples, in the tumor
samples, the two were increased and LDH had a greater
Vmax than MDH, demonstrating the more significant role
of LDH in tumorigenesis. Thus, increasing the activity of LDH and
MDH may be a strategy of tumorigenesis and proliferation for
adapting to the stressful tumor environment.
Malic enzyme (ME) produces more pyruvate from the
abundant quantity of malate generated by C-MDH. Pyruvate is used as
an LDH substrate and supports high aerobic glycolysis (20). Therefore, MDH, through producing a high
level of malate, sustains a high glycolysis rate in an indirect
model. Thus, MDH supports high aerobic glycolysis in tumors by
generating two metabolites: NAD+ and malate. In order to
verify the role of MDH in supporting glycolysis, the MDH activity
was measured in breast cancer cell lines, treated by oxamate (the
inhibitor of LDH). Notably, the reduction of LDH activity by
oxamate was concomitant with the increase of the MDH activity in
MDA-MB-231 cells, although the MDH activity in the MCF-7 cells was
stable following oxamate treatment. Furthermore, the ratio of LDH
to MDH demonstrated the pattern of the MDH activity increment
subsequent to oxamate treatment. The ratio of LDH to MDH decreased
in the MDA-MB-231 cells following oxamate treatment; however, no
change was observed in the MCF-7 cells (Figs. 1 and 2).
Increasing the MDH activity in MDA-MB-231 cells, compared with the
MCF-7 cells, is associated with the glycolysis patterns in the two
cancer cell types. MDA-MB-231 cells have a higher rate of
glycolysis than MCF-7 cells and are more dependent on glycolysis as
the main energy source (21). Thus,
MDA-MB-231 cells were more susceptible to glycolysis and LDH
inhibition. It is conceivable that cancer cells increase the MDH
activity to compensate for the LDH deficiency and produce
NAD+ for sustaining a high rate of aerobic glycolysis,
even though the production rate of NAD+ by MDH in
comparison with LDH is negligible. This finding can also be
considered in LDH-associated cancer therapy, where LDH is targeted
to disrupt the glycolysis pathway as the mainstream energy pathway
in cancer cells. In recent years, efforts have been made to
eliminate LDH activity and gene expression (22,23).
Evaluation of alternative metabolism pathways is required, where
LDH activity is inhibited to halt the glycolysis pathway, as the
cancer cells employ various approaches (such as MDH) to repair
their deficiency in order to support the glycolysis pathway. Given
the role of MDH in supporting glycolysis, inhibition of MDH
activity and gene expression, concomitant with LDH, may assist with
halting the glycolysis pathway and enhancing LDH removal
efficiency. Furthermore, the obtained results regarding increasing
MDH activity in the presence of oxamate are consistent with a
previous report (21), which indicated
the more malignant MDA-MB-231 cells are more dependent on
glycolysis and LDH than the benign MCF-7 cells.
The current data are not consistent with the
findings of Balinsky et al (24) where they demonstrated that cytosolic
MDH in tumor and healthy breast tissues exerts the same activity,
whereas the current results demonstrate that MDH has higher maximal
activity in tumor tissue samples when compared with healthy tissue
samples. The difference between these two studies may be associated
with the various methods applied, for example Balinsky et al
(24) used an electrophoretic method
for enzyme activity detection whereas a spectrophotometric assay of
LDH and MDH activities was used in the current study. The results
regarding the Km of the forward reaction are in contrast
to the results of the study by Grisham et al (13), which is one of the initial studies on
the kinetics of MDH in tumors. The current results indicated that
the affinity of MDH in forward reaction is the same in tumor and
healthy tissue samples. By contrast, Grisham et al (13) expressed that the Km of OAA
in tumor samples was higher than that in healthy tissue samples.
Therefore, it can be concluded that the difference between these
two studies is justified by considering the fact that Grisham et
al (13) used 0.2 and 2 mM of OAA
as the highest concentration of OAA in healthy and tumor tissue
samples, respectively, whereas in the current study, the highest
OAA concentration in the two tissue types was 1 mM. The present
data demonstrated that the concentrations >1 mM exerted an
inhibitory effect on MDH activity and that 1 mM was the optimum
concentration in the two types of tissue.
The kinetic differences indicated that MDH from
healthy and tumor tissue samples may exist in distinct structural
states, which may be associated with the various tumor
microenvironmental conditions. The condition of the milieu, where
the enzyme operates, may affect the enzyme kinetics. It is
important to note that the kinetic diversity of MDH may be due to
post-translational modification during tumorigenesis. Further
investigations are required to detect the post-translational
modification of MDH in cancer tissue samples and recognize the
effect on the MDH structure and the kinetic parameters.
In conclusion, the hypothesis of the present study,
which addressed the kinetics and role of MDH in supplying
NAD+, remains unanswered as the enzyme were not purified
and fully characterized. However, the results obtained in the
current study are, to the best of our knowledge, the first step in
this field. The results highlight another approach to support
glycolysis in MDA-MB-231 and propose that cancer cells adapt to a
situation, where the energy generation pathway is targeted through
LDH inhibition.
Acknowledgements
The present study was funded by grants from Shahid
Beheshti University of Medical Sciences (Tehran, Iran; grant no.
93/10/09/13061) and Shahid Chamran University of Ahvaz Research
Council (Ahvaz, Iran; grant no. 94/3/02/31580).
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Talaiezadeh A, Shahriari A, Tabandeh MR,
Fathizadeh P and Mansouri S: Kinetic characterization of lactate
dehydrogenase in normal and malignant human breast tissues. Cancer
Cell Int. 15:192015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koukourakis MI, Giatromanolaki A,
Simopoulos C, Polychronidis A and Sivridis E: Lactate dehydrogenase
5 (LDH5) relates to up-regulated hypoxia inducible factor pathway
and metastasis in colorectal cancer. Clin Exp Metastasis. 22:25–30.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D
and Lou W: Lactate dehydrogenase A is overexpressed in pancreatic
cancer and promotes the growth of pancreatic cancer cells. Tumour
Biol. 34:1523–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Israël M and Schwartz L: The metabolic
advantage of tumor cells. Mol Cancer. 10:702011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murray RK, Granner DK, Mayes PA and
Rodwell VW: Harper's illustrated biochemistry. 26th edition.
McGraw-Hill; New York, NY: 2003
|
|
10
|
Storey KB and Brooks SPJ: The basis of
enzymatic adaptation. Principles of Medical Biology. 4:Part
1147–169. 1995. View Article : Google Scholar
|
|
11
|
Fukumura D and Jain RK: Tumor
microenvironment abnormalities: Causes, consequences, and
strategies to normalize. J Cell Biochem. 101:937–949. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grisham MB, Bernstein LH and Everse J: The
cytoplasmic malate dehydrogenase in neoplastic tissues; presence of
a novel isoenzyme? Br J Cancer. 47:727–731. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Belfiore F, Borzi V, Vecchio LL, Napoli E
and Rabuazzo AM: Enzyme activities of NADPH-forming metabolic
pathways in normal and leukemic leukocytes. Clin Chem. 21:880–883.
1975.PubMed/NCBI
|
|
15
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brooks SPJA: A simple computer program
with statistical tests for the analysis of enzyme kinetics.
Biotechniques. 13:906–911. 1992.PubMed/NCBI
|
|
17
|
Brooks SPJA: A program for analyzing
enzyme rate data obtained from a microplate reader. Biotechniques.
17:1154–1161. 1994.PubMed/NCBI
|
|
18
|
Koukourakis MI, Kontomanolis E,
Giatromanolaki A, Sivridis E and Liberis V: Serum and tissue LDH
levels in patients with breast/gynaecological cancer and benign
diseases. Gynecol Obstet Invest. 67:162–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koukourakis MI, Giatromanolaki A, Winter
S, Leek R, Sivridis E and Harris AL: Lactate dehydrogenase 5
expression in squamous cell head and neck cancer relates to
prognosis following radical or postoperative radiotherapy.
Oncology. 77:285–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deberardinis RJ, Sayed N, Ditsworth D and
Thompson CB: Brick by brick: Metabolism and tumor cell growth. Curr
Opin Genet Dev. 18:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robey IF, Lien AD, Welsh SJ, Baggett BK
and Gillies RJ: Hypoxia-inducible factor-1α and the glycolytic
phenotype in tumors. Neoplasia. 7:324–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allison SJ, Knight JR, Granchi C, Rani R,
Minutolo F, Milner J and Phillips RM: Identification of LDH-A as a
therapeutic target for cancer cell killing via (i)
p53/NAD(H)-dependent and (ii) p53-independent pathways.
Oncogenesis. 3:e1022014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi Y and Pinto BM: Human lactate
dehydrogenase a inhibitors: A molecular dynamics investigation.
PLoS One. 9:e863652014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balinsky D, Platz CE and Lewis JW: Isozyme
patterns of normal, benign, and malignant human breast tissues.
Cancer Res. 43:5895–5901. 1983.PubMed/NCBI
|