Introduction
Maple syrup, which is produced by boiling down sap
collected from the sugar maple (Acer saccharum), is among
the most commonly consumed natural sweeteners worldwide (1,2). The sugar
maple is distributed throughout northeastern North America, and
maple syrup is mainly produced in this region (2). The North American maple tree has served
an important role in traditional medicine among Native Americans
(3).
Maple syrup mainly comprises sucrose, but also
contains various other components, such as oligosaccharides,
polysaccharides, organic acids, amino acids, vitamins and minerals
(1,2,4–8). Previous studies, some recent, reported
that maple syrup also contains several phytochemicals, including
phenolic compounds that present hypoglycemic, antioxidant,
antimutagenic, anticancer, anti-inflammatory, antibiotic and
anti-neurodegenerative effects (9–17). Moreover,
biological effects have been noted following maple syrup
consumption. In a model of type 2 diabetes mellitus (the Otsuka
Long-Evans Tokushima Fatty rat), oral administration of maple syrup
leads to a lower increase in plasma glucose compared to oral
administration of sucrose (18).
Additionally, maple syrup administration inhibits colorectal cancer
cell proliferation and invasion via inhibition of protein kinase B
(AKT) activation (19).
The composition and sugar content of maple sap are
associated with climatic conditions of the production season
(20–23). Therefore, the color, aroma and taste of
maple syrup varies based on differences in growth conditions and on
the season of sap collection. Maple syrup grade is primarily
determined based on flavor and color, ranging from very
light-colored and delicately flavored to very dark-colored and
strongly flavored (2). This suggests
that different grades of maple syrup could have different
biological effects; however, this subject has been scarcely
studied. The authors previously reported that maple syrup
demonstrates an inhibitory effect on colorectal cancer cell growth
and invasion, and that this anticancer effect correlates with
darker maple syrup color (19).
In the present study, the authors examined the
effects of different grades of maple syrup on gastrointestinal
cancer cell proliferation. The two types of maple syrup that
indicated the strongest and weakest anticancer effects in our
previous study of colon cancer cells were used. The aim of the
present work was to investigate whether the dark-color maple syrup
is suitable as a phytomedicine and whether it may be useful in the
development of novel anticancer drugs for gastrointestinal
cancer.
Materials and methods
Materials
Chemicals and reagents of the highest grade
available were purchased as follows: urea from GE Healthcare Life
Sciences (Chalfont, UK); 3-(3-cholamidepropyl)
dimethylammonio-1-propanesulphonate (CHAPS) from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan); and thiourea and Triton X-100 from
Nacalai Tesque, Inc. (Kyoto, Japan). All other chemicals and
reagents were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany).
Maple syrup samples
Maple syrups were purchased at a local grocery store
(Osaka, Japan) in 2015. The authors selected two maple syrups of
different colors: Maple syrup A was slightly golden, and maple
syrup B was very dark brown.
High-performance liquid chromatography
(HPLC) analysis
To determine the carbohydrate concentrations of each
maple syrup type, an LC-10Advp HPLC system was used (Shimadzu
Corporation, Kyoto, Japan) equipped with a Corona Veo detector
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and an Asahipak
NH2P-50 4E (Shimadzu Corporation) column at room temperature
(~23°C). The mobile phase was acetonitrile/milliQ water (3:1; v/v),
at a flow rate of 1 ml/min. A total of 20 µl sample solution was
then injected, which was prepared as follows. The authors
evaporated 10 µl maple syrup using a Spin Dryer mini VC-15S (Taitec
Corporation, Saitama, Japan). The residue was resuspended in 250 µl
water, and then we extracted the hydrophobic components of the
maple syrup solution using ethyl acetate. The aqueous phase was
ultrafiltered using an Amicon Ultra 10K device (EMD Millipore,
Billerica, MA, USA) to remove high-molecular-weight components.
Finally, the filtrate was diluted 1:100 in water.
Gastrointestinal cancer cell
lines
The SW480 colorectal cancer cell line, KATO III
gastric cancer cell line, OE33 esophageal cancer cell line, and
PANC-1 pancreatic cancer cell lines were obtained from the American
Type Culture Collection (Manassas, VA, USA). All cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) in an atmosphere
containing 5% CO2.
Cell proliferation assays
Cell lines were cultured in six-well plates at a
density of 5×104 cells/well. At 24 h of growth, the
normal medium was replaced with culture medium containing 1% (v/v)
maple syrup, based on the author's previous study (19). Following 24, 48, 72 and 96 h of growth,
the number of cells was counted using a Countess Automated Cell
counter (Thermo Fisher Scientific, Inc.).
Protein preparation
Cells were plated at a density of 5×105
cells per 100 mm dish. At 24 h of growth, the normal medium was
replaced with culture medium containing maple syrup. Following 72
h, the cells were solubilized in urea lysis buffer (7 M urea, 2 M
thiourea, 5% CHAPS and 1% Triton X-100). The protein concentration
was measured using the Bio-Rad Protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Western blot analysis
A total of 10 µg protein was added to each well and
was subjected to 10% SDS-PAGE under reducing conditions, and the
separated proteins were transferred to polyvinylidene fluoride
transfer membranes. Following blocking in TBS-Tween-20 (0.1%)
buffer with 5% skim milk for 2 h at room temperature, the membranes
were incubated at 4°C overnight with an anti-phospho-AKT (1;1,000;
cat. no. 4051; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-phospho-p44/42 MAPK (1;1,000; cat. no. 4370; Cell Signaling
Technology, Inc.), anti-phospho-SAPK/JNK (1;1,000; cat. no. 4668;
Cell Signaling Technology, Inc.), or anti-phospho-p38 MAPK antibody
(1;1,000; cat. no. 4511; Cell Signaling Technology, Inc.). Then the
membranes were washed and incubated with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse IgG antibody
(American Qualex, San Clemente, CA, USA). Following washing, the
blots were visualized using SuperSignal West Dura Extended Duration
substrate (Thermo Fisher Scientific, Inc.), and bands were detected
using a myECL Imager system (version 2.0; Thermo Fisher Scientific,
Inc.). Next, the same membranes were re-probed with anti-β-actin
(Sigma-Aldrich; Merck KGaA), anti-AKT, anti-p44/42 MAPK (Erk1/2),
anti-SAPK/JNK or anti-p38 MAPK antibody (Cell Signaling Technology,
Inc.) to confirm equal loading of the proteins. All western blot
analyses were performed in triplicate.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. The data were analyzed using one-way analysis of
variance followed by Dunnett's test. P≤0.05 was considered to
indicate a statistically significant difference. Computations were
performed using GraphPad Prism software (version 5; GraphPad
Software, La Jolla, CA, USA).
Results
Carbohydrate concentrations in the two
maple syrup types
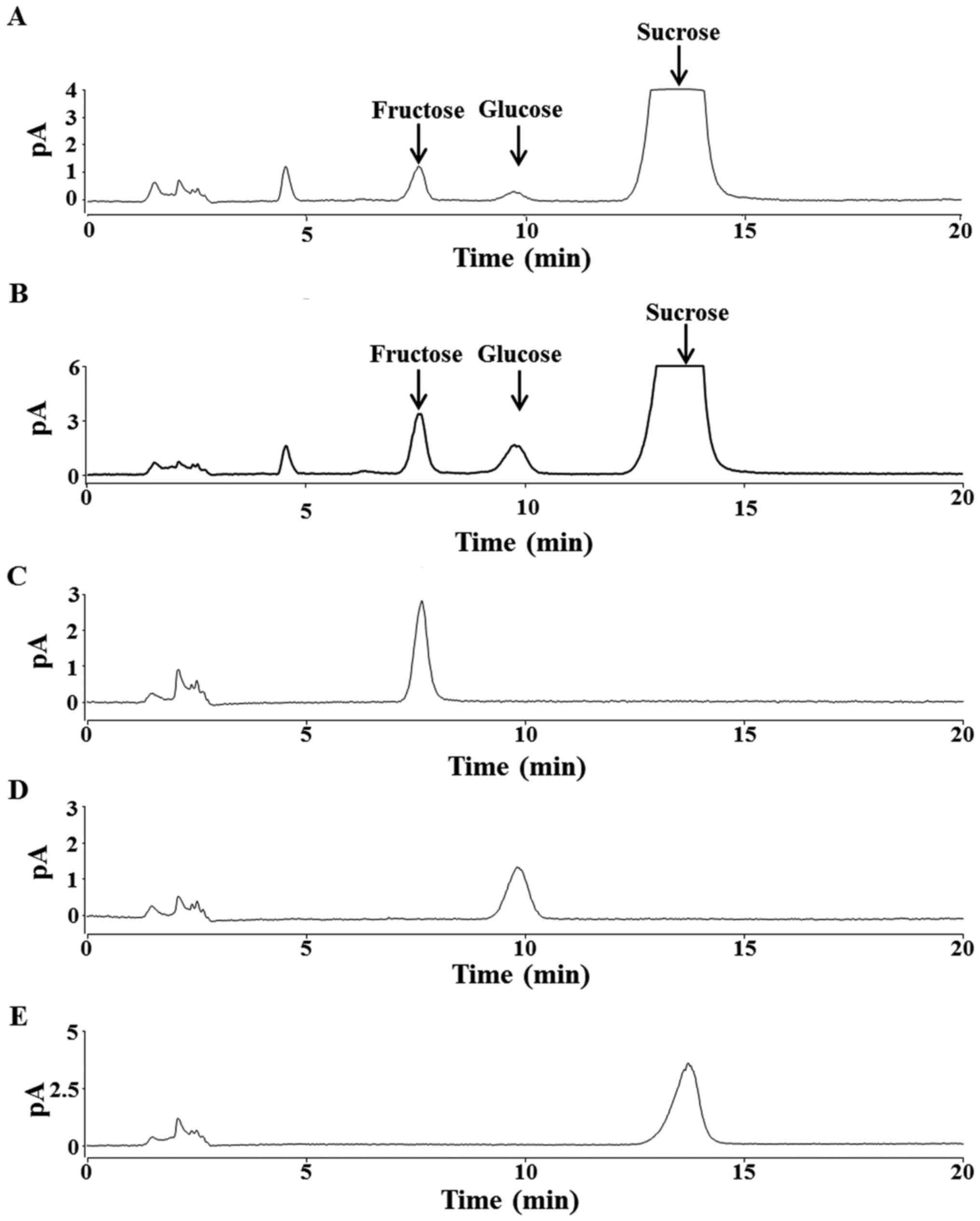
HPLC analysis revealed that both maple syrup A and B
contained abundant sucrose, and smaller concentrations of glucose
and fructose (Fig. 1A and B). Sucrose
concentration did not significantly differ between the two types of
maple syrup. Compared to maple syrup A, maple syrup B contained
significantly higher concentrations of glucose and fructose
(P<0.05; Table I).
 | Table I.Maple syrup color and carbohydrate
concentrations. |
Table I.
Maple syrup color and carbohydrate
concentrations.
| Variables | Color | Sucrose (g/100
ml) | Glucose (g/100
ml) | Fructose (g/100
ml) |
|---|
| Maple syrup A | Slightly golden | 53.02±2.20 | 0.28±0.03 | 0.82±0.05 |
| Maple syrup B | Very dark brown | 49.83±0.16 |
1.91±0.05a |
2.38±0.02a |
Effect of maple syrup on
gastrointestinal cancer cell growth
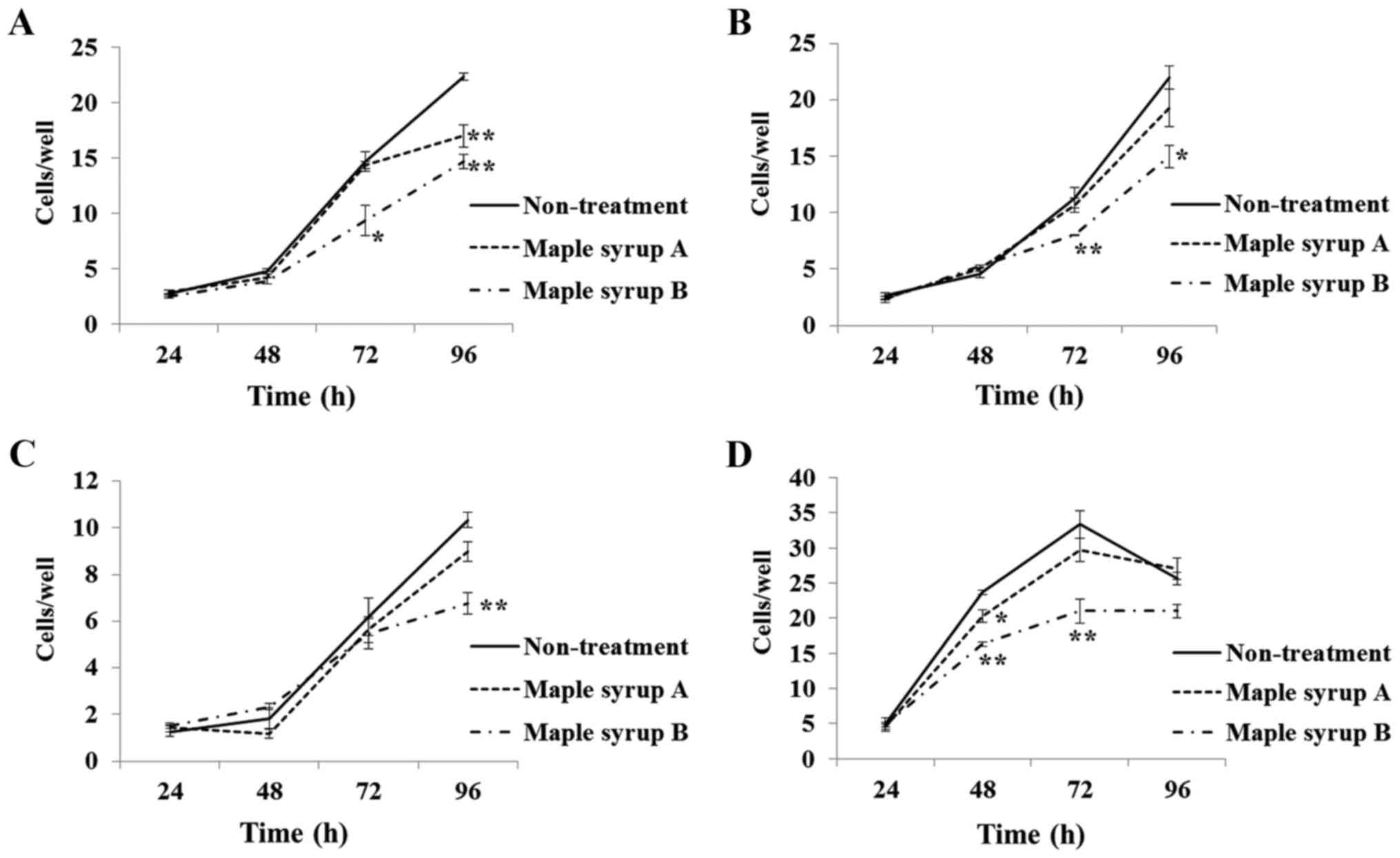
Compared to non-treated control cells,
administration of maple syrup B significantly inhibited the growth
of SW480 (P<0.01; Fig. 2A; 72 and
96 h), KATO III (P<0.05; Fig. 2B;
72 and 96 h), OE33 (P<0.01; Fig.
2C; 96 h), and PANC-1 (P<0.01; Fig.
2D; 48 and 72 h) cells. Administration of maple syrup A also
significantly inhibited the growth of SW480 (P<0.01; Fig. 2A; 96 h) and PANC-1 (P<0.05; Fig. 2D; 48 h) cells as compared to
non-treated control cells.
Effects of maple syrup on signaling
pathways in gastrointestinal cancer cells
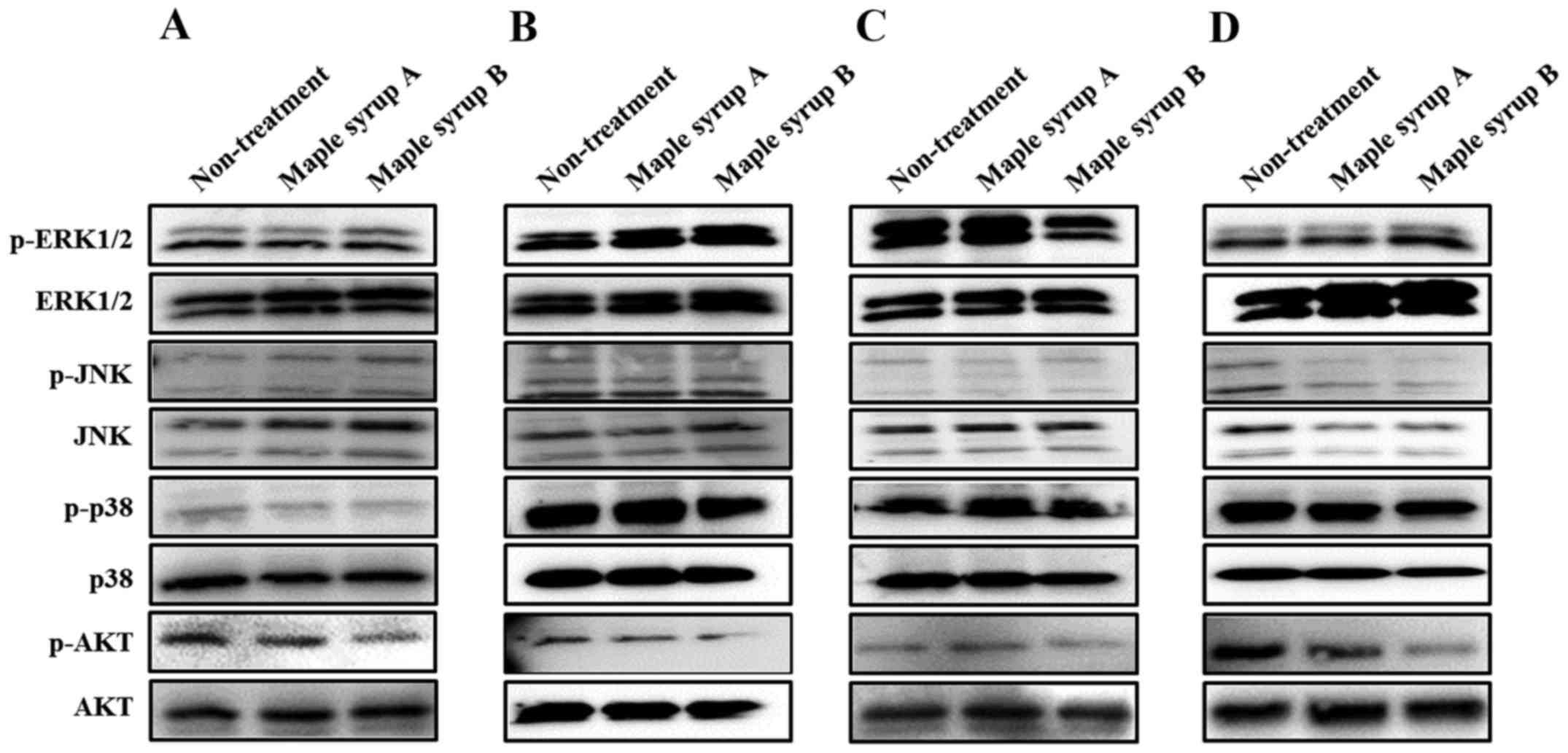
To determine which signaling pathway was affected by
maple syrup administration, the authors examined the
phosphorylation of ERK, JNK, p38 and AKT, which serve important
roles in gastrointestinal cancer cell proliferation. Administration
of maple syrup B visibly inhibited AKT phosphorylation in all
tested gastrointestinal cancer cell lines, as compared to
non-treated cells and cells treated with maple syrup A (Fig. 3A-D). Conversely, administration of
maple syrup B inhibited phosphorylation of ERK1/2 in OE33 cells
(Fig. 3C). Maple syrup administration
did not influence MAPK activation in any other tested
gastrointestinal cancer cell lines.
Discussion
In the present study, the authors examined the
inhibitory effects of two different grades of maple syrup on cell
proliferation of esophageal cancer (OE33), gastric cancer (KATO
III), colon cancer (SW480) and pancreatic cancer (PANC-1) cell
lines. As carbohydrates are the primary components of maple syrup,
the authors first examined the carbohydrate composition of each
maple syrup type to determine a suitable maple syrup dosage to
avoid cytotoxic effects from excessive sucrose. HPLC analysis of
each maple syrup type presented three peaks, corresponding to
sucrose, glucose and fructose, with sucrose being the primary
component, which is consistent with previous reports (Fig. 1A and B) (1,24). Maple
syrup B had significantly higher concentrations of glucose and
fructose, compared to maple syrup A (Table
I). Conversely, sucrose concentration was similar in both
grades of maple syrup (Table I), and
also similar to the authors' previous analysis of maple syrup that
was purchased in a different year (19). Thus, for further studies, the authors
used the same dose of maple syrup as was administered in previous
reports, which did not induce cytotoxicity due to high sucrose
concentration (19).
It was identified that administration of maple syrup
B led to a significant decrease in the cell growth rate of all
examined gastrointestinal cancer cells compared to non-treated
control cells (Fig. 2). Additionally,
administration of maple syrup A led to significant inhibition of
cell growth among colon and pancreatic cancer cells compared to
non-treated control cells (Fig. 2A and
D). Interestingly, the dark-color maple syrup had an
anti-proliferative effect on all investigated gastrointestinal
cancer cells, even though it contained a higher amount of glucose
than the golden-color maple syrup. In contrast, the golden-color
maple syrup did not significantly inhibit cell proliferation for
all investigated gastrointestinal cancer cells, but it did present
a tendency to suppress cell proliferation. These data suggested
that the maple syrup contained an active ingredient that suppressed
cancer cell proliferation, and that the amount of this active
ingredient increased with darkening syrup color.
Following this, the authors examined how maple syrup
affected the phosphorylation status of ERK, JNK, p38 and AKT, which
each serve important roles in cancer cell proliferation.
Administration of dark-color maple syrup inhibited AKT activation
in colon cancer cells, consistent with previous data (19). Moreover, it demonstrated similar
inhibitory effects in the other gastrointestinal cancer cells used
in this study. Maple syrup contains various phenolic compounds,
including lignans and coumarins. Such phenolic compounds have been
previously reported to present inhibitory effects on AKT activation
in cancer cells (25–31). The present data suggested that
dark-color maple syrup may contain larger amounts of bioactive
compounds, such as phenolic compounds.
On the other hand, administration of dark-color
maple syrup did not affect MAPK activation, except for an
inhibitory effect on ERK activation in esophageal cancer cells
(Fig. 3). Notably, despite suppression
of two signaling pathways related to cell proliferation, the
inhibitory effect on esophageal cancer cell proliferation following
administration of dark-color maple syrup was to a lesser extent
than the effect observed in other gastrointestinal cancer cells.
These data suggested that administration of dark-color maple syrup
in esophageal cancer cells may have other effects, such as
inhibition of cancer cell invasion, particularly since a previous
study of the authors demonstrated that administration of dark-color
maple syrup inhibited colon cancer cell invasion (19).
The current results indicated that dark-color maple
syrup had anti-cancer effects in upper digestive tract cancer cell
lines, such as esophageal and gastric cancer. This suggests that
routine intake of dark-color maple syrup could potentially suppress
the progression of esophageal and gastric cancer. Moreover,
administration of dark-color maple syrup presented anti-cancer
effects in pancreatic cancer cells, which is a type of cancer that
is refractory to current treatments. Therefore, the bioactive
compounds in dark-color maple syrup may be useful in the
development of novel anti-cancer drugs for treatment of
gastrointestinal cancer as well as refractory cancers. Further
studies are required to clarify the optimal dosage and
administration method for using dark-color maple syrup as a
phytomedicine for cancer treatment. Future studies are also
warranted to identify the bioactive compounds in dark-color maple
syrup that are responsible for repressing cancer cell proliferation
through inhibition of the AKT signaling pathway.
In conclusion, the present data demonstrated that
dark-color maple syrup reduced gastrointestinal cancer cell growth
through inhibition of the AKT signaling pathway. These findings
suggested that dark-color maple syrup may be useful as
phytomedicine, potentially preventing cancer progression. The
present data may also be useful for future development of novel
anti-cancer drugs for gastrointestinal cancer treatment with fewer
adverse effects than traditional chemotherapy.
Acknowledgements
The present study was supported in part by a MEXT
(Ministry of Education, Culture, Sports. Science and
Technology)-Supported Program for the Strategic Research Foundation
at Private Universities (grant no. S1411037), and by a Grant-in-Aid
for Scientific Research from the Japan Society for the Promotion of
Science to T.Y. (grant no. 15K09054).
References
|
1
|
Ball DW: The chemical composition of maple
syrup. J Chem Educ. 84:1647–U1646. 2007. View Article : Google Scholar
|
|
2
|
Perkins TD and van den Berg AK: Maple
syrup-production, composition, chemistry, and sensory
characteristics. Adv Food Nutr Res. 56:101–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnason T, Hebda RJ and Johns T: Use of
plants for food and medicine by Native Peoples of eastern Canada.
Can J Bot. 59:2189–2325. 1981. View
Article : Google Scholar
|
|
4
|
Davison RM and Young H: Abscisic-acid
content of xylem sap. Planta. 109:95–98. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taga A, Sato A, Suzuki K, Takeda M and
Kodama S: Simple determination of a strongly aromatic compound,
sotolon, by capillary electrophoresis. J Oleo Sci. 61:45–48. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taga A and Kodama S: Analysis of reducing
carbohydrates and fructosyl saccharides in maple syrup and maple
sugar by CE. Chromatographia. 75:1009–1016. 2012. View Article : Google Scholar
|
|
7
|
Storz G, Darvill AG and Albersheim P:
Characterization of polysaccharides isolated from maple syrup.
Phytochemistry. 25:437–441. 1986. View Article : Google Scholar
|
|
8
|
Sun J, Ma H, Seeram NP and Rowley DC:
Detection of Inulin, a Prebiotic Polysaccharide, in Maple Syrup. J
Agric Food Chem. 64:7142–7147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Apostolidis E, Li LY, Lee C and Seeram NP:
In vitro evaluation of phenolic-enriched maple syrup extracts for
inhibition of carbohydrate hydrolyzing enzymes relevant to type 2
diabetes management. J Funct Foods. 3:100–106. 2011. View Article : Google Scholar
|
|
10
|
Legault J, Girard-Lalancette K, Grenon C,
Dussault C and Pichette A: Antioxidant activity, inhibition of
nitric oxide overproduction, and in vitro antiproliferative effect
of maple sap and syrup from Acer saccharum. J Med Food. 13:460–468.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
González-Sarrías A, Li L and Seeram NP:
Effects of maple (Acer) plant part extracts on proliferation,
apoptosis and cell cycle arrest of human tumorigenic and
non-tumorigenic colon cells. Phytother Res. 26:995–1002. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L and Seeram NP: Maple syrup
phytochemicals include lignans, coumarins, a stilbene, and other
previously unreported antioxidant phenolic compounds. J Agric Food
Chem. 58:11673–11679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Theriault M, Caillet S, Kermasha S and
Lacroix M: Antioxidant, antiradical and antimutagenic activities of
phenolic compounds present in maple products. Food Chem.
98:490–501. 2006. View Article : Google Scholar
|
|
14
|
Hawco CLA, Wang Y, Taylor M and Weaver DF:
A maple syrup extract prevents β-amyloid aggregation. Can J Neurol
Sci. 43:198–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aaron C, Beaudry G, Parker JA and Therrien
M: Maple Syrup Decreases TDP-43 Proteotoxicity in a Caenorhabditis
elegans Model of Amyotrophic Lateral Sclerosis (ALS). J Agric Food
Chem. 64:3338–3344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maisuria VB, Hosseinidoust Z and Tufenkji
N: Polyphenolic extract from maple syrup potentiates antibiotic
susceptibility and reduces biofilm formation of pathogenic
bacteria. Appl Environ Microbiol. 81:3782–3792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma H, DaSilva NA, Liu W, Nahar PP, Wei Z,
Liu Y, Pham PT, Crews R, Vattem DA, Slitt AL, et al: Effects of a
Standardized Phenolic-Enriched Maple Syrup Extract on β-Amyloid
Aggregation, Neuroinflammation in Microglial and Neuronal Cells,
and β-Amyloid Induced Neurotoxicity in Caenorhabditis elegans.
Neurochem Res. 41:2836–2847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagai N, Ito Y and Taga A: Comparison of
the enhancement of plasma glucose levels in type 2 diabetes Otsuka
Long-Evans Tokushima Fatty rats by oral administration of sucrose
or maple syrup. J Oleo Sci. 62:737–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto T, Uemura K, Moriyama K, Mitamura
K and Taga A: Inhibitory effect of maple syrup on the cell growth
and invasion of human colorectal cancer cells. Oncol Rep.
33:1579–1584. 2015.PubMed/NCBI
|
|
20
|
Kim YT and Leech RH: Effects of climatic
conditions on sap flow in sugar maple. For Chron. 61:303–307. 1985.
View Article : Google Scholar
|
|
21
|
Marvin JW and Erickson RO: A statistical
evaluation of some of the factors responsible for the flow of sap
from the sugar maple. Plant Physiol. 31:57–61. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plamondon AP and Bernier PY: Modélisation
de la coulée de l'érable à sucre (Acersaccharum Marsh.) à partir
d'éléments météorologiques. Can J Res. 10:152–157. 1980. View Article : Google Scholar
|
|
23
|
Houle D, Paquette A, Côté B, Logan T,
Power H, Charron I and Duchesne L: Impacts of Climate Change on the
Timing of the Production Season of Maple Syrup in Eastern Canada.
PLoS One. 10:e01448442015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stuckel JG and Low NH: The chemical
composition of 80 pure maple syrup samples produced in North
America. Food Res Int. 29:373–379. 1996. View Article : Google Scholar
|
|
25
|
Huang JS, Yao CJ, Chuang SE, Yeh CT, Lee
LM, Chen RM, Chao WJ, Whang-Peng J and Lai GM: Honokiol inhibits
sphere formation and xenograft growth of oral cancer side
population cells accompanied with JAK/STAT signaling pathway
suppression and apoptosis induction. BMC Cancer. 16:2452016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y, Zhang Q, Bao J, Du CH, Wang J,
Tong Q and Liu C: Schisandrin B suppresses glioma cell metastasis
mediated by inhibition of mTOR/MMP-9 signal pathway. Biomed
Pharmacother. 74:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hyun S, Kim MS, Song YS, Bak Y, Ham SY,
Lee DH, Hong J and Yoon DY: Peroxisome proliferator-activated
receptor-gamma agonist 4-O-methylhonokiol induces apoptosis by
triggering the intrinsic apoptosis pathway and inhibiting the
PI3K/Akt survival pathway in SiHa human cervical cancer cells. J
Microbiol Biotechnol. 25:334–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zilla MK, Nayak D, Amin H, Nalli Y, Rah B,
Chakraborty S, Kitchlu S, Goswami A and Ali A:
4′-Demethyl-deoxypodophyllotoxin glucoside isolated from
Podophyllum hexandrum exhibits potential anticancer activities by
altering Chk-2 signaling pathway in MCF-7 breast cancer cells. Chem
Biol Interact. 224:100–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeon YJ, Cho JH, Lee SY, Choi YH, Park H,
Jung S, Shim JH and Chae JI: Esculetin Induces Apoptosis Through
EGFR/PI3K/Akt Signaling Pathway and Nucleophosmin Relocalization. J
Cell Biochem. 117:1210–1221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsieh CJ, Kuo PL, Hou MF, Hung JY, Chang
FR, Hsu YC, Huang YF, Tsai EM and Hsu YL: Wedelolactone inhibits
breast cancer-induced osteoclastogenesis by decreasing Akt/mTOR
signaling. Int J Oncol. 46:555–562. 2015.PubMed/NCBI
|
|
31
|
Kim WJ, Lee MY, Kim JH, Suk K and Lee WH:
Decursinol angelate blocks transmigration and inflammatory
activation of cancer cells through inhibition of PI3K, ERK and
NF-kappaB activation. Cancer Lett. 296:35–42. 2010. View Article : Google Scholar : PubMed/NCBI
|