Introduction
Helicobacter pylori (Hp) infection is a major
cause of gastric cancer (1–3). It has been previously demonstrated that
Hp eradication significantly reduces the incidence of metachronous
gastric cancer following endoscopic resection of early gastric
cancer (4).
Therefore, eradication therapy for patients with
Hp-positive chronic gastritis, now covered by the social insurance
systems in Japan, is crucial in the assessment of the Hp status
when undergoing endoscopic examination (4). Furthermore, the use of proton-pump
inhibitors (PPIs), anti-platelet and anti-coagulant has become
widespread in the clinic. Consequently, it is imperative to
establish diagnostic criteria to distinguish Hp-positive stomachs
on the basis of endoscopic findings alone.
Many clinical studies have reported on the
diagnostic performance of image-enhanced endoscopy (IEE) techniques
such as narrow-band imaging (NBI) for diagnosing endoscopic lesions
(5–8).
NBI enhances the visualization of the surface vascular pattern
using optical filters against a xenon lamp that allows narrow-band
light to pass at wavelengths of 415 and 540 nm. Combining the NBI
and magnifying endoscopy provides accurate real-time diagnostic
performance in gastric neoplastic lesion as well as Hp gastritis
compared to conventional white light endoscopy (9,10).
Recently, Fujifilm developed an endoscope system
with a semiconductor laser as a light source. The system includes
two types of lasers with wavelengths of 410 and 450 nm. The 450 nm
laser irradiates phosphor to produce illumination light similar to
that obtained with a xenon lamp. The combination of strong 410 nm
laser light, weak 450 nm laser light, and fluorescent light enables
blue laser imaging (BLI) via narrow-band light observation.
Magnifying endoscopy with BLI is useful for evaluating mucosal
surface information such as surface blood vessel and structure
patterns (11–15). The present study reported that the
diagnostic ability of magnifying BLI endoscopy in early gastric
cancer is higher than that of conventional white light endoscopy
and was similar to that of magnifying NBI endoscopy (15).
Magnifying BLI is also potentially useful in
distinguishing Hp-positive stomachs as magnifying NBI. We aimed to
investigate the diagnostic ability of magnifying BLI endoscopy to
distinguish Hp-positive stomach in cancer-free subjects. The data
were also compared to the diagnostic ability of magnifying NBI
endoscopy.
Patients and methods
Patients
Study participants were prospectively enrolled from
cancer-free patients of the endoscopy Center of Fujita Health
University (Toyoake, Japan) and Ieda Hospital (Toyota, Japan) from
December 2012 to December 2015. In total, 215 participants were
initially invited, and all agreed to participate. All the
participants underwent upper gastroscopy for various indications,
including annual screening for gastric cancer, secondary complete
check-up after barium radiographic examination due to a suspicion
of gastric cancer or peptic ulcer disease, and complaints of
abdominal discomfort. Of the 215 participants, 112 and 113
participants were assigned to the NBI and BLI groups, respectively,
in a random manner. Patients were excluded from the study if they
had malignancy, severe systemic disease or advanced chronic liver
disease; were receiving non-steroidal anti-inflammatory drugs, PPIs
or H2 receptor antagonists; had undergone Hp eradication
therapy; and had a history of gastric surgery.
The Fujita Health University School of Medicine
approved the protocol (IRB no. HM16-225). Written informed consent
was obtained from all participants.
Endoscopic procedure
Prior to endoscopic examination being initiated,
20,000 units of pronase (Pronase MS; Kaken Pharmaceutical Products
Inc., Tokyo, Japan) were administered to each participant to remove
gastric mucus. The magnifying video endoscope used in the present
study was a GIF-H260Z (Olympus Medical Systems Co., Tokyo, Japan)
for the NBI group and an EG-L590ZW (Fujifilm Corp., Tokyo, Japan)
for the BLI group, respectively.
After the endoscope was inserted, the entire stomach
was initially observed with conventional white light to exclude
obvious lesions. At least 40 images were captured from the entire
stomach, even if there was no obvious lesion.
After the initial survey, the NBI or BLI light
source was turned on, and the non-pathological mucosa of the
greater curvature of the gastric middle and upper corpus were
carefully evaluated with magnification view, the distal tip of the
endoscope being attached to the mucosa. During the procedure, a
transparent hood, Elastic Touch (Top Corp., Tokyo, Japan), was
attached approximately 1.5 mm distal to the tip of the endoscope to
maintain the focal distance. This attachment allowed us to obtaine
clear, magnified images more quickly. For the BLI system, the high
contrast mode (BLI-contrast) was used to evaluate the gastric
mucosal pattern.
To determine Hp status by magnifying NBI or BLI
endoscopy, small, round pits, accompanied with regular
honeycomb-like subepithelial capillary networks (SECNs), being
regularly interspersed with collecting venules were considered to
indicate Hp infection-negative gastric mucosa (9,10) (Fig. 1). On the other hand, enlarged or
elongated pits with unclear SECNs or dense fine irregular vessels
were considered to indicate a Hp infection-positive gastric mucosa
(9) (Fig.
1). All of the endoscopic examinations were performed by a
single expert endoscopist (TT). Using the endoscopic image of the
most predominant magnifying NBI or BLI pattern, mucosal patterns of
each case were assessed via consensus among the two expert
endoscopists (TT and TS).
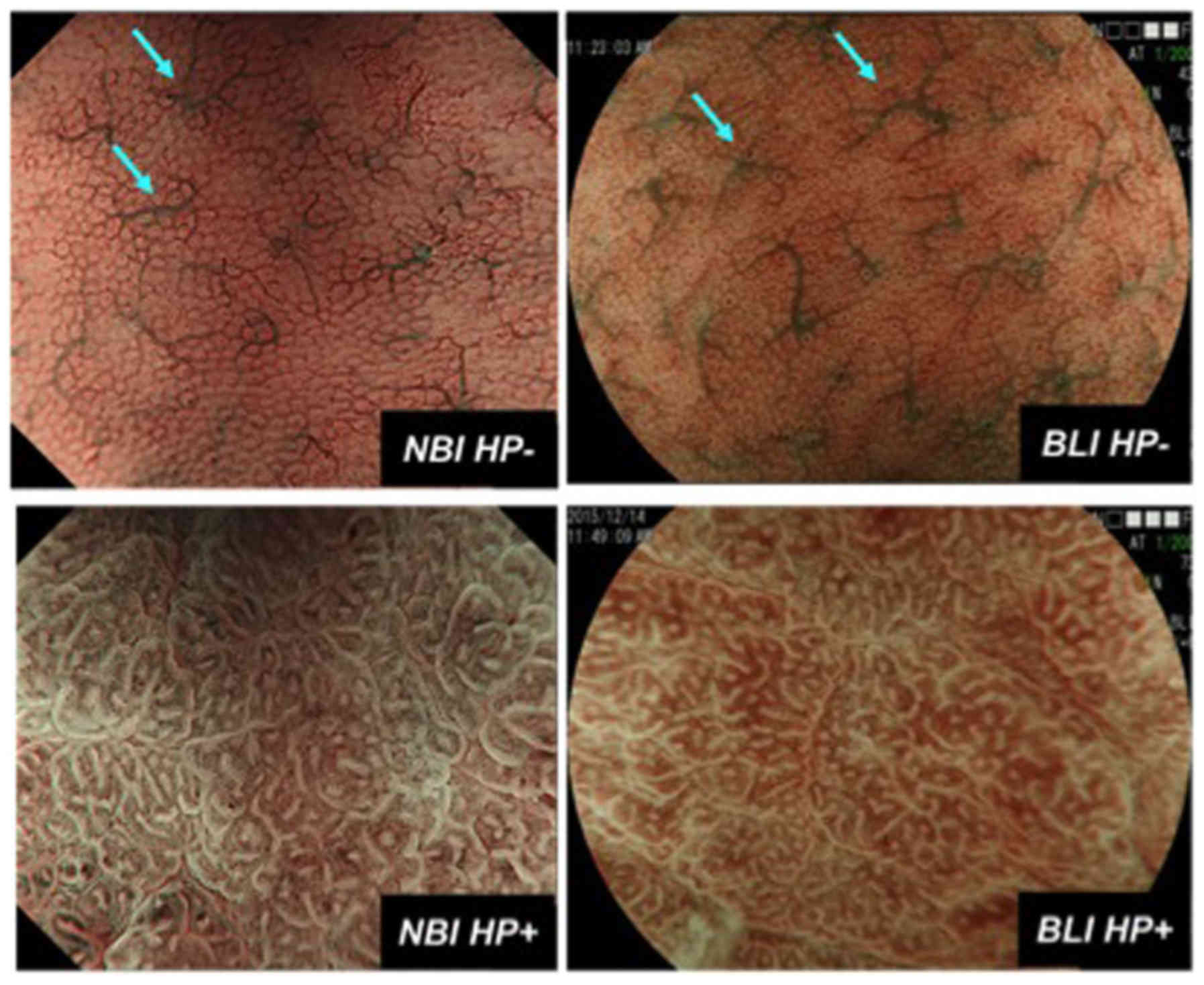 | Figure 1.Magnifying NBI (left) and BLI (right)
features of Hp infection negative (Hp−, upper) and
positive (Hp+, lower) gastric mucosa. Hp−
gastric mucosa is characterized as small, round pits, accompanied
with regular honeycomb-like SECNs, being regularly interspersed
with collecting venules (light blue arrow). On the other hand,
Hp+ gastric mucosa is characterized as enlarged or
elongated pits with unclear SECNs or dense fine irregular vessels.
NBI, narrow-band imaging; BLI, blue laser imaging; Hp,
Helicobacter pylori; SECNs, subepithelial capillary
networks. |
Detection of Hp infection
Urea breath test (UBT), histology using two biopsy
specimens from the greater curvature of the gastric antrum and the
corpus, as well as the serum titer, were used for the diagnosis of
Hp infection. A positive result in either of these tests was
diagnosed as Hp infection. A negative result in any of the tests
was diagnosed as Hp infection negative.
Data correction and statistical
analysis
The association between endoscopic mucosal patterns
using magnifying NBI or BLI endoscopy and Hp infection was
evaluated in terms of sensitivity, specificity, positive predictive
value (PPV) and negative predictive value (NPV). Sensitivity,
specificity, PPV and NPV among NBI and BLI groups were compared
using the Fisher's exact test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinicopathological
characteristics
Clinicopathological characteristics of subjects in
the NBI and BLI groups are shown in the Table I. Age and sex were not significantly
different between the two groups. During the endoscopy, neoplastic
lesions were not detected for either group, while 7 and 9 patients
were diagnosed as having gastric and duodenal ulcer in the NBI
group. On the other hand, 2 and 4 patients were diagnosed as having
gastric and duodenal ulcer in the BLI group, albeit the prevalence
of gastric and duodenal ulcers identified in the two groups was not
statistically significant (P>0.05).
 | Table I.Clinicopathological characteristics of
subjects. |
Table I.
Clinicopathological characteristics of
subjects.
| Variables | NBI group | BLI group |
|---|
| No. of subjects | 112 | 113 |
| Age in years, median
(range) | 64 (22–87) | 63 (24–86) |
| Sex, male (%) | 48 (42.9) | 44 (38.9) |
| Gastric ulcer, n
(%) | 7 (6.3) | 2 (1.8) |
| Duodenal ulcer, n
(%) | 9 (8) | 4 (3.5) |
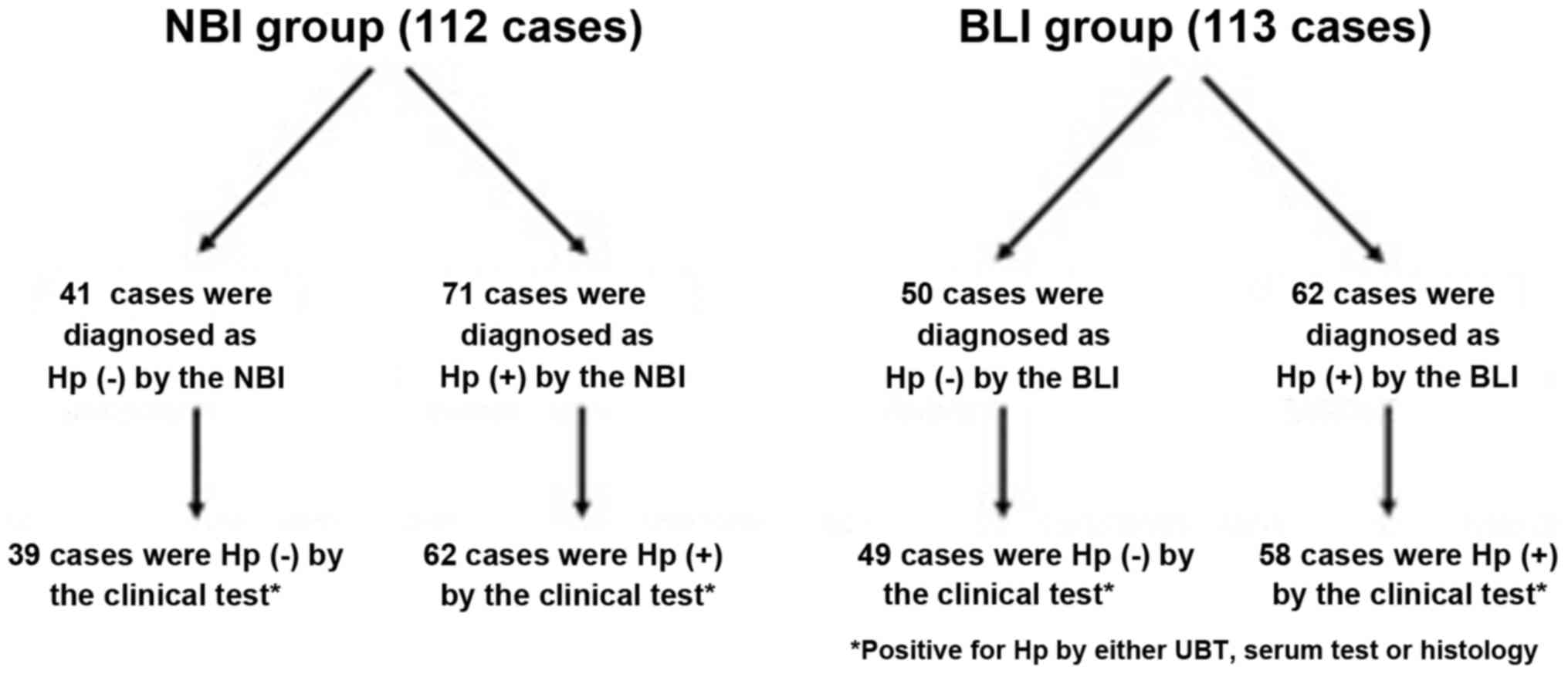
Hp infection in the NBI and BLI
groups
The mucosal patterns of the greater curvature of the
gastric corpus were clearly visualized in all cases in the NBI and
BLI groups (Fig. 1). In the NBI groups
(n=112), 41 cases were considered as Hp infection negative based on
the magnifying NBI pattern and 39 cases were diagnosed as Hp
infection negative by the clinical test (UBT, histology and serum
titer). On the other hand, 71 cases were considered as Hp infection
positive based on the magnifying NBI pattern and 62 cases were
diagnosed as Hp infection positive by the clinical test. As for the
BLI group (n=113), 50 cases were considered as Hp infection
negative based on the magnifying BLI pattern and 49 cases were
diagnosed as Hp infection negative by the clinical test. On the
other hand, 62 cases were considered as Hp infection positive based
on the magnifying NBI pattern and 58 cases were diagnosed as Hp
infection positive by the clinical test (Fig. 2). Sensitivity, specificity, PPV and NPV
for diagnosing Hp infection for the NBI group was 0.97, 0.81, 0.87
and 0.95, respectively. Sensitivity, specificity, PPV and NPV for
the BLI group was 0.98, 0.92, 0.93 and 0.98, respectively. There
was no significant difference in values between the NBI and BLI
groups (all P>0.2; Table II).
 | Table II.Sensitivity, specificity, PPV and NPV
in magnifying NBI and BLI endoscopy for predicting Helicobacter
pylori status. |
Table II.
Sensitivity, specificity, PPV and NPV
in magnifying NBI and BLI endoscopy for predicting Helicobacter
pylori status.
| Variables | Magnifying NBI
endoscopy | Magnifying BLI
endoscopy | P-value |
|---|
| Sensitivity | 0.97 | 0.98 | 1 |
| Specificity | 0.81 | 0.92 | 0.24 |
| PPV | 0.87 | 0.93 | 0.26 |
| NPV | 0.95 | 0.98 | 0.59 |
Discussion
Results of the present study have shown that the
diagnostic ability of magnifying BLI to distinguish Hp-positive
stomach seemed to be acceptable for clinical settings, which was
similar to the diagnostic ability of magnifying NBI. Magnifying BLI
uses narrow-band laser light combined with illumination light. The
system provides a high contrast clear view of fine mucosal and
capillary structures. Diagnostic effectiveness of BLI endoscopy has
been suggested in colorectal tumors and early gastric cancers
(11,15). The current study presents new evidence
that magnifying BLI endoscopy is useful in distinguishing
Hp-positive stomach. Similar to NBI endoscopy, the magnifying BLI
feature of Hp-negative gastric mucosa was characterized as small,
round pits, accompanied with regular honeycomb-like SECNs, being
regularly interspersed with collecting venules, while Hp-positive
gastric mucosa was characterized as various sizes of enlarged or
elongated pits with unclear SECNs or dense fine irregular vessels.
Previous findings have shown that the magnifying NBI patterns of
gastric mucosa are closely associated with histological degrees of
inflammation and atrophy (9). This is
because the histological condition of Hp-positive gastric mucosa is
characterized as chronic inflammation with enhanced infiltration of
neutrophils and mononuclear cells. Additionally, the continuous
destruction and regeneration of new vessels and edema due to severe
active inflammation may reflect increased density of irregular
microvessels and enlarged or prolonged pits, seen in Hp-positive
gastric mucosa. It is possible that magnifying NBI and BLI
visualizes fine mucosal patterns and capillary patterns in detail,
which suggests the more detailed histological condition of gastric
mucosa than the conventional white light endoscopy. Indeed, it has
been reported that white light endoscopy itself has limited ability
to distinguish Hp-infected subjects (10). It is essential to use IEE such as
magnifying NBI and BLI for the endoscopic diagnosis of Hp
infection.
Since the diagnostic ability was similar among
magnifying NBI and BLI, the magnifying BLI system would serve as a
promising tool for the diagnosis of Hp-positive stomach. The use of
PPIs, anti-platelet and anti-coagulant has recently become
widespread in the clinic. Diagnostic accuracy of a UBT, which is
thought to be most sensitive clinical test for detecting Hp, may be
decreased by the use of PPIs (16)
while the risk of bleeding due to biopsy should not be disregarded
for culture of Hp or histological analysis in patients taking
anti-platelet and anti-coagulant. Therefore, the magnifying BLI
system would play an important role for patients undergoing such
medications. In the present study, we assessed the magnifying NBI
and BLI mucosal patterns in the greater curvature of the gastric
middle and upper corpus, where the gastric atrophy would be mildest
in the stomach. It has also been suggested that magnifying
endoscopic assessment of Hp status should be carried out in mucosa
without atrophy (17). The
participants in the present study were all cancer-free patients.
However, it should be noted that endoscopic assessment of Hp status
may become difficult in patients of gastric cancer, especially
those that have spread of severe atrophy in the entire stomach.
In Japan, eradication therapy for patients with
Hp-positive chronic gastritis is now covered in medical insurance
schemes. It has been shown that the magnifying NBI endoscopy can be
useful in the assessment of Hp status following eradication therapy
(10,18). Our findings may lead to further
longitudinal investigation of magnifying BLI endoscopy to determine
its clinical utility in such settings.
In conclusion, the present finidngs show that the
diagnostic ability of magnifying BLI for predicting Hp status is
acceptable, since it is similar to that of magnifying NBI.
References
|
1
|
Parsonnet J, Friedman GD, Vandersteen DP,
Chang Y, Vogelman JH, Orentreich N and Sibley RK: Helicobacter
pylori infection and the risk of gastric carcinoma. N Engl J
Med. 325:1127–1131. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang JQ, Sridhar S, Chen Y and Hunt RH:
Meta-analysis of the relationship between Helicobacter
pylori seropositivity and gastric cancer. Gastroenterology.
114:1169–1179. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukase K, Kato M, Kikuchi S, Inoue K,
Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S and Asaka M:
Japan Gast Study Group: Effect of eradication of Helicobacter
pylori on incidence of metachronous gastric carcinoma after
endoscopic resection of early gastric cancer: An open-label,
randomised controlled trial. Lancet. 372:392–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayoshi T, Tajiri H, Matsuda K, Kaise M,
Ikegami M and Sasaki H: Magnifying endoscopy combined with narrow
band imaging system for early gastric cancer: Correlation of
vascular pattern with histopathology (including video). Endoscopy.
36:1080–1084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ezoe Y, Muto M, Uedo N, Doyama H, Yao K,
Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, et al: Magnifying
narrowband imaging is more accurate than conventional white-light
imaging in diagnosis of gastric mucosal cancer. Gastroenterology.
141:2017–2025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goda K, Tajiri H, Ikegami M, Urashima M,
Nakayoshi T and Kaise M: Usefulness of magnifying endoscopy with
narrow band imaging for the detection of specialized intestinal
metaplasia in columnar-lined esophagus and Barrett's
adenocarcinoma. Gastrointest Endosc. 65:36–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Machida H, Sano Y, Hamamoto Y, Muto M,
Kozu T, Tajiri H and Yoshida S: Narrow-band imaging in the
diagnosis of colorectal mucosal lesions: A pilot study. Endoscopy.
36:1094–1098. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahara T, Shibata T, Nakamura M, Yoshioka
D, Okubo M, Arisawa T and Hirata I: Gastric mucosal pattern by
using magnifying narrow-band imaging endoscopy clearly
distinguishes histological and serological severity of chronic
gastritis. Gastrointest Endosc. 70:246–253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yagi K, Saka A, Nozawa Y and Nakamura A:
Prediction of Helicobacter pylori status by conventional
endoscopy, narrow-band imaging magnifying endoscopy in stomach
after endoscopic resection of gastric cancer. Helicobacter.
19:111–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida N, Hisabe T, Inada Y, Kugai M,
Yagi N, Hirai F, Yao K, Matsui T, Iwashita A, Kato M, et al: The
ability of a novel blue laser imaging system for the diagnosis of
invasion depth of colorectal neoplasms. J Gastroenterol. 49:73–80.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshida N, Yagi N, Inada Y, Kugai M,
Okayama T, Kamada K, Katada K, Uchiyama K, Ishikawa T, Handa O, et
al: Ability of a novel blue laser imaging system for the diagnosis
of colorectal polyps. Dig Endosc. 26:250–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osawa H and Yamamoto H: Present and future
status of flexible spectral imaging color enhancement and blue
laser imaging technology. Dig Endosc. 26 Suppl 1:105–115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyaki R, Yoshida S, Tanaka S, Kominami Y,
Sanomura Y, Matsuo T, Oka S, Raytchev B, Tamaki T, Koide T, et al:
A computer system to be used with laser-based endoscopy for
quantitative diagnosis of early gastric cancer. J Clin
Gastroenterol. 49:108–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dohi O, Yagi N, Majima A, Horii Y,
Kitaichi T, Onozawa Y, Suzuki K, Tomie A, Kimura-Tsuchiya R, Tsuji
T, et al: Diagnostic ability of magnifying endoscopy with blue
laser imaging for early gastric cancer: A prospective study.
Gastric Cancer. 20:297–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shirin H, Frenkel D, Shevah O, Levine A,
Bruck R, Moss SF, Niv Y and Avni Y: Effect of proton pump
inhibitors on the continuous real time (13)C-urea breath test. Am J
Gastroenterol. 98:46–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yagi K, Nakamura A and Sekine A:
Magnifying endoscopy of the gastric body: A comparison of the
findings before and after eradication of Helicobacter
pylori. Dig Endosc. 14:S76–S82. 2002. View Article : Google Scholar
|
|
18
|
Okubo M, Tahara T, Shibata T, Nakamura M,
Yoshioka D, Maeda Y, Yonemura J, Ishizuka T, Arisawa T and Hirata
I: Changes in gastric mucosal patterns seen by magnifying NBI
during H. pylori eradication. J Gastroenterol. 46:175–182.
2011. View Article : Google Scholar : PubMed/NCBI
|